Abstract
Despite the effective suppression of viremia with antiretroviral therapy, HIV can still replicate in the central nervous system (CNS). This was a longitudinal study of the cerebrospinal fluid (CSF) and serum dynamics of several biomarkers related to inflammation, the blood-brain barrier, neuronal injury, and IgG intrathecal synthesis in serial samples of CSF and serum from a patient infected with HIV-1 subtype C with CNS compartmentalization.
The phylogenetic analyses of plasma and CSF samples in an acute phase using next-generation sequencing and F-statistics analysis of C2-V3 haplotypes revealed distinct compartmentalized CSF viruses in paired CSF and peripheral blood mononuclear cell samples. The CSF biomarker analysis in this patient showed that symptomatic CSF escape is accompanied by CNS inflammation, high levels of cell and humoral immune biomarkers, CNS barrier dysfunction, and an increase in neuronal injury biomarkers with demyelization. Independent and isolated HIV replication can occur in the CNS, even in HIV-1 subtype C, leading to compartmentalization and development of quasispecies distinct from the peripheral plasma. These immunological aspects of the HIV CNS escape have not been described previously. To our knowledge, this is the first report of CNS HIV escape and compartmentalization in HIV-1 subtype C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the effective suppression of viremia with antiretroviral (ARV) therapy, HIV can still replicate in the central nervous system (CNS), with the development of resistant strains in the CNS in patients with acute and subacute neurological manifestations (Haggerty and Stevenson 1991; Zarate et al. 2007; Harrington et al. 2009). Disagreement between the HIV viral loads in the plasma and CSF is defined by detectable levels of HIV RNA in the cerebrospinal fluid (CSF), indicative of a viral load of >200 copies/mL, when the viral load in the plasma is <50 copies/mL or by an HIV RNA viral load in the CSF that is ≥1 log higher than that in the plasma (Canestri et al. 2010).
HIV-1 subtypes present many structural and functional differences that may influence cellular tropism and organ involvement, including CNS involvement and cognitive impairment. The HIV-1 subtype C has been proposed to be less neuropathogenic than subtype B (Satishchandra et al. 2000), based on an in vitro defective trans-activator of transcription (Tat) chemokine dimotif in the position (C30C31) that might influence cellular trafficking and CNS inflammation (Ranga et al. 2004).
Compartments are defined as anatomical regions that restrict the genetic flow of HIV, thereby enabling viral evolution and divergence from the virus circulating in the peripheral blood. On the other hand, reservoirs are cells or anatomical sites where HIV or HIV-infected cells survive because the viral kinetics is slower than that in the peripheral blood. Compartments and reservoirs protect HIV from specific immune responses, ARV therapy, and biochemical changes, thereby providing an environment for pathogen-host interactions (North et al. 2010; Karris and Smith 2011). The CNS serves as an important reservoir for HIV, due to several specific constitutional characteristics (Haggerty and Stevenson 1991; Zarate et al. 2007; Karris and Smith 2011).
The aims of this study were to study the dynamics of several biomarkers related to inflammation, CNS barriers, neuronal injury, and IgG intrathecal synthesis. The biomarkers were measured in serial samples of CSF and serum from a patient infected with HIV-1 subtype C with CNS escape and compartmentalization. These immunological aspects of HIV CNS escape have not been described before by previous reports. As far as the authors know, this is the first report of CNS HIV escape in a HIV-1 subtype C infected patient.
Methods
HIV(+) CSF and serum samples
This study was approved by institutional review boards (IRB) at Hospital de Clínicas—UFPR, and Brazil National IRB (CONEP).
CSF samples were collected by lumbar puncture: sample 0 (September 28, 2008) AIDS diagnostic; sample 1 (January 13, 2011); sample A (February 25, 2011) collected at the time CSF and blood HIV RNA discordance (HIV CNS escape) was identified, before ARV change; sample B (May 11, 2011) 2.5 months after ARV change; sample C (August 18, 2012) was collected 18 months after ARV change; and sample D (September 18, 2013) was collected 31 months after ARV change (Table 1). Samples 0 and 1 were not included in the biomarker dynamic study.
CSF samples were collected sequentially from a 27-year-old heterosexual male Caucasian patient. He was a truck driver with 7 years of education. He had the disease since 29 months; HIV/AIDS was diagnosed in 2008, with the nadir CD4+ 6 cells/mm3; hemogram and CSF (Table 1; sample 0); HCV and HBV serology negative. ARV therapy was started with a combination of tenofovir (TDF), lamivudine (3TC), efavirenz (EFZ), and a CNS penetration-effectiveness (CPE) rank (Letendre et al. 2010) 1 + 2 + 3 = 6. Three months later (December 17, 2008), due to dizziness and somnolence, ARV therapy was changed to the following: atazanavir (ATV), ritonavir (RTV), TDF, CPE 2 + 1 + 1 = 4. He was also administered prophylaxis medication with sulfamethoxazole, trimethoprim, and azithromycin. Clinical and laboratorial (CSF and blood) follow-up characteristics are shown in Table 1.
Current medical history
The patient was hospitalized in January 2011 for 12 days with a history of progressive cognitive impairment, somnolence, confusion, weight loss, and fever starting a month ago. On neurologic examination, there was the presence of paratonia in the four limbs and a palmomental reflex was present. Both signs indicated impairment in the frontal lobe and impairment of intercommunications. Deep tendon reflexes had hyperreflexia (3+/5 in the upper limbs and 4+/5 in the lower limbs). The Glasgow Coma Scale (GCS) score was 14/15. The neurologic abnormalities started subacutely (>2 weeks). CSF cytology and biochemistry, immunological status, and CSF HIV RNA are shown in Table 1 (sample 1). There was recovery without intervention, and the patient was discharged.
Brain magnetic resonance imaging (MRI) at the time of presentation showed white matter hyperintensities on T2-weighted and FLAIR sequences; basal cistern, fissures, and cortical sulci slightly increased with age. The morphology of the IV ventricle was preserved in the midline. There were supratentorial ventricular dilations. Diffuse and symmetrical changes in the intensity of the signal through the subcortical white matter, periventricular, and semi-oval centers were characterized by a hyperintense signal on T2-weighted sequences and without significant changes in the T1-weighted sequence. Alterations in the supratentorial white matter were suggestive of diffuse encephalopathy (with demyelination and gliosis components). Subcortical atrophy was present and there were no signs of encephalic expansive processes intra- or extra-axis.
After 40 days, the patient was readmitted into the infectious diseases unit of the Hospital de Clínicas da UFPR in a coma, GCS 7 in 15. He was currently adherent to ARV (described above). Empiric treatment to neurotuberculosis was initiated (rifampicin, isoniazid, pyrazinamide, and ethambutol) due to the critical clinical status and CSF findings (Table 1, samples 1 and A), although PCR for Mycobacterium tuberculosis was negative and CSF lactic acid was in the normal range. HIV encephalitis was diagnosed and there was escape of HIV in the CNS. The log CSF HIV RNA was 4.4 and log plasma HIV RNA was 2.9 (CSF/plasma = 1.50). The genotypic testing for HIV-1 ARV resistance (sequencing of gag and pol region) showed no ARV resistance; HIV-1 genotyping indicated subtype C (Rotta et al. 2014). The ARV scheme was changed to a regimen with better CNS penetration (zidovudine [AZT], EFZ, 3TC; CPE 4 + 2 + 3 = 9. There was progressive clinical improvement. After 16 days of hospitalization (GCS 14/15), the patient was discharged from the hospital. After a change in the ARV scheme, the HIV viral load in the blood persisted and HIV in the CSF was undetectable.
Thirty-one months after the ARV scheme was changed, the patient had good clinical evolution, important improvement in neurologic deficits, persistent difficulty walking, a necessity for orthosis, and he began working again. MRI results remained unaltered, but there was clinical and CSF cytochemistry improvement (Fig. 1).
Selected MRI images (July 24, 2012); a–d (from up left to down right) show MRI at 17 months after the time when discordance was identified. Even though neurologic symptoms had resolved, imaging still showed volumetric reduction in the brain parenchyma, large basal cisterns, fissures, cortical sulci, and cerebellar follies. Hippocampal and para-hippocampal areas showed anatomic aspects and normal intensity signals. Large VI ventricles (a), a pervious cerebral aqueduct, a third ventricle and the lateral ventricles were slightly dilated. Confluent areas of hyperintense signal in T2-weighted and Flair were seen that were hypo-intense in T1 (b, c). Involvement of internal and external capsules was seen in the periventricular areas (d). There are no expansive brain processes intra- or extra-axis. Leukoencephalopathy with periventricular predominance was observed. There was a reduction in leukoencephalopathy compared with previous MRI results
HIV(−) control group (n = 19)
Although this was a descriptive study, an HIV(−) control group was added because the normal range of the majority of the biomarkers studied were not defined. The HIV(−) group was described previously (de Almeida et al. 2016a, b).
Clinical laboratory parameters
HIV RNA levels in the serum and CSF were quantified using a branched DNA assay (Siemens) with a nominal limit of detection at 50 copies/mL. CD4 counts were quantified by flow cytometry (FACSCalibur-Multitest), while nadir CD4 level information was extracted from the medical records.
CSF and serum biomarkers
Regulated on activation, normal T cell expressed and secreted (RANTES); urokinase plasminogen activator receptor (uPAR) and sCD-14 (R&D Systems, Minneapolis, MN); neopterin (Thermo Scientific); neurofilament light (NFL-light, UmanDiagnostic); and myelin basic protein (MBP, Ansh Labs) were quantified by high-sensitivity enzyme-linked immunosorbent assays.
Tumor necrosis factor α (TNFα), interferon γ (IFNγ), interleukin (IL-1β, IL-2, IL-4, IL-6, IL-7, and IL-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 α and β (MIP-1α and β), interferon gamma-induced protein-10 (IP-10) and sE-Selectin, soluble cell adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1), and metalloproteinase 2, 3, and 9 (MMP-2, MMP-3, and MMP-9) were quantified by multiplex bead suspension array immunoassays (EMD Millipore, Billerica, MA). Phosphorylated Tau-181 (pT-181, Invitrogen) in which primary antibodies were immobilized to dyed fluorescent microspheres. Total Tau, amyloidβ (Aβ 38, 40, 42), soluble amyloid precursor proteins α and β (sAPPα and sAPPβ), and tissue inhibitor metalloproteinases 1 and 2 (TIMP-1 and 2) were measured using an electrochemiluminescence assay (Meso Scale Discovery, Rockville, MD). Human β2 microglobulin (β2M) was quantified using the nephelometric method (Dade Behring BNII, Deerfield, IL) with reagent N Latex β2 microglobulin (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). Ferritin (Architect, Abbott, Longford, Ireland) was used as microglia injury marker.
All samples were assayed concurrently in duplicate according to the manufacturers’ instructions.
Blood-CSF barrier function
CSF and serum albumin were quantified using the nephelometric method (Dade Behring BNII, Deerfield, IL) using antiserum N human albumin (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) and VR 7.0–34 mg/dL (Breebaart et al. 1978). The functional integrity of the blood-CSF barrier was then assessed by the CSF albumin/serum albumin quotient, which was calculated as QAlb = AlbCSF/Albserum. The upper limit of the reference range was age-dependent, and it was calculated for each sample with the following equation: QAlb ref. = (4 + age (years)/15) × 10−3 (Reiber and Peter 2001). Albumin leakage across the blood-brain barrier over 24 h was also calculated. In normal individuals, the albumin BBB leakage range is −5 mg/day to 75 mg/day (Tourtellotte et al. 1989).
IgG intrathecal synthesis
IgG intrathecal synthesis in the CSF was assayed by quantitative and qualitative methods. IgG in the CSF and serum intrathecal was quantified using the nephelometric method (Dade Behring BNII, Deerfield, IL) with antiserum N-human-immunoglobulin (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany).
Quantitative IgG intrathecal synthesis was assayed by the following:
-
1)
IgG intrathecal synthesis for 24 h (mg/day). To estimate the amount of IgG intrathecal synthesis during the 24 h (mg/day), the Tourtellotte intrathecal IgG synthesis rate formula was calculated. The reference value was ≤10 mg/day (Tourtellotte 1970; Caroscio et al. 1983).
-
2)
The IgG hyperbolic function was calculated using Reibergram plots (Reiber 1995).
Qualitative analysis of IgG intrathecal synthesis was assayed in the CSF and serum with an oligoclonal band search using isofocusing in agarose gels followed by IgG immunofixation (Hydragel CSF Isofocusing—Sebia, Norcross, GA).
Viral sequencing, sequence filtering, and bioinformatics analysis
Subsequently, we amplified the HIV-1 env C2-V3 (HXB2 coordinates 6928–7344) region from DNA extracted from CSF cellular pellets and peripheral blood mononuclear cell (PBMC) by Nested PCR using specific primers. Sequencing was performed using next-generation sequencing (NGS) 454 GS FLX Titanium (454 Life Sciences, Roche, Branford, CT, USA). Read (FASTA) and quality score files produced by the 454 instruments were further analyzed using a purpose-built bioinformatics pipeline, used by our group in numerous published studies (Fisher et al. 2015; Carter et al. 2015; Wagner et al. 2014). The pipeline is available at https://github.com/veg/HIV-NGS.
Viral compartmentalization was assessed by the F-statistics (Fst), p < 0.01 (Hudson et al. 1992), used to measure of genetic distance between populations (Chen and Wang 2015). Statistical significance was derived via 1000 population-structure randomization/permutation test. Viral compartmentalization analysis via Fst was repeated using representative haplotypes to guard against possible skewing of allelic frequencies due to PCR amplification.
Results
Phylogenetic analyses of CNS compartmentalization
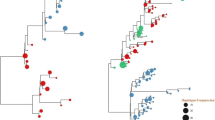
Phylogenetic analyses of C2-V3 region revealed distinct compartmentalized CSF viruses in paired CSF and PBMC samples (Fig. 2).
Maximum likelihood tree of partial HIV env sequences (C2-V3) from cerebrospinal fluid (CSF) and peripheral blood mononuclear cells (PBMC). It was included on this analysis samples collected when HIV CNS escape was identified (sample A). Tree topology revealed evidence of monophyletic HIV variants in PBMC (black dots) and CSF (gray dots). HIV compartmentalization between PBMC and CSF was confirmed by Fst approach, p < 0.01 (Hudson et al. 1992). Viral compartmentalization analysis via Fst was repeated using representative haplotypes to guard against possible skewing of allelic frequencies due to PCR amplification. Viral haplotypes from partial HIV env (C2-V3), generated from cleaned mapped NGS reads with in-house pipeline (https://github.com/veg/HIV-NGS) (Fisher et al. 2015; Carter et al. 2015; Wagner et al. 2014), were realigned using MUSCLE (Edgar 2004), piped to FastTree 2 (Guindon and Gascuel 2003) for maximum likelihood trees reconstruction, and subjected to codon-based (MG94) phylogenetic analyses in HyPhy
Dynamic study of CSF and serum biomarkers
This was a descriptive, longitudinal study of the dynamics of inflammation, cellular and humoral immune responses, the BBB, and neuronal injury in serially obtained samples of CSF and serum.
Monocyte and macrophage immune activation markers
The levels in the CSF of sCD14, neopterin, and β2M were increased in the acute phase (sample A), and levels in sample D were similar to the control. The levels of sCD14 and β2M were normal in the serum, indicating independent CNS production (Table 4).
Cellular immune response (cytokines and chemokines)
The values of cytokines and chemokines in the CSF and serum are shown in Table 2.
Almost all cytokines that were quantified in the CSF were increased in the acute phase (sample A) compared to the control group. TNFα was 179 times increased in the acute phase. The values progressively and slowly decreased to the normal range.
In serum, the cytokines IFNγ, IL-10, IL-6, IL-7, and TNFα were increased in sample A. The levels in sample B were similar to the control ranges and were less than TNFα, which reached normal values, in sample C.
All the chemokines that were quantified in the CSF were increased in the acute phase (sample A). IP-10, MCP-1, and MIP-1a were higher in the CSF than the serum indicating intrathecal synthesis. IP-10 was 1000 times higher than the control group, and RANTES was increased 30 times in the acute phase.
IP-10 and MIP-1β levels decreased progressively, although they were persistently higher than control group in sample C, indicating that they would reach normal values. MCP-1 and RANTES in the CSF were decreased in sample B, increased again in sample C, and were higher than sample A. RANTES was 593 times higher than controls and 19 times than sample A.
In serum, in sample A, there was increase only for IP-10 and MIP-1α, which was less than 10 times the control group. Only IP-10 persisted as higher than normal compared with the control group, but with tendency to normalize.
Values in the CSF samples were higher than in serum, indicating intrathecal synthesis of these cytokines independent from serum. This delay in CSF biomarkers decreasing to normal values more than 18 months after the change in the ARV scheme is a clear indication of the persistence of the immunological stimuli in the CNS, despite the control of HIV RNA in the CSF.
Blood CSF and blood-brain barriers evaluation
There was great BCSFB dysfunction in the first CSF sample before the ARV change (sample A). It was normal in samples B and D, and there was slight dysfunction in sample C (Table 1; Fig. 3). The rate of trans-BBB albumin leakage in 24 h (Table 1) was increased for samples A (843 mg/day) and B (77 mg/day). Samples C (15 mg/day) and D (12 mg/day) were both normal. The rate of trans-BBB albumin leakage in the control group was, median (IQR), 5 (−14.1; 26.5).
Hyperbolic function (Reibergram plot) of blood-CSF barrier function and intrathecal IgG synthesis. The graphic shows the dynamics of intrathecal IgG synthesis and blood CSF barrier (BCSFB) dysfunction reconstitution. Hyperbolic functions are a consequence of nonlinear interactions of molecular flux with the CSF flow rate as derived from the laws of diffusion (Reiber 1995). BCSFB dysfunction is indicated on the graphic with the area with vertical stripes; the age-corrected normal range for QAlb was 6 × 10−3. The plot was generated with CSF Research software. Sample A collected at the time when CSF and blood HIV RNA discordance was identified (HIV CNS escape), before ARV change, showed BCSFB dysfunction and 20% of IgG synthesis. Sample B collected 2.5 months after the ARV change showed normal BCSFB and 80% of IgG synthesis, besides clinical neurologic improvement. Sample C 18 months after the ARV change showed BCSFB dysfunction and 75% IgG synthesis. Sample D 31 months after the ARV change showed normal BCSFB and persistence of IgG synthesis (60%)
The BBB was evaluated by assessing cell adhesion molecules (sE-Selectin, sVCAM-1, sICAM-1, and uPAR), metalloproteinases (MMP-2, MMP-3, and MMP-9), and the respective tissue inhibitor metalloproteinases (TIMP-1 and TIMP-2) (Table 3). Almost all the biomarkers for BBB disruption were increased in the CSF. MMP-9 was 1050 times higher than the control group, although it was lower than in serum. This indicated influx of MMP-9 from the serum to the CSF. uPAR and MMP-2 levels were higher in the CSF than serum indicating intrathecal synthesis (Table 3). TIMP-1 and TIMP-2 levels were in the normal range compared with the control group in the acute phase (sample A).
In the CSF, sVCAM-1 and MMP-9 decreased progressively in the second and third CSF samples although they were still higher than that control group. Levels of CSF TIMP-1 and TIMP-2 were higher in sample C, indicating a physiological response to inhibit the respective MMPs. MMP-9 and MMP-2 levels decreased in sample C and were inhibited by TIMP-1 and TIMP-2, respectively. In the serum, TIMP-1 and TIMP-2 were in the normal range, indicating that the higher values in the CSF were due to intrathecal synthesis.
The types of proteins stimulated in the serum were more limited than in the CSF, and only the levels of sVCAM-1, UPAR, and MMP-9 in serum were increased in sample A. Only uPAR was persistently increased in sample C, although with a tendency to normalize.
Humoral immune response (IgG intrathecal synthesis)
There was IgG intrathecal synthesis in all four samples, and this was higher in the acute phase. There was concordance in all the formulas and in graphic analysis (Table 1; Fig. 3). There was presence of oligoclonal bands type 3, analyzed by isoelectric focusing in an agarose gel (Hydragel CSF Isofocusing—Sebia, Norcross, GA). Oligoclonal band type 3 is defined by the presence of IgG band in CSF and serum, with additional bands in the CSF not present in serum (Andersson et al. 1994). The pattern of oligoclonal bands in the CSF persisted similar in the three sequential samples studied including the sample (C) collected 18 months after ARV schema change (Supplementary Fig. S 1). The IgG quantitative and qualitative analyses indicated persistence of the immunological stimulus in the CNS, with persistent IgG intrathecal production.
CSF and serum neuronal injury biomarkers
There was a neuronal lesion without phosphorylation of Tau proteins (pT-181) in the acute phase. There was a decrease on Aβ total and Aβ-42 in the CSF. This was important when these values were compared with that in sample A, which were in the control group range (Table 4). This can indicate an increase in amyloidal deposits over time.
NFL is an important marker of axonal injury. It was 50 times higher than controls in the acute phase with slow progression to the normal range reached only in sample D.
CSF MBP is a marker of demyelination. In sample A, it was 10 times higher than the reference values (Greene et al. 2012), reaching normal values in sample C, despite brain MRI (Fig. 1), showing intense demyelization signs even with normal CSF MBP values.
CSF cell and biochemistry characteristics
CSF WBCs were reduced to the normal range already in the sample (B) after the change in ARV, together with the CSF HIV RNA that was undetectable. The total protein reached the normal range only 18 months after the ARV change (sample C; Table 1).
Longitudinal neuropsychological and functional independence assessment
The neuropsychological (NP) assessment was investigated at three different times. The global deficit score (GDS) was calculated based on a well-established HIV battery of neuropsychological tests, as described previously (de Almeida et al. 2013). The cutoff for GDS was ≥0.5. During the hospitalization when CNS HIV escape was diagnosed, it was not possible to perform the NP assessment due to the neurological status of the patient (Table 1). Three months later, the hospitalization NP assessment was measured by GDS. Visit 1 was 2.85, visit 2 (December 2, 2011) was GDS 2.05, and visit 3 (February 19, 2014) was GDS 1.06. The comparison of results from the three assessments suggested that the patient’s performance improved over time. Colored Trail Making scores, a measure of executive function (cognitive flexibility), seemed to show the greatest improvement when comparing all measures. Moreover, scores on the Stroop Test, also a measure of executive function (inhibition), also revealed better results. On the other hand, patient’s scores on both modalities of memory tests, verbal and visual, were still considerably lower than during the previous evaluation as he was able to remember half of the items from the word list and only one of the figures of the stimulus page. The results from the Wisconsin Card Sorting Test also suggested executive function difficulties. In summary, results suggested that the patient improved his performance in specific domains, such as attention, language, and information processing and maintained impaired results on memory and learning.
The functional independence was measured by the Functional Independence Measure (FIM) (Granger and Hamilton 1987); on the first evaluation, the patient was dependent for all the activities considered by FIM. There was worsening of functional independence on the second evaluation with dependence in all areas. After the diagnosis of HIV CNS escape and the ARV scheme change, in the fourth evaluation (18 months after the diagnosis), there was great improvement with functional independence in the majority of activities evaluated by the FIM (scores 6 or 7 in 11 of the 18). In seven areas, the patient showed dependence; in five, he needed direct supervision (score 5). He needed direct help only for those that required the greatest physical effort and balance, such as walking (score 4) and stair climbing (score 3).
Discussion
Our study is the first to show CNS escape and genetic compartmentalization in a patient with HIV subtype C.
This paper will add to the contributions of previous reports (Canestri et al. 2010; van Lelyveld et al. 2010; Katlama et al. 2010; Bogoch et al. 2011; Bingham et al. 2011; Del Palacio et al. 2012; Peluso et al. 2012) by further investigating the dynamics of several biomarkers in the CSF and serum samples of HIV subtype C with genetic HIV CNS compartmentalization. The overall biomarker longitudinal analysis showed the following: (1) during the acute phase, the majority of the neuroinflammation biomarkers studied, including all the ß-chemokines, were increased in the CSF, with a slow decrease to normal usually at 18 months after the change in the ARV scheme. (2) The persistence of increased biomarker levels in the CSF during a long period did not follow the improvement in clinical symptoms and control of HIV replication in the CNS but were consistent with the persistence of MRI alterations. (3) This could be interpreted as persistence of the immunological stimulus in the CNS. (4) The same can be considered for persistent IgG intrathecal synthesis with the presence of oligoclonal bands in all samples. (5) There was very high BCSFB dysfunction that also normalized 18 months after the change in the ARV scheme. The restoration of the function of the BCSFB in this patient followed the improvement of clinical symptoms. (6) There was great neuronal injury in the acute phase with demyelization, which was reduced in the following samples. (7) There was an increase in the β-amyloid deposits over time and was higher 31 months after the change in the ARV compared to the acute phase, indicating a great impact and interaction on amyloidal metabolism, and persistence of the injury mechanisms. (8) The cellular immune response to HIV in the CSF was different from that in serum. It was stronger in the CNS than in the peripheral blood, indicating that the CNS cellular immune response in HIV infection was compartmentalized, in accordance with previously described (de Almeida et al. 2016B). (9) Several biomarkers had levels higher in the CSF than serum, clearly showing intrathecal production.
HIV genetic compartmentalization is defined when genetic differences, characterizing quasispecies, between HIV in the compartments is detected. Extensive HIV-1 genetic compartmentalization between the periphery and the CNS has been reported in subjects with HAD (Harrington et al. 2005; Schnell et al. 2010). This patient had some described risk factors to CNS escape, despite a relatively reconstituted immune status at the time of evaluation. This patient had CD4+ T cell nadir at 6 cells/mm3, which was lower than a previous report of a median nadir CD4+ T cell count of 55 cells/mm3 (Canestri et al. 2010, Peluso et al. 2012). A history of advanced immunosuppression may confer increased risk for prior enhanced local CNS infection and compartmentalization (Ritola et al. 2005), which, despite peripheral CD4+ T cell improvement, fails to be entirely suppressed by ART. Clinically, the CD4+ T cell nadir might be an important factor to consider in the assessment of a patient with new neurologic abnormalities.
The CSF biomarker analysis of this patient showed that symptomatic CSF escape is accompanied by CNS inflammation, and a moderately reconstituted immune system may play an important role in both eliciting a symptomatic inflammatory response and providing a substrate for ongoing discordant HIV replication within the CNS. The combination of persistent CNS infection and a relatively preserved immune response, including an HIV-specific response, may generate immunopathology in the cases of CSF escape. This is analogous to immune reconstitution inflammatory syndrome, IRIS (Miller et al. 2004), but may differ in that it represents, not the effects of immune reconstitution, but rather a “stable state” of antigen and immune response within the CNS (Peluso et al. 2012).
The hypothesis that β-chemokines would be less stimulated by HIV-1C than in HIV-1B, due to a deletion in the CC dimotif in HIV-1C (Ranga et al. 2004) has been offered. In this case, we showed that there was high stimulation of these chemokines mainly in the acute phase. MCP-1 (CCL2) and RANTES (CCL5), both β-chemokines, were respectively 1.4 and 19 times higher than the first sample at 18 months after the ARV change. Some possibilities to explain this could be considered, such as (1) ARV CNS toxicity, as the scheme included EFV. (2) IRIS is another possible diagnosis, although this patient did not fulfill the criteria for IRIS because there was immunological restoration at the time of the change in the ARV scheme and because he was asymptomatic during the period of increase in MCP-1 and RANTES. IRIS can occur few months from the ARV initiation or change in the ARV regimen and the nervous system is a frequent target (Riedel et al. 2006; Venkataramana et al. 2006; Berkeley et al. 2008; Johnson and Nath 2010). The frequency of CNS IRIS is 0.6% 1 year after the start of highly active antiretroviral therapy (HAART) (Subsai et al. 2006) and 1.2% 2 years after HAART initiation (Lipman and Breen 2006; Murdoch et al. 2007). (3) CD8 encephalitis must be considered as a differential diagnosis (Lescure et al. 2013).
The increase in TINP-1 and TINP-2 18 months after the ARV change probably indicated a physiologic reaction to control the increase of MMPs, as TIMP-1 is the tissue inhibitor of MMP-9 and MMP-3 and TIMP-2 is the tissue inhibitor of MMP-2. As a response to TIMP increases, MMPs are normal or have a trend to normalize in the third CSF sample.
This patient had very high CSF PBM levels in the acute phase indicating high levels of demyelization, which is suggestive of a destructive process in the brain (Jacque et al. 1982). Viral encephalitis of different causes determines the highest levels of PBM, which were higher than 10 ng/mL (Jacque et al. 1982). Similar to the levels of the present case in the acute phase, the normal range upper limit was 1.1 ng/mL (Greene et al. 2012). Studies on CSF PBM in CNS HIV infection are not common. It has been shown that participants with HIV encephalitis had mild to severe PBM levels (5–5.3 ng/mL) (Pfister et al. 1989; Liuzzi et al. 1992). In the present case, there was normalization of CSF PBM levels despite MRI demyelization.
MRI findings were consistent with those reported in previous cases (Canestri et al. 2010; Bogoch et al. 2011; Del Palacio et al. 2012; Peluso et al. 2012). The MRI alterations persisted during long term follow-up in addition to the clinical improvement and CSF cell, biochemistry, HIV RNA, and some biomarker normalization; although, there was improvement compared to the initial and previous MRI results. This suggests that this process is associated with findings on imaging that may persist after the resolution of symptoms and may take months to years to resolve completely (Peluso et al. 2012). The nature of these imaging findings remains incompletely understood (Peluso et al. 2012). MRI finding with white matter hyperintensity on T2-weighted and FLAIR imaging suggest a generalized inflammatory process consistent with diffuse encephalitis and similar to findings reported in patients failing ART (Langford et al. 2002). The increase in inflammatory biomarkers in the present patient supports the hypothesis of a generalized inflammatory process and diffuse encephalitis.
The CSF pleocytosis in this patient was consistent with cases described previously. Increased WBCs were present in 9 of 10 patients with a median of 14.5 cells/mm3 (range 0–200 cells/mm3). In addition, biochemical abnormalities were also found in 8 of 9 patients and they had elevated CSF protein levels. The median protein level was 105 mg/dL (range, 46–170 mg/dL) (Peluso et al. 2012).
The CSF WBC count was moderately elevated in the acute phase (sample A) as described in the other cases in the same range (Canestri et al. 2010; Peluso et al. 2012). CSF total protein in sample A was very high; that is, 7.5-fold higher than the normal range for lumbar puncture and higher than the levels described by Peluso et al. (2012). CSF total protein with HIV infection can be increased due to BCSF dysfunction, intrathecal immunoglobulin synthesis or both. In this specific case, the cause was both processes.
In the present patient, the presence of CSF oligoclonal bands with a pattern very similar in all samples indicated the persistence of the immunological stimulus, besides denormalization of basic CSF cell and biochemistry characteristics and CSF HIV RNA as undetectable, and with clinical improvement. Oligoclonal bands are markers of antibody intrathecal synthesis. They have been detected in all stages of HIV infection, regardless of the presence or absence of HIV neurological disease (Andersson et al. 1988; Gallo et al. 1988). They are present in 18 to 44% of HIV(+) patients (Goudsmit et al. 1986; McArthur et al. 1988; Gallo et al. 1989).
Increased IgG daily synthesis was observed in 22 to 93% of patients in all clinical stages of HIV infection (Andersson et al. 1988; Marshall et al. 1991; Resnick et al. 1998). The presence of intrathecal IgG synthesis does not necessarily imply neurological damage and may simply indicate the persistence of the virus in the CNS (Sönnerborg et al. 1989; Goswami et al. 1991). HAART does not significantly reduce immune activation, as a significant proportion of patients continue to have macrophage activation signals and an elevated IgG index after treatment (Abdulle et al. 2005; Edén et al. 2007). This was described as not invariably associated with clinical HIV neurological disease (Goudsmit et al. 1986; Elovaara et al. 1987; Reboul et al. 1989; Marshall et al. 1991). All the previous studies of IgG intrathecal synthesis were in HIV-1 subtype B.
This study is different from the previous studies in that (1) it was a longitudinal dynamic study of humoral and cell-mediated immunological responses, CNS barrier analysis, and neuronal injury biomarkers. (2) In previous reports, some patients were on atypical or incomplete regimens (Canestri et al. 2010; van Lelyveld et al. 2010; Bogoch et al. 2011) and our patient was on standard ARV regimen. (3) Poor adherence was considered a predisposing factor, as partially reduced adherence may lead to insufficient drug concentrations in the CSF while maintaining satisfactory concentrations in the plasma (Peluso et al. 2012). Our patient had good adherence with reconstituted immune status at the time of evaluation. (4) In the cases reported by Peluso et al. (2012), all patients had a CD4+ T cell nadir <250 cells/mm3, with many below 100 cells/mm3, which was similar to another report of a median nadir CD4+ T cell count of 55 cells/mm3 (Canestri et al. 2010; Del Palacio et al. 2012). In our patient, the nadir was very low (6 cells/mm3), which was in accordance. (5) In the previous reports, the subtype of the patient was not reported. They possibly were subtype B because the cases were from the USA (Peluso et al. 2012) or Europe (Canestri et al. 2010). This patient was an HIV-1 subtype C infected patient. (6) This patient was in coma, which is not a frequent neurological presentation, and it was present in 1 of 10 participants (Peluso et al. 2012), and aphasia was present in 2 of 10 or 1 of 11 participants (Peluso et al. 2012; Canestri et al. 2010, respectively). (7) Usually, the reported cases have not had HIV RNA in the CSF as high as in the present case, which was a strong indication of CNS genetic compartmentalization, which was demonstrated using NGS.
The results of this report must be seen with cautiousness and cannot be extended to other HIV participants or groups; the present study is not free of limitations. It was a descriptive study with a limited number of samples from the same participant. These findings will serve future investigations and hypothesis. Future studies with larger sample size with adequate statistical analysis are necessary to validate the results presented here.
Conclusions
Physicians should be aware of this unusual but clinically significant manifestation of HIV disease. The appearance of new neurologic symptoms in the context of standard ART regimens and well-controlled plasma HIV infection warrants an evaluation of the CSF to determine whether CNS isolated viral replication is occurring. Considering the important limitations of the study, we were still able to show that independent and isolated HIV replication can occur in the CNS in HIV-1 subtype C, also leading to compartmentalization and the development of quasispecies distinct from the peripheral plasma. These CSF samples were associated with strong cell and humoral immunological responses and brain lesions. Optimizing ART by using drugs with improved CNS penetration may achieve viral suppression in the CSF with improvement of neurological symptoms. The significance of this case description is on evidencing CNS escape and compartmentalization in other HIV-1 subtypes different than B, and its effects on the CNS including neurological, neuroimaging, BBB function, and inflammation.
References
Abdulle S, Hagberg L, Gisslén M (2005) Effects of antiretroviral treatment on blood-brain barrier integrity and intrathecal immunoglobulin production in neuroasymptomatic HIV-1-infected patients. HIV Medicine 6:164–169
Andersson MA, Bergström TB, Blomstrand C, Hermodsson SH, Håkansson C, Löwhagen GB (1988) Increasing intrathecal lymphocytosis and immunoglobulin G production in neurologically asymptomatic HIV-1 infection. J Neuroimmunol 19:291–304
Andersson LM, Alvarez-Cermeño J, Bernardi G, Cogato I, Fredman P, Frederiksen J, Fredrikson S, Gallo P, Grimaldi LM, Grønning M (1994) Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. Journal of Neurology, neurosurgery, Psychiatry 57:897–902
Berkeley JL, Nath A, Pardo CA (2008) Fatal immune reconstitution inflammatory syndrome with human immunodeficiency virus infection and Candida meningitis: case report and review of the literature. Journal of Neurovirology 14:267–276
Bingham R, Ahmed N, Rangi P, Johnson M, Tyrer M, Green J (2011) HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. Int J STD AIDS 22:608–609
Bogoch II, Davis BT, Venna N (2011) Reversible dementia in a patient with central nervous system escape of human immunodeficiency virus. J Infect 63:236–239
Breebaart K, Becker H, Jongebloed FA (1978) Investigation of reference values of components of cerebrospinal fluid. J Clin Chem Clin Biochem 16:561–565
Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, Peytavin G, Tubiana R, Pialoux G, Katlama C (2010) Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 50:773–778
Caroscio JT, Kochwa S, Sacks H, Cohen JA, Yahr MD (1983) Quantitative CSF IgG measurements in multiple sclerosis and other neurological diseases. Arch Neurol 40:409–413
Carter CC, Wagner GA, Hightower GK, Caballero G, Phung P, Richman DD, Pond SL, Smith DM (2015) HIV-1 neutralizing antibody response and viral genetic diversity characterized with next generation sequencing. Virology 474:34–40
Caudie C, Bancel J, Dupont M, Matanza D, Poitevin F, Honnorat J (2005) CSF levels and diagnostic utility of cerebrospinal fluid beta2-microglobulin. Ann Biol Clin 63:631–637
Chen W, Wang H (2015) Variance estimation for nucleotide substitution models. Mol Phylogenet Evol 90:97–103
de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, Maich I, Raboni SM, Rotta I, Barbosa FJ, Heaton RK, Umlauf A, Ellis RJ (2013) Neurocognitive impairment in HIV-1 subtype C- versus B-infected individuals in Southern Brazil. J Neurovirol 19:550–556
de Almeida SM, Rotta I, Jiang Y, Li X, Raboni SM, Ribeiro CE, Smith D, Vaida F, Letendre S, Ellis RJ (2016a) Biomarkers of chemotaxis and inflammation in cerebrospinal fluid and serum in individuals with HIV-1 subtype C versus B. J Neurovirol 22:715–724. doi:10.1007/s13365-016-0437-4
de Almeida SM, Rotta I, Ribeiro CE, Smith D, Wang R, Judicello J, Potter M, Vaida F, Letendre S, Ellis R (2016b) Blood-CSF barrier and compartmentalization of CNS cellular immune response in HIV infection. J Neuroimmunol. doi:10.1016/j.jneuroim.2016.10.009 [Epub ahead of print]
Edén A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslén M (2007) Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. Journal of Infectious Disease 196:1779–1783
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113
Elovaara I, Iivanainen M, Valle SL, Suni J, Tervo T, Lähdevirta J (1987) CSF protein and cellular profiles in various stages of HIV infection related to neurological manifestations. J Neurol Sci 78:331–342
Fisher RG, Smith DM, Murrell B, Slabbert R, Kirby BM, Edson C, Cotton MF, Haubrich RH, Kosakovsky Pond SL, Van Zyl GU (2015) Next generation sequencing improves detection of drug resistance mutations in infants after PMTCT failure. J Clin Virol 62:48–53
Gallo P, de Rossi A, Cadrobbi P, Francavilla E, Chieco-Bianchi L, Tavolato B (1988) Intrathecal synthesis of anti-H1V oligoclonal IgG in H1V-seropositive patients having no signs of H1V-induced neurological diseases. Ann N Y Acad Sci 540:615–618
Gallo P, Frei K, Rordorf C, Lazdins J, Tavolato B, Fontana A (1989) Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol 23:109–116
Goswami KK, Kaye S, Miller R, McAllister R, Tedder R (1991) Intrathecal IgG synthesis and specificity of oligoclonal IgG in patients infected with HIV-1 do not correlate with CNS disease. J Med Virol 33:106–113
Goudsmit J, Wolters EC, Bakker M, Smit L, Van der Noordaa J, Hische EA, Tutuarima JA, Van der Helm HJ (1986) Intrathecal synthesis of antibodies to HTLV-III in patients without AIDS or AIDS related complex. Br Med J (Clin Res Ed) 292:1231–1234
Granger C, Hamilton B (1987) Development of a uniform national data system for medical rehabilitation 1984–97. Final report for grant number G008435062. Buffalo: National Institute on Disability and Rehabilitation Research, Office of Special Education and Rehabilitative Services, Department of Education
Greene DN, Schmidt RL, Wilson AR, Freedman MS, Grenache DG (2012) Cerebrospinal fluid myelin basic protein is frequently ordered but has little value, a test utilization study. Am J Clin Pathol 138:262–272
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Haggerty S, Stevenson M (1991) Predominance of distinct viral genotypes in brain and lymph node compartments of HIV-1-infected individuals. Viral Immunol 4:123–131
Harrington PR, Haas DW, Ritola K, Swanstrom R (2005) Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by short-lived cells. J Virol 79:7959–7966
Harrington PR, Schnell G, Letendre SL, Ritola K, Robertson K, Hall C, Burch CL, Jabara CB, Moore DT, Ellis RJ, Price RW, Swanstrom R (2009) Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS 23:907–915
Hudson RR, Slatkin M, Maddison WP (1992) Estimation of levels of gene flow from DNA sequence data. Genetics 132:583–589
Jacque C, Delassalle A, Rancurel G, Raoul M, Lesourd B, Legrand JC (1982) Myelin basic protein in CSF and blood, relationship between its presence and the occurrence of a destructive process in the brains of encephalitic patients. Arch Neurol 39:557–560
Johnson T, Nath A (2010) Neurological complications of immune reconstitution in HIV-infected populations. Ann N Y Acad Sci 1184:106–120
Karris M, Smith DM (2011) Tissue-specific HIV-1 infection: why it matters. Future Virol 6:869–882
Katlama C, Valantin MA, Algarte-Genin M, Duvivier C, Lambert-Niclot S, Girard PM, Molina JM, Hoen B, Pakianather S, Peytavin G, Marcelin AG, Flandre P (2010) Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS 24:2365–2374
Langford TD, Letendre SL, Marcotte TD, et al. (2002) Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS 16:1019–2
Lescure FX, Moulignier A, Savatovsky J, Amiel C, Carcelain G, Molina JM, Gallien S, Pacanovski J, Pialoux G, Adle-Biassette H, Gray F (2013) CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis 57:101–108
Letendre S, Ellis R, Deutsch R, Clifford D, Marra C, McCutchan A, Morgello S, Simpson D, Heaton R, Grant I, the CHARTER Group (2010) Correlates of time-to-loss-of-viral response in CSF and plasma in the CHARTER cohort. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 16–19 February (poster 430)
Lipman M, Breen R (2006) Immune reconstitution inflammatory syndrome in HIV. Curr Opin Infect Dis 19:20–25
Liuzzi GM, Mastroianni CM, Vullo V, Jirillo E, Delia S, Riccio P (1992) Cerebrospinal fluid myelin basic protein as predictive marker of demyelination in AIDS dementia complex. J Neuroimmunol 36:251–254
Marshall DW, Brey RL, Butzin CA, Lucey DR, Abbadessa SM, Boswell RN (1991) CSF changes in a longitudinal study of 124 neurologically normal HIV-1-infected U.S. Air Force personnel. J Acquir Immune Defic Syndr 4:777–781
McArthur JC, Cohen BA, Farzedegan H, Cornblath DR, Selnes OA, Ostrow D, Johnson RT, Phair J, Polk BF (1988) Cerebrospinal fluid abnormalities in homosexual men with and without neuropsychiatric findings. Ann Neurol 23:–37
Miller RF, Isaacson PG, Hall-Craggs M, Lucas S, Gray F, Scaravilli F, An SF (2004) Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol 108:17–23
Murdoch DM, Venter WDF, Van Rie A, Feldman C (2007) Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther 4:9
North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, Shacklett BL, Schinazi RF, Luciw PA (2010) Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol 84:2913–2922
Palacio D, Tamarit M, Quereda C, Gonzalez-Rozas M, Corral I, Casado JL (2012) HIV type 1 viral encephalitis after development of viral resistance to plasma suppressive antiretroviral therapy. AIDS Res Hum Retrovir 28:83–86
Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, Gisslén M, Angoff N, Price RW, Cinque P, Spudich S (2012) Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well-controlled plasma viral load. AIDS 26(14):1765–1774
Petzold A, Eikelenboom MJ, Gveric D, Keir G, Chapman M, Lazeron RH, Cuzner ML, Polman CH, Uitdehaag BM, Thompson EJ, Giovannoni G (2002) Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain 125:1462–1473
Pfister HW, Einhäupl KM, Wick M, Fateh-Moghadam A, Huber M, Schielke E, Goebel FD, Matuschke A, Heinrich B, Bogner JR (1989) Myelin basic protein in the cerebrospinal fluid of patients infected with HIV. J Neurol 236:288–291
Ranga U, Shankarappa R, Siddapa NB, Ramakrishna L, Nagendran R, Mahalingam M, Mahadevan A, Jayasuryan N, Satishchandra P, Shankar SK, Prasad VR (2004) Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virology 78:2586–2590
Reboul J, Schuller E, Pialoux G, Rey MA, Lebon P, Allinquant B, Brun-Vezinet F (1989) Immunoglobulins and complement components in 37 patients infected by HIV-1 virus: comparison of general (systemic) and intrathecal immunity. J Neurol Sci 89:243–252
Reiber H (1995) External quality assessment in clinical neurochemistry: survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin Chem 41:256–263
Reiber H, Peter JB (2001) Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 184:101–122
Resnick L, Berger JR, Shapshak P, Tourtellotte WW (1998) Early penetration of the blood brain barrier by HIV. Neurology 38:9–14
Riedel DJ, Pardo CA, McArthur J, Nath A (2006) CNS manifestations of HIV-associated immune reconstitution inflammatory syndrome. Nat Clin Pract Neurol 2:557–565
Ritola K, Robertson K, Fiscus SA, Hall C, Swanstrom R (2005) Increased human immunodeficiency virus type 1 (HIV-1) env compartmentalization in the presence of HIV-1-associated dementia. J Virol 79:10830–10834
Rotta I, Raboni SM, Ribeiro CEL, Riedel M, Winhescki MG, Smith DM, Ellis RJ, de Almeida SM (2014) Cerebrospinal fluid can be used for HIV genotyping when it fails in blood. Arquivos de Neuro-Psiquiatria 72(7):506–509
Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E (2005) The international HIV dementia scale: a new rapid screening test for HIV dementia. AIDS 19:1367–1374
Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Rasvi V, Desai A, Chandramuki A, Jayakumar PN, Shankar SK (2000) Profile of neurologic disorders associated with HIV/AIDS from Bangalore, South India (1989-96). Indian J Med Res 111:14–23
Schnell G, Price RW, Swanstrom R, Spudich S (2010) Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol 84:2395–2407
Sönnerborg AB, von Sydow MA, Forsgren M, Strannegård OO (1989) Association between intrathecal anti-HIV-1 immunoglobulin G synthesis and occurrence of HIV-1 in cerebrospinal fluid. AIDS 3:701–705
Subsai K, Kanoksri S, Siwaporn C, Helen L, Kanokporn O, Wantana P (2006) Neurological complications in AIDS patients receiving HAART: a 2-year retrospective study. Eur J Neurol 13:233–239
Tourtellotte WW (1970) On cerebrospinal fluid immunoglobulin-G (IgG) quotients in multiple sclerosis and other diseases. A review and a new formula to estimate the amount of IgG synthesized per day by the central nervous system. J Neurol Sci 10:279–304
Tourtellotte WW, Shapshak P, Osborne MA, Rubinshtein G, Lee M, Staugaitis K (1989) New Fomula to calculate the rate of albumin blood brain barrier leakage. Ann Neurol 26:176
van Lelyveld SF, Nijhuis M, Baatz F, Wilting I, van den Bergh WM, Kurowski M, de Jong D, Hoepelman AI, Wensing AM (2010) Therapy failure following selection of enfuvirtide resistant HIV-1 in cerebrospinal fluid. Clin Infect Dis 50:387–390
Venkataramana A, Pardo CA, McArthur JC, Kerr DA, Irani DN, Griffin JW, Burger P, Reich DS, Calabresi PA, Nath A (2006) Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 67:383–388
Wagner GA, Pacold ME, Kosakovsky Pond SL, Caballero G, Chaillon A, Rudolph AE, Morris SR, Little SJ, Richman DD, Smith DM (2014) Incidence and prevalence of intrasubtype HIV-1 dual infection in at-risk men in the United States. J Infect Dis 209:1032–1038
Zarate S, Pond SLK, Shapshak P, Frost SDW (2007) Comparative study of methods for detecting sequence compartmentalization in human immunodeficiency virus type 1. J Virol 81:6643–6651
Acknowledgements
The authors would like to thank Professor Albert Anderson, Emory School of Medicine, Atlanta, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by institutional review boards (IRB) at Hospital de Clínicas—UFPR in Brazil and Brazil National IRB (CONEP).
Funding
This work was supported by the following grants: National Institute of Health, NIH R21 MH76651 (Ellis, Ronald J.; Almeida, Sergio M.), NIH R01 MH83552 (Smith, David M.), S10 RR31646 (Letendre, Scott), and K24 MH097673 (Letendre, Scott); University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK; and Ministério da Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico, MCT/CNPq-Universal 014/2008, Brazil (Almeida, Sergio M.).
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH. The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System and includes the following: Director: Robert K. Heaton, Ph.D.; Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., Scott Letendre, M.D.; Center Managers: Thomas D. Marcotte, Ph.D., Jennifer Marquie-Beck, M.P.H., Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D. (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D. (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; and Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Figure S1
(DOC 214 kb)
Rights and permissions
About this article
Cite this article
de Almeida, S.M., Rotta, I., Ribeiro, C.E. et al. Dynamic of CSF and serum biomarkers in HIV-1 subtype C encephalitis with CNS genetic compartmentalization—case study. J. Neurovirol. 23, 460–473 (2017). https://doi.org/10.1007/s13365-017-0518-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-017-0518-z