Abstract
In northern Italy, the native European hare (Lepus europaeus) and the introduced Eastern cottontail (Sylvilagus floridanus) can occur together at a local scale, as a result of cottontail introduction and expansion into the European hare range. Hare populations are limited in Italy by habitat loss, diseases, and most important by overhunting, and many areas within hare range in northern Italy are undergoing increasing anthropogenic impact. Therefore, quantitative studies on resource selection and exploitation by both species will be of great interest to evaluate the degree of habitat overlap and to search for exploitation competition evidences. We studied habitat selection during resting time by both species in two areas where they occur alone and in one area where they occur together. Habitat selection by the two species was examined at micro- and macro-habitat scales during autumn–winter and spring–summer. Both species selected ecotonal zones between arboriculture stands and crops and between arboriculture stands and spontaneous vegetation (i.e., herbaceous, bush, and woody permanent species), which were the less available in the area of sympatry. No habitat shifts were evident at macro-habitat level because the two species showed a differential micro-habitat use within patches. On the whole, it seems that habitat heterogeneity promoted daytime segregation between the two species. In particular, edges between crops and canopy habitats should be improved, thus reducing chance of intra- and inter-specific encounters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European hare (Lepus europaeus) is common in arable farmlands; however, the habitat changes caused by agricultural intensification have negatively affected hare abundance through Europe since 1960s (Smith et al. 2005; Jennings et al. 2006; Reichlin et al. 2006; Pépin and Angibault 2007). European hare is widespread in Italy with the exception of the two major islands, but its populations are limited by habitat loss, diseases, and mainly by overhunting (Meriggi et al. 2001; Santilli and Galardi 2006).
The Eastern cottontail (Sylvilagus floridanus) was successfully introduced in northern Italy by several illegal releases starting in the late 1960s. Since its introduction, the species has chiefly expanded along the river network of the Po Plain. Thus, at a local scale, both species can occur together, as a result of cottontail expansion into the range of European hare.
Locally, cottontails can reach grater densities than hares because of their smaller home range sizes and their better reproductive performances, therefore the species may have greater dispersal and exploitation ability than the autochthonous lagomorph (Chapman et al. 1977; Swihart 1986; Hansen 1992; Vidus-Rosin et al. 2008). In arable farmlands, both lagomorph species use similar macro-habitats and macro-structural features (both patchy and linear features) to forage, to rest, and to shelter or raise young. For these reasons, competition for resources between the two species (food, shelter) may be taken into account particularly during limiting seasons and in marginal habitats. Moreover, anthropogenic habitat alteration, mostly habitat fragmentation and simplification which deeply modified arable landscape in northern Italy, can emphasize the negative effect of competition on the persistence of hares within agro-ecosystems (Barbour and Litvaitis 1993; Swihart et al. 2006; Manor and Saltz 2008).
Intra- or inter-specific competition, as well as predation or parasitism, could influence the abundance and distribution of animals forcing them into less preferred habitats, but it is generally agreed that coexistence within the same geographical range is determined by some ecological differences between species, involving mainly niche dimensions, activity scales, timings of activity, and different habitat or resource perception in space and time, which could lead to differences in habitat selection (Rosenzweig 1991; Morris 1996; Bonesi and Macdonald 2004; Bonesi et al. 2004; Manor and Saltz 2008).
Because many areas within hare range in northern Italy are undergoing increasing anthropogenic impact, quantitative studies on resource selection and exploitation (food and cover) by hares and their potential competitors will be of great interest to find evidence of exploitation competition. One way to examine this type of competition is to compare the habitat use of a species in areas where it occurs alone (allopatry) with other areas where it occurs with another competing species (sympatry).
If the two areas are basically similar, niche shifts observed in sympatry should reflect the response to interspecific competition (Pianka 1981; Belovsky 1984; Bonino et al. 1997; Bonesi et al. 2004).
Indeed, the difference between the fundamental niche (the entire set of resources used by a species) and the realized niche (the subset of resources exploited by a species), or the niche change due to competitors, can reflect the effects of interspecific competition. Consequently, the presence of competitors may be viewed as a reduction of habitat quality (Partridge 1978).
Habitat use by both lagomorph species can be quite different between day and night; they concentrate their feeding activity during nocturnal hours and their resting activity during day-time. Therefore, more information on both strategies of habitat selection is needed to understand the potential interactions between them on the whole. In particular, habitat selection by hares and cottontails during resting time is not well documented. Thus, we focused on form characteristics to study macro- and micro-habitat variables associated with resting sites of both lagomorph species.
We studied habitat selection by the European hare and the Eastern cottontail during resting time from September 2007 to August 2008 with the aims of: (1) quantify the degree of habitat overlap between both species in sympatry, (2) evaluate any evidence of exploitation competition by comparing habitat use and breadth of both species in areas of allopatry and sympatry, and (3) individuate the key factors allowing coexistence of the two species. Moreover, we improved the discussion of our results considering the main findings of the previous research on the habitat selection and overlap between the two species during feeding activity carried out in the same study areas from September 2006 to August 2007 (Vidus-Rosin et al. 2011).
Material and methods
Study area
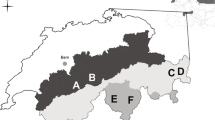
The three study areas (A: allopatry for hares, 4.3 km2; B: sympatry, 4.8 km2; C: allopatry for cottontails, 8.2 km2) were located in the Province of Pavia, northern Italy (A: 45° 05′ 09.40″ N, 9° 13′ 22.94″ E; B: 45° 00′ 57.78″ N, 8° 56′ 27.98″ E; C: 45° 04′ 25.84″ N, 9° 00′ 55.71″ E). The average distance between study areas was 14.3 km, with the minimum of 8.1 km between B and C and the maximum of 19.6 km between C and A. The climate was continental-temperate; annual rainfall averaged 700 mm and was concentrated in spring and autumn. The yearly temperature averaged 12°C (January 1.0°C and July 22.5°C). Crops were the most represented habitat type in all the areas (A, 81.9 %; B, 76.1 %; C, 55.2 %), especially winter cereals (35.5 %, 47.8 %, 28.6 %, respectively). Spontaneous vegetation (i.e., herbaceous, bush, and woody permanent species) was present in woods, fallow fields, and along hedgerows, field edges, basins, and streams, and it was more developed in area C (24.8 %) compared to area B (18 %) and area A (7.7 %). Farmsteads, road networks, and barren areas occupied 10.5 % of area A, 3.5 % of area B, and 7.7 % of area C. Arboriculture stands were present in area B (2.5 %) and in area C (12.3 %).
Methods
We conducted spotlight counts from a moving car (5 km/h maximum speed) along a permanent transect in each study area. The transect route was selected from the existing road network to sample each area in a representative manner with respect to available habitat types (at least 10 %). For this reason, we mapped each area covered by the hand-light in order to make sure of the reliability of each nocturnal survey (Meriggi 1989; Langbein et al. 1999). Moreover, we considered that road networks in our study areas did not significantly influence hare and cottontail distribution with respect to the transect route during feeding activity: herbaceous strips, which were usually present along roads in our study areas and could attract lagomorphs especially in winter, were available and scattered within the study areas; moreover, roads were unpaved and characterized by very low traffic disturbance (only few agricultural machineries; Roedenbeck and Voser 2008). Spotlight counts were conducted monthly from August–December 2007 and from January–April 2008; we sampled during late spring and early summer months but deemed the counts unreliable because of the growth of crops and herbaceous cover. Each count started at least 2 h after sunset and ended no later than 2 h before sunrise. Groups (two or more individuals feeding together) were considered as singular observations, and their perpendicular distances were measured from the center of each group to transect; sightings on the transect were registered at “0 m”.
We examined macro- and micro-habitat characteristics of forms used by hares and cottontails during two seasons: autumn–winter (October 2007–February 2008) and spring–summer (May–August 2008). We mapped macro-habitat types in both seasons by direct surveys and digitalized them using ArcView 3.2. Then, we randomly chose 10 % of patches in each area. The edges of every patch were walked between 9.00 and 15.00 h in both seasons (i.e., when the two lagomorphs were usually at their resting places) to flush hares and cottontails to locate forms. In each area, every patch was sampled twice a season; forms were recorded only after identifying the flushing species. This sampling design allowed us to investigate every habitat type in each season, without crossing crops during spring and early summer when crops were growing and during autumn when fields were sown with winter cereals and the soil was too wet because of the rainy weather. Edge types were individuated on the basis of the two macro-habitats adjacent to each edge: crops-crops (C-C); crops-spontaneous vegetation (C-SV), crops-arboriculture stands (C-AS), spontaneous vegetation-spontaneous vegetation (SV-SV), spontaneous vegetation-arboriculture stands (SV-AS).
We classified forms on the basis of four qualitative characteristics (soil condition, aspect, cover type, form type), and we measured the height of vegetation at form sites. Hare and cottontail forms were considered as “permanent” when they showed evidence of handling, with the posterior part excavated and the anterior part scraped; otherwise, they were considered as “temporary”. Moreover, we measured directly 11 micro-habitat variables within 1-m radius plots centered on forms (Althoff et al. 1997); successively, we measured 13 macro-habitat variables within a buffer of 100-m radius from the form centers using ArcView 3.2. Distances from forms to the nearest spontaneous feature were measured using ArcView 3.2, and they were considered as micro-habitat variables (Appendix 1).
Statistical analyses
Density
In each study area, data on all spotlight surveys were used in the analysis to estimate hare and cottontail densities (individuals per square kilometer) and their standard errors. Data were analyzed using the software Distance 5.0 (Burnham et al. 1985; Buckland et al. 2001). The best detection function among the key functions (half-normal, hazard rate, negative exponential) was selected based on the minimum AICc value of each candidate model. Since we analyzed our data as intervals, a single goodness-of-fit test was performed for each model using those intervals. The Chi-square test assessed the adequacy of each fit by comparing the observed distribution of observations with the expected one within each interval.
Buckland et al. (2001) suggested that line transect should not be positioned along roads because avoidance/attraction behavior for the road transect by animals could bias the density estimation. However, Butler et al. (2007) showed that distance method applied to roads can provide an efficient and inexpensive technique for monitoring wild population trends across large ecoregion scales. In our case, the application of distance method to roads allowed us to easily and inexpensively monitor hare and cottontail populations through years in arable landscape. In our study areas, it may be difficult to cover random transects by car or feet because most patches are usually ploughed or cultivated. The only way to perform nocturnal counts is along the existing road networks, most of whom are linear transect roads.
Form characteristics
We used the chi-square permutation test to assess significant differences between the observed frequencies of hare and cottontail forms in relation to qualitative variables of the forms. Moreover, we performed one-way ANOVA to detect significant differences between average values of the height of vegetation measured at hare and cottontail form sites.
Edge use
We calculated habitat breadth of hares and cottontails using the similarity proportion (SP) index proposed by Feinsinger et al. (1981), which measured the similarity between the frequency distribution of resources used by a population and the frequency distribution of resources available when the population utilizes each resource in proportion to its abundance (SP = 1). If the population uses the less available resources more intensively than the others, SP = min (q i ), with q i as the proportion of habitat i available in the study area.
We used Manly's preference index (α) to compare habitat preference by the two species in sympatry (Manly et al. 1993; Krebs 1999). This index measured the preference for each resource upon the relative preference of those remaining. When preference do not occur, α i = 1/M for each i = 1, …, M resources. If α i is greater than 1/M, then resource type i is preferred. Conversely, if α i is less than 1/M, resource type i is avoided. In our case 1/M was 0.2.
Regarding each index, we calculated the usage proportion of each edge type as the ratio between the number of forms found along each edge type and the total number of forms found and the availability proportion of each edge type in the study area as the ratio between the total length of each edge type and the total length of all edges.
To test the reliability of the above indexes with respect to test values, we re-sampled the forms 1,000 times by the bootstrap method (Dixon 1993). Then, we calculated the average values and 95 % confidence intervals of each index to evaluate significant differences between seasons, study areas, and species by the superimposition of paired confidence intervals.
Macro- and micro-habitat characteristics
We compared macro- and micro-habitat variables measured in hare and cottontail forms between areas of allopatry and sympatry using the multivariate analysis of variance (MANOVA). Logistic regression analysis (LRA, stepwise forward selection, maximum likelihood method, Norusis 1992) was performed to detect the predictive macro- and micro-habitat variables of cottontail form with respect to those of hare forms in each season. We chose the stepwise method (LRA) in accordance with Smith et al. (2009) that pointed out that standardized partial regression coefficients were unbiased estimates of the relative importance of habitat variables (cover, amount, and fragmentation) even when predictors were highly correlated. Moreover, we calculated the variance inflation factor (VIF) in order to detect collinearity among predictor variables both for MANOVA and LRA models (Smith et al. 2009; Zuur et al. 2009).
All cover-type analyses were conducted with ArcView 3.2. Statistical analyses were performed by SPSS/PC+Version 15.0.
Results
Density
Hare density was 103.8 individuals per square kilometer (SE = 9.72) in area A and 47.8 individuals per square kilometer (SE = 7.23) in area B, whereas cottontail densities were 53.3 (SE = 8.04) and 170.4 individuals per square kilometer (SE = 17.3) in area C and B, respectively. The best detection probability function was the half-normal in every study area. Regarding hares, in area A, the function was characterized by three parameters and gave an effective strip wide (ESW) of 69.15 m (χ 2 = 1.12, df = 3, P = 0.77), while in area B, the function included two parameters, and it estimated an ESW of 73.96 m (χ 2 = 1.33, df = 3, P = 0.72). Concerning cottontails, in area B, the function included three parameters, and it calculated an ESW of 32.21 m (χ 2 = 0.15, df = 1, P = 0.70), and in area C, the function was characterized by two parameters and gave an ESW of 29.29 m (χ 2 = 1.12, df = 3, P = 0.95).
Form characteristics
We found 168 forms of hares (100 in autumn–winter and 68 in spring–summer) and 104 of cottontails (55 in autumn–winter and 49 in spring–summer). Cottontails rested more frequently in wet soils than hares (likelihood ratio = 9.61, df = 3, P = 0.030), and their forms were more frequently exposed to North and East than those of hares (likelihood ratio = 8.68, df = 3, P = 0.050). The percentage of “temporary” forms was greater for cottontail than for hares (likelihood ratio = 30.92, df = 1, P < 0.0001). Cottontail forms were more associated with canopy, bush, and herbaceous vegetation with respect to hares that rested on bare ground more often than cottontails (likelihood ratio = 64.63, df = 8, P < 0.0001) (Table 1). The average height (±SE) of vegetation at form sites was greater for cottontails (1.5 ± 0.20) than for hares (0.7 ± 0.9) (F (1, 231) = 14.94; P < 0.0001).
Edge use
Hare habitat breadth in area A (allopatry) was similar to that in area B (sympatry) in autumn–winter, but in spring–summer, it was narrower in area B with respect to area A. Cottontail habitat breadth did not show significant differences between study areas and seasons. Hares had slightly wider habitat breadth than cottontails except in spring–summer in area B (Table 2).
In area B during autumn–winter, cottontails preferred edges between two patches of spontaneous vegetation contrarily to hares that avoided them. Both species avoided edges between crops and between crops and spontaneous vegetation (Fig. 1). In spring–summer, cottontails preferred edges between crops and arboriculture stands that were used as available by hares; edges between two patches of spontaneous vegetation were used by cottontails relative to their availability and avoided by hares. Both species avoided edges between two crop fields and between crops and spontaneous vegetation (Fig. 2).
Average values of Manly's index for habitat preference of hares and cottontails in autumn–winter (area B, bars represent 95 % confidence intervals) (October–February 2007; Province of Pavia, northern Italy; C-C crops-crops, C-SV crops-spontaneous vegetation, C-AS crops-arboriculture stands, SV-SV spontaneous vegetation-spontaneous vegetation, SV-AS spontaneous vegetation-arboriculture stands)
Average values of Manly's index for habitat preference of hares and cottontails in spring–summer (area B, bars represent 95 % confidence intervals) (May–August 2008; Province of Pavia, northern Italy; C-C crops-crops, C-SV crops-spontaneous vegetation, C-AS crops-arboriculture stands, SV-SV spontaneous vegetation-spontaneous vegetation, SV-AS spontaneous vegetation-arboriculture stands)
Macro- and micro-habitat characteristics of forms
MANOVA showed significant differences between allopatry and sympatry areas of habitat characteristics of forms for both species (hares: Wilks' λ = 0.370, F (25, 140) = 9.55, P < 0.0001; cottontails: Wilks' λ = 0.417, F (28, 73) = 3.64, P < 0.0001). Two macro- and seven micro-habitat variables of hare forms differed significantly between area A and B: distances from the nearest wood and forage crops were greater in area A, while canopy, litter, and dead leaf cover, height of canopy, distance from the nearest herbaceous edge, and edges had greater means in area B (Table 3). Regarding cottontail forms, six macro- and three micro-habitat variables showed significant differences between area C and B: distances from the nearest herbaceous and non-herbaceous edge, arboriculture stands, and woods had greater means in area C, while distance from the nearest arboriculture stand, ploughed fields, fallow fields, basins and streams, and edges had greater means in area B (Table 4).
In area B, the LRA carried out on micro- and macro-habitat variables of cottontail and hare forms generated two models for the two seasons. The distance from the nearest wood entered the autumn–winter model with negative effect on the probability of forms to be of cottontails. The same variable also entered the spring–summer model together with the percentage of herbaceous cover with negative effect on the probability of forms to be of cottontails. The third variable that entered the model was the distance from the nearest fallow field with a positive effect on cottontail forms (Table 5). The autumn–winter model correctly classified 75.4 % of the original cases (63.0 % of hare forms and 86.7 % of cottontail ones), whereas 79.5 % of cases were correctly classified by the spring–summer model (84.6 % of hare forms and 69.2 % of cottontail ones).
The VIF values showed no collinearity among predictors in all models (VIFs < 3).
Discussion
The estimated densities of hares recorded on our study areas were comparable to the highest values reported by Smith et al. (2005) on several areas of arable farming through Europe even in sympatry when cottontail abundance was twice that of hares. Considering hares, population density estimated in allopatry was higher than that estimated in sympatry, but no difference in hare detection probability was evident between the two study areas. Therefore, it seemed that cottontails did not directly influence hare abundance and distribution in sympatry during nocturnal activity.
Although in simplified agro-ecosystem, the two species living in sympatry at high densities might frequently exploit simultaneously the same habitats, thus enhancing the chance of exploitation competition (Vidus-Rosin et al. 2008, 2011); resource overlap alone does not predict the degree of exploitation competition because the availability and allocation of resources in space and time as well as habitat preferences and niche segregation at different scales should be considered (Fa et al. 1992; Morris 1996; Katona et al. 2004).
At forms, hares and cottontails used different habitat features that could be explained taking into account the proximity of resting sites to their feeding sites (Reitz and Leonard 1994). During feeding activity, the two species segregated intensively on the basis of their adaptability to exploit foraging habitats at different distances from the nearest patch of permanent cover; in particular, the differential use of shared fields was much evident during spring, when growing crops and spontaneous vegetation increased open field suitability for hares and improved underground cover along ecotonal zones for cottontails, respectively (Vidus-Rosin et al. 2011). The contrasting dependence on cover during feeding is due to their different anti-predator strategies: hares use the long-distance running to escape from terrestrial predators while cottontails run away for short distances sheltering in the nearest dense underground cover (Cowan and Bell 1986; Fa et al. 1992; Althoff et al. 1997).
As a matter of fact, in our study areas, hares located forms in open fields as well as in association with cover, mainly herbaceous vegetation. On the contrary, cottontail forms were found in more developed bush and herbaceous vegetation, which also protected them sufficiently against the harsh temperatures registered in our study areas (Althoff et al. 1997). For these reasons, we supposed that cottontails do not require to build “permanent” forms as frequently as hares.
Regarding edges, our results suggested that hares were slightly generalist with respect to cottontails with the exception of spring-summer in sympatry, when hares exhibited a habitat contraction. In sympatry, the two species shared the same habitats, with the exception of edges between patches of spontaneous vegetation. In particular, during spring–summer, the use by hares for edges with at least arboriculture stands on one side strongly coincided with the preference of cottontails for the same habitats. Pépin and Angibault (2007) focused the attention on non-cropped habitats at the between-field scale, such as field margins, edges, ecotones, as optimal habitats for resting hares. On the other hand, cottontails are known to be attracted to the structural qualities of cover provided by bush hedges, tree-lined margins, woods, as well as of arboriculture stands (Swihart and Yahner 1982, 1984; Scribner and Warren 1990; Mankin and Warner 1999; Vidus-Rosin et al. 2010). Therefore, patches of permanent cover interspersed with crops provided optimal resting habitats for both lagomorphs mostly in summer, when these habitats buffer the negative effects of the seasonal changes of land use, in particular the reduction of cover after harvesting. Because of their linear features (i.e., narrow strips), edges can force the two species to rest at relatively high chance of intra- and inter-specific encounters and prevent habitat segregation between them at a finer scale (Bonesi and Macdonald 2004). In our case, no evidence of hare habitat shift at the between-patch scale was evident and due to cottontail preference for the same edge types.
The availability of high structural ecotonal zones was much important for cottontails with respect to hares during feeding activity; cottontails preferred patches of permanent cover (hedgerows, woodlots, arboriculture stands) all year round and used the other macro-habitat types as available, both in allopatry and in sympatry. Conversely, hares were used to feed in alfalfa and crops and used permanent cover and edges relative to their availability. As a consequence, hare habitat breath was broader than that of cottontails both in allopatry and sympatry with the exception of summer and autumn, when probably the sudden reduction of suitable habitats due to crop harvesting and field sowing promoted hare habitat contraction. Even during feeding activity, no evidence of hare habitat shift was evident due to cottontail exploitation of the same macro-habitat types in sympatry, rather habitat breath and preference of both species seemed to reflect the seasonal change of the carrying capacity (Vidus-Rosin et al. 2011).
Considering the macro- and micro-habitat characteristics around form sites, our results did not provide any evidence of exploitation competition between the two species. Differential habitat use by hares in sympatry with respect to allopatry seems mainly due to different habitat amount between the two areas. In sympatry, where permanent cover was more developed than in allopatry, hares located forms closer to or more frequently within patches of canopy cover, whereas in allopatry, hares rested more in herbaceous habitats. Regarding cottontails, it seems that habitat selection during resting time depends partially upon population density (Rosenzweig 1981, 1991). In sympatry, where the population was greater and arboriculture stands and woods were concentrated in small lots with respect to allopatry, cottontails rested more in herbaceous and open habitats, as well as along edges (Swihart and Yahner 1982; Althoff et al. 1997; Smith and Litvaitis 2000).
Even though intra-specific competition among cottontails might force the species to rest within habitats frequently used by hares, in sympatry, the two species appear to spatially segregate within-patches through differential utilization of micro-habitats (Abramsky 1981). In particular, the closeness to woody habitats enhanced the habitat suitability for cottontails to rest, while the availability and proximity to herbaceous cover enhanced the one for hares. The differential habitat use within macro-habitat types by the two species came out even during feeding activity, and it seemed to be motivated by maximum acquisition of nutrients and predation avoidance. In particular, cottontails were highly associated with bush and canopy cover, and within woodlots, the species occupied also the central area where thick layers of plant litter were more developed. On the contrary, hares were more likely to exploit open fields' mostly herbaceous-dominated habitats than cottontails (Vidus-Rosin et al. 2011).
On the whole, it seems that hares and cottontails may exploit the same macro-habitat features in agro-ecosystems even at high population levels but with good habitat heterogeneity that allows spatial segregation during feeding and daytime between the two species. In particular, arboriculture stands and patches of spontaneous vegetation (woods, fallow fields, field boundaries with bush and canopy cover) interspersed with crops should be promoted to enhance habitat diversity and edge differentiation, therefore to maintain their differential perception of habitat structure during feeding activity, and to ensure optimal resting habitats for hares all year round and limit intra- and inter-specific encounters.
Future research may shed more light on the relationship between lagomorphs and habitat structure. In particular, we need to clarify the interactions between the two lagomorph species mediated by habitat availability and population density; therefore, replicates of allopatric and sympatric sites with a range of density and habitat heterogeneity would be required to provide the necessary data to evaluate the role of habitat structure in facilitating the coexistence between the two species. Moreover, it would be important to carry out field experiments followed by enclosure experiments because the first approach allows to evaluate the relationships between habitat availability, species density, and temporal variation of the carrying capacity, and the second approach is necessary to validate the potential competitive relationships between the two species.
References
Abramsky Z (1981) Habitat relationships and competition in two Mediterranean Apodemus spp. Oikos 36:219–225
Althoff DP, Storm GL, Dewalle DR (1997) Daytime habitat selection by cottontails in central Pennsylvania. J Wildl Manage 61:450–459
Barbour MS, Litvaitis JA (1993) Niche dimensions of New England cottontails in relation to habitat patch size. Oecologica 95:321–327
Belovsky GE (1984) Moose and snowshoe hare competition and a mechanistic explanation from foraging theory. Oecologica 61:150–159
Bonesi L, Macdonald DW (2004) Differential habitat use promotes sustainable coexistence between the specialist otter and the generalist mink. Oikos 106:509–519
Bonesi L, Chanin P, Macdonald DW (2004) Competition between Eurasian otter Luta lutra and American mink Mustela vison probed by niche shift. Oikos 106:19–26
Bonino N, Sbriller A, Manacorda MM, Larosa F (1997) Food partitioning between the mara (Dolichotius patagonum) and the introduced hare (Lepus europaeus) in the Monte Desert, Argentina. Stud Neotr Fauna Environ 32:129–134
Buckland ST, Anderson DR, Burnham KP, Laake JL, Thomas L (2001) Introduction to distance sampling: estimating abundance of biological populations. Oxford University Press, Oxford, pp 1–432
Burnham KP, Anderson DR, Laake JL (1985) Efficiency and bias in strip and line transect sampling. J Wildl Manage 49:1012–1018
Butler MJ, Ballard WB, Wallace MC, Demaso SJ (2007) Road-based survey for estimating wild turkey density in the Texas rolling plain. J Wildl Manage 71:1646–1653. doi:10.2193/2006-270
Chapman JA, Harman AL, Samuel DE (1977) Reproductive and physiological cycles in cottontail complex in Western Maryland and nearby West Virginia. Wildl Monogr 56:1–73
Cowan D, Bell DJ (1986) Leporid social behaviour and social organization. Mamm Rev 16:169–179
Dixon PM (1993) The bootstrap and the jackknife: describing the precision of ecological indices. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 290–318
Fa JA, Romero FJ, Paniagua LP (1992) Habitat use by parapatric rabbits in a Mexican high-altitude grassland system. J Anim Ecol 29:357–370
Feinsinger P, Spears EE, Poole RW (1981) A simple measure of niche breath. Ecology 62:27–32
Hansen K (1992) Reproduction in European hare in a Danish farmland. Acta Theriol 37:27–40
Jennings N, Smith RK, Hacklander K, Harris S, White PCL (2006) Variation in demography, condition and dietary quality of hares Lepus europaeus from high-density and low-density populations. Wildl Biol 12:179–189
Katona K, Biro Z, Hahn I, Kertesz M, Altbacker V (2004) Competition between European hare and European rabbit in a lowland area, Hungary: a long-term ecology study in the period of rabbit extinction. Folia Zool 53:255–268
Krebs CJ (1999) Ecological methodology. Second Edition. Addison-Welsey Educational Publishers, Inc., Canada, pp 1-607
Langbein J, Hutchings MR, Harris S, Stoate C, Tapper SC, Wray S (1999) Techniques for assessing the abundance of Brown Hares Lepus europaeus. Mamm Rev 29:93–116
Mankin PC, Warner RE (1999) Responses of Eastern cottontails to intensive row-crop farming. J Mammal 80:890–949
Manly BFJ, McDonald LL, Thomas DL, McDonald TL, Erickson WP (1993) Resource selection by animals: statistical design and analysis for field studies. Kluwer, Dordrecht
Manor R, Saltz D (2008) Conservation implications of competition between generalist and specialist rodents in Mediterranean afforested landscape. Biodivers Conserv 17:2513–2523. doi:10.1007/s10531-008-9397-1
Meriggi A (1989) Analisi critica di alcuni metodi di censimento della fauna selvatica (Aves, Mammalia). Aspetti teorici ed applicativi Ricerche di Biologia della Selvaggina 83:1–59
Meriggi A, Ferloni M, Geremia R (2001) Studio sul successo dei ripopolamenti di Lepre. Dipartimento di Biologia Animale, Università di Pavia, Pavia
Morris DW (1996) Coexistence of specialist and generalist rodents via habitat selection. Ecology 77:2352–2364
Norusis MJ (1992) SPSS/PC+Base system user’s guide, Vers. 5.0. SPSS Inc., Chicago
Partridge L (1978) Habitat selection. In: Krebs JR, Davies NB (eds) Behavioural ecology. An evolutionary approach. Blackwell, Oxford, pp 351–376
Pépin D, Angibault JM (2007) Selection of resting sites by the European hare as related to habitat characteristics during agricultural changes. Eur J Wildl Res 53:183–189. doi:10.1007/s10344-007-0087-1
Pianka ER (1981) Competition and niche theory. In: May RM (ed) Theoretical ecology. Principles and applications. Blackwell, Oxford, pp 167–196
Reichlin T, Klansek E, Hacklander K (2006) Diet selection by hares (Lepus europaeus) in arable land and its implications for habitat management. Eur J Wildl Res 52:109–118. doi:10.1007/s10344-005-0013-3
Reitz F, Leonard Y (1994) Characteristics of European hare Lepus europaeus use of space in a French agricultural region of intensive farming. Acta Theriol 39:143–157
Roedenbeck P, Voser P (2008) Effects of roads on spatial distribution, abundance and mortality of brown hare (Lepus europaeus) in Switzerland. Eur J Wildl Res 54:425–437. doi:10.1007/s10344-007-0166-3
Rosenzweig ML (1981) A theory of habitat selection. Ecol 62:327–335
Rosenzweig ML (1991) Habitat selection and population interactions: the search for mechanism. Am Nat 137:S5–S28
Santilli F, Galardi L (2006) Factors affecting Brown hare (Lepus europaeus) hunting bags in Tuscany region (central Italy). Hystrix 17:143–153
Scribner KT, Warren RJ (1990) Seasonal demography and movements of cottontail rabbits on isolated Playa Basins. J Wildl Manage 54:403–409
Smith AC, Koper N, Francis CM, Fahrig L (2009) Confronting collinearity: comparing methods for disentangling the effects of habitat loss and fragmentation. Landsc Ecol 29:1271–1285. doi:10.1007/s10980-009-9383-3
Smith DF, Litvaitis JA (2000) Foraging strategies of sympatric lagomorphs: implications for differential success in fragmented landscapes. Can J Zool 78:2134–2141
Smith RK, Jennings NV, Harris S (2005) A quantitative analysis of the abundance and demography of European hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mamm Rev 35:1–24
Swihart KR (1986) Home range-body mass allometry in rabbits and hares (Leporidae). Acta Theriol 31:139–148
Swihart RK, Yahner RH (1982) Habitat features influencing use of farmstead shelterbelts by eastern cottontail (Sylvilagus floridanus). Am Midl Nat 107:411–414
Swihart RK, Yahner RH (1984) Winter use of insular habitat patches by the Eastern cottontail. Acta Theriol 29:45–56
Swihart RK, Lusk JJ, Duchamp JE, Rizkalla CE, Moore JE (2006) The roles of landscape context, niche breadth, and range boundaries in predicting species responses to habitat alteration. Diversity Distribution 12:277–287
Vidus-Rosin A, Gilio N, Meriggi A (2008) Introduced Lagomorphs as a threat to native Lagomorphs: the case of the Eastern cottontail (Sylvilagus floridanus) in northern Italy. In: Alves PC, Ferrand N, Hacklander K (eds) Lagomorph biology. Evolution, ecology and conservation. Springer, Eidenberg, pp 153–165
Vidus-Rosin A, Meriggi A, Serrano-Perez S (2010) Density and habitat requirements of introduced Eastern cottontail Sylvilagus floridanus in northern Italy. Acta Theriol 55:139–151. doi:10.4098/j.at.0001-7051.029.2010
Vidus-Rosin A, Meriggi A, Cardarelli E, Serrano-Perez S, Mariani M-C, Corradelli C, Barba A (2011) Habitat overlap between sympatric European hares (Lepus europaeus) and Eastern cottontails (Sylvilagus floridanus) in northern Italy. Acta Theriol 56:53–61. doi:10.1007/s13364-010-0005-x
Zuur AF, Ieno EN, Elphick CS (2009) A protocol for data exploration to avoid common statistical problems. Meth Ecol Evol. doi:10.1111/j.2041-210x.2009.00001.x
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Emerson Vieira
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Vidus-Rosin, A., Lizier, L., Meriggi, A. et al. Habitat selection and segregation by two sympatric lagomorphs: the case of European hares (Lepus europaeus) and Eastern cottontails (Sylvilagus floridanus) in northern Italy. Acta Theriol 57, 295–304 (2012). https://doi.org/10.1007/s13364-012-0084-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-012-0084-y