Abstract
Multidrug resistance (MDR) is a complex phenomenon caused by numerous reasons in cancer chemotherapy. It is related to the abnormal tumor metabolism, precisely increased glycolysis and lactic acid production, extracellular acidification, and drug efflux caused by transport proteins. There are few strategies to increase drug delivery into cancer cells. One of them is the inhibition of carbonic anhydrases or certain proton transporters that increase extracellular acidity by proton extrusion from the cells. This prevents weakly basic chemotherapeutic drugs from ionization and increases their penetration through the cancer cell membrane. Another approach is the inhibition of MDR proteins that pump the anticancer agents into the extracellular milieu and decrease their intracellular concentration. Physical methods, such as ultrasound-mediated sonoporation, are being developed, as well. To increase the efficacy of sonoporation, various microbubbles are used. Ultrasound causes microbubble cavitation, i.e., periodical pulsation of the microbubble, and destruction which results in formation of temporary pores in the cellular membrane and increased permeabilization to drug molecules. This review summarizes the main approaches to reverse MDR related to the drug penetration along with its applications in preclinical and clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to World Health Organization, 8.8 mln people died from cancer in 2015 and it was one of the leading causes of death worldwide [1]. Although during the last two decades mortality rates decline, they still remain high [2]. Therefore, it is very important to improve cancer diagnostics and enhance the efficacy of anticancer therapy. To ensure the therapeutic efficacy of an anticancer drug, sufficient concentration of the compound must be achieved in tumor cells. It becomes a challenge for the drug developers due to certain characteristics of the tumor microenvironment [3]. An increasing attention is given to the transport of drugs to tumors.
Due to increased extracellular acidity of tumor, basic drugs tend to ionize. Positive charge limits drug ability to permeate cellular membrane and reach the target site [4]. The efficacy of chemotherapy is also associated with MDR which is partially caused by various drug efflux proteins, that transport drug molecules from the cytoplasm to the extracellular fluid [5, 6]. Multidrug resistance protein-1 (MRP-1/ABCC1) has been shown to be associated with MDR in stage I and II hormone positive breast cancer (n = 516). Patients who administered cyclophosphamide, methotrexate, and fluorouracil and who had increased expression of MRP-1 experienced increase in relapse rate and mortality when compared to those patients, who had negative MRP-1 expression in tumors [7]. In other clinical study (n = 59), high phosphoglycoprotein (P-gp/ABCB1) expression level was associated with a poor prognosis of a disease and decreased length of progression-free survival [8]. It is important that cell sensitivity to chemotherapy and the mechanisms of resistance vary between different types of cancer and different cell lines of the same type of cancer. Kibria et al. investigated the sensitivity of 15 different cell lines to doxorubicin. They found that the sensitivity of cancer cells to doxorubicin was not always proportional to the intracellular concentration of doxorubicin. Also, the inhibition of P-gp in certain cell lines significantly increased cytotoxicity of doxorubicin, while in other cell lines, no effect was observed. This means that, besides P-gp, there are some other mechanisms that determine drug resistance [9]. Various types of tumors differ in the rates and mechanisms of chemoresistance. For example, a characteristic feature of pancreatic cancer is a very rich stroma with a high number of fibroblasts and macrophages. It was found that these cells contribute to chemoresistance by secreting various growth factors which leads to increased cell survival and proliferation [10, 11]. Furthermore, cancer stroma is like a physical barrier to drug penetration. High number of stroma cells and increased interstitial pressure cause the constriction of blood vessels and limited drug penetration into target site [12]. Also, pancreatic cancer is usually highly hypoxic. Hypoxia may increase chemoresistance by activation of certain signaling pathways [13]. Pancreatic cancer is very poorly vascularized [14]. It is considered to be one of the most hypoxic types of cancer [14]. Also, increased expression of drug efflux transporters multidrug resistance protein-5 (MRP-5/ABCC5) [15], multidrug resistance protein-5 (MRP-8/ABCC11) [16], and human equilibrative nucleoside transporter-1 [17] contributes to chemoresistance to gemcitabine and 5-fluorouracil.
In order to overcome these problems of inefficient cancer chemotherapy, pH modulators (vacuolar-H+-ATPase (V-ATPase) inhibitors, carbonic anhydrase (CA) inhibitors, sodium-hydrogen exchanger-1 (NHE-1) inhibitors), inhibitors of MDR proteins P-gp, MRP-1, and breast cancer resistance protein (BCRP/ABCG2)), nanocarrier systems, and physical methods (sonoporation, electroporation) are being developed and widely investigated.
Tumor microenvironment
There are some significant differences between normal and tumor tissues and one of the main discrepancies is a pH gradient. Because of anaerobic metabolism in tumor tissues, extracellular fluid is more acidic than in normal tissues [18]. It was estimated that extracellular pH in tumor varies between is about 6.8 [19, 20]. Activation of oncogenes, hypoxia-inducible factor-1 activation arises in cancer cells, and this leads to induced expression of glycolytic enzymes [21]. Upregulation of glucose transporters occurs, as well [22]. Therefore, cellular energy metabolism shifts from oxidative phosphorylation towards anaerobic glycolysis even in the presence of oxygen. This phenomenon is called the Warburg effect and was discovered by Otto Heinrich Warburg in 1920s [23]. High rate of glycolysis results in increased lactic acid production and various transporters, such as V-ATPase, NHE, monocarboxylate transporter, extrude protons into the extracellular tissue, thus increasing its acidity [24, 25].
It is known that neutral molecules penetrate cell membrane easier than positively or negatively charged ions [26]. According to pH-partition theory, at lower pH basic drugs, e.g., doxorubicin, undergo ionization, therefore, the penetration of these compounds declines and their therapeutic efficacy decreases (Fig. 1) [18, 27]. This is called “ion trapping” phenomenon. The same problem is typical with other basic compounds such as anthracyclines, anthraquinones, and Vinca alkaloids. Weakly basic drugs also tend to accumulate in lysosomes and endosomes that have an acidic lumen [28, 29].
There are two main strategies to reduce this acidity-related chemoresistance. One of them is increasing pH of extracellular milieu by basic substances, such as sodium bicarbonate [27, 30]. Robey et al. investigated that oral administration of 200 mM sodium bicarbonate slightly increased extracellular pH from 7.0 to 7.4, whereas did not affect intracellular pH in murine breast cancer models [31]. Other study showed that oral administration of sodium bicarbonate in mice may enhance the activity of basic anticancer drugs, such as doxorubicin [27]. However, it is associated with a risk of metabolic alkalosis, hypernatremia, electrolyte imbalance, and other side effects [32]. Therefore, other more modern approaches, based on the inhibition of proton transporters, CAs or ATP-binding cassette (ABC) transporters are being developed. These enzymes and transporters contribute to chemoresistance by pumping protons from cytoplasm to extracellular tissue and acidic vesicles, thus increasing extracellular acidity, and by mediating drug efflux out of cancer cell (Fig. 2). Compounds that inhibit proton pumps prevent proton transport from the cell cytoplasm to extracellular milieu and suppress the acidification of the extracellular tissue [33].
Transporters and enzymes involved in multidrug resistance. Proton transporters V-ATPase and NHE extrude protons out of cells thus decreasing pH of extracellular milieu; CA IX contributes to the extracellular acidity by carbon dioxide hydration resulting in bicarbonate ion and proton release. ATP-binding cassette transporters P-gp, MRP-1, and BCRP mediate anticancer drugs efflux out of the cell. Abbreviations: V-ATPase, vacuolar-H + -ATPase; NHE, Na+/H+ exchanger; CA IX, carbonic anhydrase IX; P-gp, phosphoglycoprotein; MRP-1, multidrug resistance protein-1; BCRP, breast cancer resistance protein
pH modulators
One of the strategies to increase the penetration of basic drugs into the cells is to reduce the extracellular acidity in tumor tissue. For this purpose, various pH modulators, such as proton pump inhibitors (PPIs), CA inhibitors, or NHE inhibitors are being applied.
V-ATPase inhibitors
V-ATPase is a proton pump which regulates H+ transport across cell membrane and maintains low pH within endosomes and lysosomes [34]. It is located in the membrane of lysosomal vesicles and in the plasma membrane of certain cells. This enzyme pumps H+ from the cell into the interstitial fluid, thus maintaining acidic extracellular pH and transport H+ from cytosol to cellular vesicles [35].
It is well known that in some tumors the activity of several isoforms of V-ATPase is increased [36,37,38]. High activity of this transport protein is associated with a poor prognosis of the disease [39]. Compounds that inhibit V-ATPase lead to pH increase in extracellular milieu and acidic vesicles [40]. Thus, basic drugs can easier penetrate into cancer cells [41]. V-ATPase inhibitors also reduce basic drug sequestration and neutralization within lysosomes and their extrusion out of cells via exocytosis [42]. It leads to decrease of drug trapping, enhanced delivery to their target site, and improved cell sensitivity to chemotherapy. Lee et al. found that siRNA-induced inhibition of V-ATPase leads to the reduced intracellular pH and increased cytotoxicity of paclitaxel in chemoresistant epithelial ovarian cancer cells [39].
Plecomacrolide antibiotics bafilomycins and conacanamycins are the earliest known V-ATPase inhibitors. It was shown that bafilomycin A1 effectively inhibits V-ATPase, thus increases extracellular pH in cancer cell cultures [43]. Similar results were found with concanamycin A. Kiyoshima et al. estimated that this compound inhibits the acidification of cellular vesicles and reduces proliferation of oral squamous carcinoma cells [44]. Nevertheless, none of these compounds was introduced to clinical trials.
Another well-known group of V-ATPase inhibitors is PPIs. Recent studies show that drugs, such as omeprazole, lansoprazole, and pantoprazole, reverse MDR by reducing extracellular acidity [39, 41, 45, 46]. PPIs tend to accumulate in acidic cell compartments where they are activated by the protonation of basic nitrogen atoms [47]. In this way, they might specifically be active in acidic tumor tissues. Patel et al. found that pantoprazole increased endosomal pH and nuclear uptake of doxorubicin within mouse mammary sarcoma cells and tumor tissue [46]. There is evidence that omeprazole pretreatment in combination with paclitaxel reduces tumor growth in chemoresistant epithelial ovarian cancer mice models compared to paclitaxel alone [39]. Similar results were reported by Luciani et al. [48].
It is important to emphasize that simultaneous treatment with PPIs and antitumor drugs did not potentiate the efficacy of chemotherapy. Authors explain this phenomenon by the competition of drugs against each other for cellular uptake [48]. However, the data about the disruption of intracellular and extracellular pH gradient caused by PPIs is controversial. Linder et al. detected no difference in intracellular and extracellular pH after 24 and 48 h of cancer cells treatment with PPI. Furthermore, increase in intracellular pH and decrease in extracellular pH were determined in these cells after 72 h of treatment with PPI [49]. These data suggest that there may be some other mechanisms through which PPIs enhance the cytotoxicity of anticancer drugs. Also, there is evidence that co-administration of PPIs with certain anticancer drugs may reduce some side effects of chemotherapy. Recently, Ikemura et al. investigated that PPIs can ameliorate the nephrotoxicity of cisplatin by inhibiting organic cation transporter 2 [50]. Furthermore, there is evidence that PPIs not only improve the efficacy of chemotherapeutics, but also exert anticancer activity themselves. Among these drugs, lansoprazole has shown the most potent cytotoxicity [51].
Because of high potential for the application in chemotherapy, PPIs gained a great interest among researchers. On the basis of existing structures, novel bisbenzimidazole derivatives were developed. These compounds showed to be potent V-ATPase inhibitors and demonstrated high antiproliferative activity against breast cancer cells [52] and are among the most promising transport modulators of basic drugs.
CA inhibitors
CA is a transmembrane zinc metalloprotein which is involved in pH homeostasis of various tissues. This enzyme catalyzes the reversible hydration of carbon dioxide to bicarbonate [53]. 16 α-CA isoforms exist in mammals. Two of them—CA IX and XII—are associated with cancer development and progression [54, 55]. According to Robertson et al., inhibition of CA IX expression results in a delay of cancer cell growth and reduction of cell survival under normoxia and hypoxia [54]. Although both isoforms are found in normal tissues, such as the gastric mucosa, duodenum, or kidney, their gene expression is highly increased in many types of tumors [56, 57]. Therefore, they are attractive targets for anticancer therapy.
It is hypothesized that inhibition of CA IX and XII reduces extracellular acidification, therefore enhancing basic drug delivery into tumor tissue. There are some preclinical studies confirming this theory. Gieling et al. found that inhibition of CA IX by acetazolamide enhances uptake and toxicity of weakly basic doxorubicin, but reduces weakly acidic melphalan penetration into cells [58].
Several CA IX and CA XII inhibitors have been developed and tested as transport modulators. However, most of them are still in preclinical studies and the results are controversial. SLC-0111, also known as U104, is a benzenesulfonamide derivative and highly selective inhibitor of CA IX and CA XII. It was shown that U104 significantly increased the efficacy of paclitaxel in orthotopic breast cancer mice models [59]. In contrast, Riemann et al. assessed that although U104 reduced cancer cell proliferation and increased apoptosis, it did not improve the cytotoxicity of daunorubicin and cisplatin in prostate cancer cells [60]. Phase I clinical study was designed in order to evaluate the pharmacokinetic profile, safety, and efficacy of U104 in anticancer therapy (clinicaltrials.gov, NCT02215850). However, the results have not been published yet.
For higher therapeutic efficacy, CA inhibitors can be incorporated into nanocarrier systems. Janoniene et al. loaded porous silicon nanoformulations with doxorubicin and conjugated with selective CA IX inhibitor VD11-4-2 [61]. It enhanced target drug delivery towards tumor tissue and improved doxorubicin penetration into cancer cells. VD11-4-2 also enhanced drug loading efficacy into nanoparticles and improved drug release profile by reducing the premature release of doxorubicin. Besides an increase in intracellular drug concentration in tumors, these systems also reduce the effect on other tissues thus reducing toxicity. Therefore, this field is currently very widely investigated and show promising results.
NHE inhibitors
NHE is a ubiquitous proton transporter that mediates Na+/H+ exchange across the cell membrane. It extrudes protons out of the cell and transports Na+ into the cytoplasm. As cellular pH regulators, NHEs also contribute to MDR. Inhibition of these transporter proteins increases basic drug penetration into tumor tissue. Thirteen isoforms of NHE exist in humans. NHE-1 is of particular interest in oncology because it is involved in cancer cell migration [62] and metastasis [63]. NHE-1 is found in many normal tissues and also is upregulated in various tumors, such as gastric [64] and breast cancer [63], hepatocellular carcinoma [65] or glioblastoma [66]. Previous studies showed that knockdown of NHE-1 results in increased sensitivity to doxorubicin in T cell acute lymphoblastic leukemia cells [67].
The first known NHE inhibitor was potassium-sparing diuretic amiloride, discovered in 1965 [68]. A few decades later, more potent amiloride derivatives specific to NHE-1 were synthesized. 5-(N-ethyl-N-isopropyl)amiloride (EIPA) is amiloride analog which is 200-fold more effective in inhibition of NHE. Some studies show that EIPA may reverse doxorubicin resistance in cancer cells. Pannocchia et al. found that EIPA significantly increased doxorubicin accumulation in doxorubicin-resistant colon cancer cells while the addition of monensin (NHE activator) significantly reduced intracellular doxorubicin concentration [69]. Miraglia et al. showed that inhibition of NHE by EIPA leads to the reduction of intracellular pH and thus increases doxorubicin concentration in colon cancer cells [70]. On the contrary, activation of NHE by phorbol 12-myristate increases intracellular pH and decreases penetration of doxorubicin into cells.
Recently, more powerful and highly selective NHE-1 inhibitors cariporide, zoniporide, and eniporide were developed. Although they showed good tolerability in humans, all the clinical trials performed were oriented in the field of cardiology because of their cardioprotective effects [71, 72].
ABC transporters
Another reason for MDR is the activity of ABC transporters. They are transmembrane proteins involved in self-defense mechanisms. These proteins actively pump various endogenous molecules and xenobiotics out of the cell. The main transporters that are linked to the resistance of many structurally unrelated anticancer agents are P-gp, MRP-1, and BCRP [73]. The substrates of these transporters include numerous anticancer agents from various groups (Table 1).
ABC transporters are normally found in many various organs such as the kidney, liver, testes, intestine, and physiological barriers [95]. Usually, their expression in tumor tissues is highly increased [96, 97]. It was shown that inhibition of these transporters may enhance delivery and efficacy of anticancer drugs [78].
P-gp inhibitors
The most widely studied ABC transporter is P-gp. It is a 170-kD transmembrane protein. P-gp overexpression causes chemoresistance against many anticancer agents, such as paclitaxel, doxorubicin [98], daunorubicin [99], etoposide [100], or vinblastine [101].
There are three generations of compounds that inhibit P-gp (Table 2). First generation includes verapamil, quinine, and cyclosporine A. These are pharmacologically active compounds, approved for various cancer unrelated indications. In 1989, it was found that verapamil competitively inhibits P-gp and enhances doxorubicin, colchicine, and vincristine retention within leukemia cells [102]. Cyclosporine A was found to enhance distribution, retention and cytotoxicity of doxorubicin and mitoxantrone in cells overexpressing P-gp, MRP-1 and BCRP [103]. However, during some studies, it was noticed that these drugs lack efficacy [104] or may improve the toxicity of chemotherapy and cause various adverse events [105, 106].
In order to reduce toxicity, first-generation P-gp inhibitors were modified using chiral switching, and second generation P-gp inhibitors were developed. Drug binding to P-gp is not stereospecific; thus, isomers of P-gp inhibitors maintain their inhibitory effect and ability to reduce MDR. Dexverapamil, the R-isomer of verapamil, was shown to exert less potent calcium channel blocking activity and lower cardiotoxicity compared to S-isomer [107, 108] while maintaining its ability to reduce doxorubicin chemoresistance in the same extent as its racemate [109]. Another second-generation P-gp inhibitor PSC833, also known as valspodar, is a 10–20-fold more potent analog of cyclosporine D, but contrary to its parent compound, valspodar does not exert immunosuppressive activity. However, these compounds showed to be potent CYP 3A4 enzyme inhibitors. Therefore, significant undesirable pharmacokinetic interactions between anticancer drugs were observed, that result in delayed elimination and increased toxicity [110,111,112,113]. It is because many anticancer agents, such as doxorubicin, paclitaxel, and vinblastine, are metabolized by CYP 3A4 enzyme.
Aforesaid limitations encouraged the development of the third-generation P-gp inhibitors, that possess low toxicity, higher specificity, and binding affinity to P-gp and do not interact with the CYP450 3A4 enzyme. These inhibitors include tariquidar, zosuiqudar, elacridar, biricodar, and ONT-093. Design of these novel compounds was based on the structure-activity relationship studies. Tariquidar is a derivative of anthranilic acid. It has 4-fold higher affinity for P-gp than vinblastine, and 20-fold higher affinity than paclitaxel [114]. Tariquidar is a non-competitive P-gp inhibitor [114]. Phase I trial showed that tariquidar is well tolerated when combined with doxorubicin, docetaxel, or vinorelbine [115]. However, two phase III clinical trials of tariquidar in combination with paclitaxel plus carboplatin or vinorelbine alone for non-small cell lung cancer were discontinued. These decisions have been made due to high levels of toxicity observed in the tariquidar arms (QLT Inc. Form 8-K). Another P-gp inhibitor biricodar showed acceptable levels toxicity and good tolerability [116], but was not very efficient [117].
Due to unsuccessful results of the third-generation inhibitors in clinical trials, screening of natural substances has been started. These plant-based compounds belong to the fourth-generation P-gp inhibitors and include alkaloids, terpenoids, flavonoids, coumarins, and saponins. It has long been known that grapefruit juice induces P-gp-related drug efflux [118]. In a recent study by Zhang et al., incubation of doxorubicin-resistant osteosarcoma cells with resveratrol for 48 h caused an almost 7-fold decrease of doxorubicin antiproliferative activity when compared to cells incubated with doxorubicin alone. Also, an increase in intracellular concentration of drug and downregulation of MDR1/P-gp gene expression was determined [119]. Another natural P-gp inhibitor, citrus methoxyflavone nobiletin, was found to inhibit P-gp efflux function and increase the efficacy of paclitaxel, doxorubicin, docetaxel, and daunorubicin in ovarian cancer and colorectal adenocarcinoma cells [120]. Moreover, it was shown that flavonoids inhibit not only P-gp but also BCRP, thus increasing an intracellular concentration of anticancer compounds that are BCRP substrates [121].
BCRP inhibitors
BCRP is the most recently found ABC transporter. The substrates of BCRP possess several common structural features such as a planar structure, hydrophobic regions, aromatic systems, 7 to 20 carbon atoms and oxygen-containing groups [136]. There are only few selective BCRP substrates. Usually, the substrates of BCRP have the high affinity to P-gp, as well. One of the first discovered BCRP inhibitors was fumitremorgin C, an indole alkaloid isolated from Aspergillus fumigatus. According to in vitro studies, it is a very effective BCRP inhibitor that almost completely reverses resistance to mitoxantrone, doxorubicin, topotecan and paclitaxel in BCRP overexpressing MCF-7 breast cancer cells [137]. Similar results were found in colon carcinoma cells S1-M1-3.2 that expressed low levels of P-gp and MRP [138].
However, fumitremorgin C has been reported to cause severe neurotoxicity, which impeded its application in clinical practice. Therefore, its nontoxic analog Ko143 was developed. It is a very potent BCRP inhibitor that exerts its effect at nanomolar concentrations [139]. Recently, it was found that at concentrations higher than 1 μM Ko143 inhibits P-gp and MRP-1 [132]. This inhibitory effect on all three ABC transporters may be favorable in order to increase the efficacy of anticancer therapy but may also increase the risk of toxicity.
MRP-1 inhibitors
MRP-1 is another transmembrane protein that belongs to the ABC transporter family and pumps drug substances out of the cell. It was first discovered in human small cell lung carcinoma cells in 1992 [77]. As previously described P-gp and BCRP, MRP-1 is also overexpressed in a wide variety of solid tumors and it is considered to be a negative prognostic factor of the disease. This protein takes part in the efflux of well-known common anticancer drugs such as doxorubicin, vinblastine, methotrexate, and recently developed compounds, for instance, tyrosine kinase inhibitors. Numerous in vitro and in vivo studies have shown that inhibition of MRP-1 or downregulation of its gene expression leads to chemosensitization of cancer cells to various anticancer agents [140,141,142]. One of the most potent MRP-1 inhibitors is a pyrazolopyrimidine derivative reversan. Reversan also inhibits P-gp, and it showed favorable toxicity profiles in murine models [143]. Regardless of favorable results in vitro or in mice, no clinical trials were conducted with MRP-1 inhibitors yet.
Physical methods
Besides inhibition of pH-regulating proteins or ABC transporters with chemical compounds, physical methods can also be used to improve drug delivery into cancer cells. These methods include sonoporation by low-intensity ultrasound and electroporation.
Sonoporation
In late 1940s, ultrasound was first applied for medicinal diagnostics [144]. In recent decades, there is an increasing interest by scientists in the application of ultrasound in anticancer therapy. There are numerous studies proving that ultrasound combined with microbubbles may enhance anticancer drug delivery into tumor cells [145,146,147].
Microbubbles consist of hydrophobic, usually fluorinated gas coated with 10–100 nm layer made of polymers, proteins, and lipids. In order to increase the specificity of microbubbles to tumor tissues, a particular ligand specific to the cell surface receptors can be attached to them. Due to the acoustic pressure of ultrasound, microbubbles start to shrink and expand periodically. This process is called cavitation. When the acoustic pressure reaches a certain threshold, a collapse of microbubbles occurs [148]. It is thought that cavitation or explosion of microbubbles creates temporary pores in the cell membrane through which the drug passively enters the cells (Fig. 3) [149,150,151]. These pores close up as soon as ultrasound exposure is terminated [149, 151]. The drug solution can be either mixed with microbubbles or added before.
According to some studies, ultrasound may improve not only passive diffusion but also the active transport of drugs. It is believed that ultrasound may cause changes of ion channels; therefore, intracellular Ca2+ concentration increases and causes cytoskeletal rearrangement [152]. These processes stimulate endocytosis and drug delivery into cells.
In order to cause microbubble cavitation, low-frequency (0.4–3.0 MHz) ultrasound is used and duty cycle may vary from less than 1 to 90%. Long duty cycle and high intensity of ultrasound may cause tissue damage [153].
Results from in vitro studies confirm the possible benefit of ultrasound application in chemotherapy. Escoffre et al. determined that ultrasound and microbubbles combination increased doxorubicin antiproliferative activity by 2.5-fold [145]. Similar results were published by Piron et al. [146]. Grainger et al. investigated the effect of ultrasound on drug delivery in 3D cancer cell cultures. It was shown that ultrasound when used in combination with microbubbles increases nanoparticle penetration into breast cancer cell spheroids [147]. There is a lack of clinical trials, though. There is only one phase I clinical trial that showed promising results on the application of ultrasound and microbubbles against pancreatic cancer. Ultrasound prolonged survival from 8.9 to 17.6 months when compared to control and did not increase drug toxicity. However, patient cohort was too small (n = 10) to make reliable conclusions [154]. In order to evaluate the impact of ultrasound on the efficacy of chemotherapy, further in vivo studies and clinical trials are needed.
Electroporation
Electroporation, also known as electropermeabilization, technique is similar to sonoporation. The main difference is that instead of ultrasound the cells are exposed with short pulses of high voltage (usually 0.5–1 V) electrical field [155]. Electric field causes structural the rearrangement of lipid molecules of the cell membrane. This results in creation of hydrophilic pores and increased permeability of the cell membrane. Electroporation can be reversible or irreversible. The pores created by reversible electroporation are temporary and remain at least several minutes, depending on their size and duration of electric pulse [156]. In contrary, during irreversible electroporation, certain threshold of the strength and duration of electrical pulse is exceeded and cell death is caused. There are many in vitro and in vivo [157,158,159] studies that demonstrate an increased efficacy of anticancer drugs, such as bleomycin, gemcitabine, or cisplatin, when combined with electric pulses [160,161,162]. In phase II clinical trial (n = 52), the influence of electroporation on the efficacy of bleomycin in treatment of superficial metastasis of various cancers was tested. One month after the first application of electroporation, the reduction in tumor size was observed in 50 of 52 patients. After the second course of electroporation, 18 of 27 patients had partial or complete response. One hundred sixty-nine of 257 tumor nodules disappeared and in 89 size reduction was determined [163]. In contrary to sonoporation, electrochemotherapy is currently used in clinical practice as palliative treatment in case of melanoma [164], basal and squamous skin cancer [165], and skin metastasis from tumors of non-skin origin [166]. Many researchers are still working in the field of electrochemotherapy in order to investigate the possible application of the method on other types of cancer, such as bladder [167] or esophageal cancer [168].
Nanocarrier systems
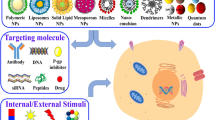
To increase drug delivery to tumor cells and to reduce toxicity against normal tissues, various targeted nanocarrier systems are being developed. They include micelles, liposomes, dendrimers, gold nanoparticles, mesoporous silica nanoparticles, superparamagnetic iron oxide nanoparticles, carbon nanotubes, and quantum dots [169]. Non-targeted nanoparticles accumulate in tumor tissues due to leaky and defective blood vessels, and reduced lymphatic drainage [170]. Targeted nanoparticles bear particular ligands that have high binding affinity to cancer cell surface molecules [171].
Nanoparticles carry an anticancer agent to the target site, where drug can be released due to various stimuli, such as pH changes, reduction reactions caused by glutathione sulfhydryl groups, enzymatic activity, magnetic or electric field, ultrasound [169]. Recently, mono-allyloxylated cucurbit[7]uril (AO1CB [7]) nanovesicles have been created [172]. It works as a nanocontainer for various drugs and proteins. When these vesicles are affected with UV light or near-infrared two-photon laser, the allyloxy tails of (AO1CB [7]) react with glutathione or other intracellular molecule containing thiol group, thus resulting in targeted drug delivery. Although this method showed to increase doxorubicin delivery into cervical cancer cells, its efficacy needs to be investigated in further in vitro and in vivo studies.
For stronger therapeutic efficacy, various transport modulators can be incorporated into these systems. Janoniene et al. combined porous silicon nanoformulations with transport modulator—selective carbonic anhydrase IX inhibitor [61]. Porous silicon nanoparticles loaded with doxorubicin and conjugated with carbonic anhydrase IX inhibitor enhanced target drug delivery towards tumor tissues and improved doxorubicin penetration into cancer cells. Carbonic anhydrase IX inhibitor also enhanced drug loading efficacy into nanoparticles and improved drug release profile by reducing the premature release of doxorubicin.
Dual delivery systems consisting of an anticancer drug and nucleic acids, that silence the expression of drug efflux transporters genes, are being developed, as well. Meng et al. showed that co-delivery of doxorubicin and siRNA, that knocks down the expression of P-gp, by mesoporous silica nanoparticles increased intracellular delivery and cytotoxicity of doxorubicin [173]. Silencing of P-gp gene leads to a reduction of drug efflux and a decrease in pump-mediated drug resistance.
At this point, there are 49 clinical trials on the field of cancer with a term “nano” listed on ClinicalTrials.gov database and most of them are still recruiting or ongoing. Two liposomal drugs—doxorubicin (Doxil®) and irinotecan (Onivyde®), one polymeric nanoparticle drug—leuprolide acetate (Eligard®), and one protein nanoparticle drug—albumin-bound paclitaxel (Abraxane®)—are currently approved for clinical use. Although, simple nanoparticles show higher efficacy and reduced toxicity, progressive trends towards more complex nanomedicine and dual delivery systems can be seen in the research field.
Conclusions
Despite various attempts to reverse MDR, it still remains one of the most important problems of chemotherapy. So far, neither pH modulators nor ABC transporter inhibitors or application of ultrasound is applied in clinical practice. However, novel V-ATPase or ABC-transporter inhibitors, especially third- and fourth-generation P-gp inhibitors (such as zosuiqudar, elacridar and resveratrol), show good efficacy in in vitro and in vivo models. CA and NHE-1 inhibitors or ultrasound in combination with microbubbles also demonstrate promising results in modulation of anticancer drug penetration. Currently, there are two ongoing clinical trials (NCT03458975 and NCT02233205) investigating the efficacy of sonoporation on the delivery of anticancer drugs, but the results are not published, yet. Instead of microbubbles, researchers are developing nanobubbles as they are smaller and can easier penetrate through blood vessels [174]. Electrochemotherapy is approved for the treatment of a few certain types of cancers, but researchers are still trying to adapt the method for the treatment of cancer in body cavities. Besides physical methods, various nanocarrier systems are gaining a great attention in an anticancer therapy, as well. Incorporation of transport modulators into these delivery systems is an unsaturated research niche area warrant for further investigation.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin [Internet]. 2018;68:7–30. https://doi.org/10.3322/caac.21442.
Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016;27:926–33.
Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. JNCI J Natl Cancer Inst [Internet]. 2007 [cited 2018 Mar 26];99:1441–54. Available from: https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/djm135
Roos A. Weak acids, weak bases, and intracellular pH. Respir Physiol [Internet]. 1978 [cited 2018 Aug 6];33:27–30. Available from: http://linkinghub.elsevier.com/retrieve/pii/0034568778900804
Chang G. Multidrug resistance ABC transporters. FEBS Lett [Internet]. No longer published by Elsevier; 2003 [cited 2018 May 22];555:102–5. Available from: https://www.sciencedirect.com/science/article/pii/S0014579303010858
Lu Q, Lu S, Huang L, Wang T, Wan Y, Zhou CX, et al. The expression of V-ATPase is associated with drug resistance and pathology of non-small-cell lung cancer. Diagn Pathol [Internet]. BioMed Central; 2013 [cited 2018 may 22];8:145. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23984887.
Filipits M, Pohl G, Rudas M, Dietze O, Lax S, Grill R, et al. Clinical Role of multidrug resistance protein 1 expression in chemotherapy resistance in early-stage breast cancer: the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol [Internet]. 2005 [cited 2018 Aug 7];23:1161–8. Available from: http://ascopubs.org/doi/10.1200/JCO.2005.03.033
Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JGM, Wiemer EAC, et al. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res [Internet]. 2003 [cited 2018 Aug 7];9:827–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12576456.
Kibria G, Hatakeyama H, Akiyama K, Hida K, Harashima H. Comparative study of the sensitivities of cancer cells to doxorubicin, and relationships between the effect of the drug-efflux pump P-gp. Biol Pharm Bull [Internet]. 2014 [cited 2018 Aug 8];37:1926–35. Available from: http://jlc.jst.go.jp/DN/JST.JSTAGE/bpb/b14-00529?lang=en&from=CrossRef&type=abstract
Ireland L, Santos A, Ahmed MS, Rainer C, Nielsen SR, Quaranta V, et al. Chemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factors. Cancer Res [Internet]. Europe PMC Funders; 2016 [cited 2018 Aug 12];76:6851–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27742686.
Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell [Internet]. NIH Public Access; 2014 [cited 2018 Aug 12];25:719–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24856586.
Dauer P, Nomura A, Saluja A, Banerjee S. Microenvironment in determining chemo-resistance in pancreatic cancer: neighborhood matters [Internet]. Pancreatology. 2017 [cited 2018 Aug 12]. p. 7–12. Available from: http://linkinghub.elsevier.com/retrieve/pii/S142439031631256X
Yokoi K, Fidler IJ. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res [Internet]. American Association for Cancer Research; 2004 [cited 2018 Aug 12];10:2299–306. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10815919.
Lee ES. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol [Internet]. 2014 [cited 2018 Aug 13];20:7864. Available from: http://www.liebertpub.com/doi/10.1089/ars.2007.1628
Hagmann W, Jesnowski R, Löhr JM. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia [Internet]. 2010 [cited 2018 Aug 14];12:740–7. Available from: www.neoplasia.com
Oguri T, Bessho Y, Achiwa H, Ozasa H, Maeno K, Maeda H, et al. MRP8/ABCC11 directly confers resistance to 5-fluorouracil. Mol Cancer Ther [Internet]. 2007 [cited 2018 Aug 14];6:122–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17237272.
Tsujie M, Nakamori S, Nakahira S, Takahashi Y, Hayashi N, Okami J, et al. Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res [Internet]. 2007 [cited 2018 Aug 14];27:2241–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17695509.
Alfarouk KO, Stock C-M, Taylor S, Walsh M, Muddathir AK, Verduzco D, et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int [Internet]. BioMed Central; 2015;15:71. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4502609&tool=pmcentrez&rendertype=abstract
Ojugo ASE, McSheehy PMJ, McIntyre DJO, McCoy C, Stubbs M, Leach MO, et al. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous19F and31P probes. NMR Biomed [Internet]. 1999 [cited 2018 May 22];12:495–504. Available from: http://doi.wiley.com/10.1002/%28SICI%291099-1492%28199912%2912%3A8%3C495%3A%3AAID-NBM594%3E3.0.CO%3B2-K
Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. J Nucl Med [Internet]. NIH Public Access; 2010 [cited 2018 May 22];51:1167–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20660380.
Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem [Internet]. American Society for Biochemistry and Molecular Biology; 1996 [cited 2018 Apr 12];271:32529–37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8955077.
Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem [Internet]. 2001 [cited 2018 Apr 12];276:9519–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11120745.
Warburg O. The metabolism of carcinoma cells. J Cancer Res [Internet]. American Association for Cancer Research Journals; 1925 [cited 2018 Apr 12];9:148–63. Available from: http://cancerres.aacrjournals.org/cgi/doi/10.1158/jcr.1925.148
Candice Sun Hong A, Graham NA, Gu W, Braas D, Graeber TG, Christofk Correspondence HR. MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4 MCT1 inhibition of cancer cells with MCT1/MCT4 reduces proliferation and tumor growth. Cell Rep [Internet]. 2016 [cited 2018 Apr 12];14:1590–601. Available from: https://doi.org/10.1016/j.celrep.2016.01.057
Xu K, Mao X, Mehta M, Cui J, Zhang C, Mao F, et al. Elucidation of how cancer cells avoid acidosis through comparative transcriptomic data analysis. PLoS One [Internet]. Public Library of Science; 2013 [cited 2018 Apr 12];8:e71177. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23967163.
Paula S, Volkov AG, Hoek AN Van, Haines TH, Deamer DW. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys J [Internet]. 1996 [cited 2018 Apr 18];70:339–48. Available from: http://www.cell.com/biophysj/pdf/S0006-3495(96)79575-9.pdf
Raghunand N, He X, Van Sluis R, Mahoney B, Baggett B, Taylor CW, et al. Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer. 1999;80:1005–11.
Gotink KJ, Broxterman HJ, Labots M, de Haas RR, Dekker H, Honeywell RJ, et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin Cancer Res [Internet]. 2011 [cited 2018 Apr 18];17:7337–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21980135.
Ishizaki J, Yokogawa K, Hirano M, Nakashima E, Sai Y, Ohkuma S, et al. Contribution of lysosomes to the subcellular distribution of basic drugs in the rat liver. Pharm Res [Internet]. 1996 [cited 2018 Apr 18];13:902–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8792430.
Robey IF, Nesbit LA. Investigating mechanisms of alkalinization for reducing primary breast tumor invasion. Biomed Res Int. 2013;2013:1–10.
Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, et al. Bicarbonate Increases Tumor pH and Inhibits Spontaneous Metastases. [cited 2018 Jul 26]; Available from: http://cancerres.aacrjournals.org/
Faes S, Dormond O. Systemic buffers in cancer therapy: the example of sodium bicarbonate; stupid idea or wise remedy? Med Chem (Los Angeles) [Internet]. 2015;5:540–4. Available from: https://www.omicsonline.org/open-access/systemic-buffers-in-cancer-therapy-the-example-of-sodium-bicarbonatestupid-idea-or-wise-remedy-2161-0444-1000314.php?aid=65744
McCarty MF, Whitaker J. Manipulating tumor acidification as a cancer treatment strategy. Altern Med Rev [Internet]. 2010 [cited 2018 Aug 6];15:264–72. Available from: http://www.altmedrev.com/archive/publications/15/3/264.pdf
Nishi T, Forgac M. The vacuolar (H+)-Atpases — nature’s most versatile proton pumps. Nat Rev Mol Cell Biol [Internet] 2002;3:94–103. Available from: http://www.nature.com/doifinder/10.1038/nrm729
Smith GA, Howell GJ, Phillips C, Muench SP, Ponnambalam S, Harrison MA. Extracellular and luminal pH regulation by vacuolar H+-ATPase isoform expression and targeting to the plasma membrane and endosomes. J Biol Chem [Internet]. 2016 [cited 2018 Apr 18];291:8500–15. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4861423/pdf/zbc8500.pdf
Xu J, Xie R, Liu X, Wen G, Jin H, Yu Z, et al. Expression and functional role of vacuolar H+-ATPase in human hepatocellular carcinoma. Carcinogenesis [Internet]. 2012 [cited 2018 Apr 18];33:2432–40. Available from: https://watermark.silverchair.com/bgs277.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAaowggGmBgkqhkiG9w0BBwagggGXMIIBkwIBADCCAYwGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMhvfRL_V9Vr_ENyv1AgEQgIIBXaWMdxvFCE4qPSeF7oIgyaozdItDbYRtYgQmPF_3kCe1u2PR
Otero-Rey EM, Somoza-Martín M, Barros-Angueira F, García-García A. Intracellular pH regulation in oral squamous cell carcinoma is mediated by increased V-ATPase activity via over-expression of the ATP6V1C1 gene. Oral Oncol. 2008;44:193–9.
Chung C, Mader CC, Schmitz JC, Atladottir J, Fitchev P, Cornwell ML, et al. The vacuolar-ATPase modulates matrix metalloproteinase isoforms in human pancreatic cancer. Lab Investig [Internet]. 2011 [cited 2018 Apr 18];91:732–43. Available from: https://www.nature.com/articles/labinvest20118.pdf
Lee Y-Y, Jeon H-K, Hong JE, Cho YJ, Ryu JY, Choi J-J, Lee SH, Yoon G, Kim WY, Do IG, Kim MK, Kim TJ, Choi CH, Lee JW, Bae DS, Kim BG Proton pump inhibitors enhance the effects of cytotoxic agents in chemoresistant epithelial ovarian carcinoma. Oncotarget [Internet] 2015;6:35040–50. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L606844230%5Cn http://dx.doi.org/10.18632/oncotarget.5319
Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin-a1, a specific inhibitor of vacuolar-type H+-Atpase, inhibits acidification and protein-degradation in lysosomes of cultured-cells. J Biol Chem. 1991;266:17707–12.
Lee CM, Tannock IF. Inhibition of endosomal sequestration of basic anticancer drugs: influence on cytotoxicity and tissue penetration. Br J Cancer [Internet] 2006;94:863–9. Available from: http://www.nature.com/doifinder/10.1038/sj.bjc.6603010
Zhitomirsky B, Assaraf YG. The role of cytoplasmic-to-lysosomal pH gradient in hydrophobic weak base drug sequestration in lysosomes. Cancer Cell Microenviron [Internet] 2015;2:3–9. Available from: http://www.smartscitech.com/index.php/CCM/article/view/807
McSheehy PMJ, Troy H, Kelland LR, Judson IR, Leach MO, Griffiths JR. Increased tumour extracellular pH induced by Bafilomycin A1 inhibits tumour growth and mitosis in vivo and alters 5-fluorouracil pharmacokinetics. Eur J Cancer [Internet]. 2003 [cited 2018 Apr 19];39:532–40. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0959804902006718
Kiyoshima T, Yoshida H, Wada H, Nagata K, Fujiwara H, Kihara M, et al. Chemoresistance to concanamycin A1 in human oral squamous cell carcinoma is attenuated by an HDAC inhibitor partly via suppression of Bcl-2 expression. Koul HK, editor. PLoS One [Internet]. Public Library of Science; 2013 [cited 2018 May 3];8:e80998. Available from: http://dx.plos.org/10.1371/journal.pone.0080998
Yu M, Lee C, Wang M, Tannock IF. Influence of the proton pump inhibitor lansoprazole on distribution and activity of doxorubicin in solid tumors. Cancer Sci. 2015;106:1438–47.
Patel KJ, Lee C, Tan Q, Tannock IF. Use of the proton pump inhibitor pantoprazole to modify the distribution and activity of doxorubicin: a potential strategy to improve the therapy of solid tumors. Clin Cancer Res. 2013;19:6766–76.
Kromer W, Krüger U, Huber R, Hartmann M, Steinijans VW. Differences in pH-dependent activation rates of substituted benzimidazoles and biological in vitro correlates. Pharmacology [Internet]. Karger Publishers; 1998 [cited 2018 Apr 18];56:57–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9494064.
Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702–13.
Lindner K, Borchardt C, Schöpp M, Bürgers A, Stock C, Hussey DJ, et al. Proton pump inhibitors (PPIs) impact on tumour cell survival, metastatic potential and chemotherapy resistance, and affect expression of resistance-relevant miRNAs in esophageal cancer. J Exp Clin Cancer Res. 2014;33:73.
Ikemura K, Oshima K, Enokiya T, Okamoto A, Oda H, Mizuno T, et al. Co-administration of proton pump inhibitors ameliorates nephrotoxicity in patients receiving chemotherapy with cisplatin and fluorouracil: a retrospective cohort study. Cancer Chemother Pharmacol. Springer Berlin Heidelberg; 2017;79:943–9.
Lugini L, Federici C, Borghi M, Azzarito T, Marino ML, Cesolini A, Spugnini EP, Fais S Proton pump inhibitors while belonging to the same family of generic drugs show different anti-tumor effect. J Enzyme Inhib Med Chem [internet]. Informa UK Ltd; 2016;31:538–45. Available from: https://doi.org/10.3109/14756366.2015.1046062
Patil R, Kulshrestha A, Tikoo A, Fleetwood S, Katara G, Kolli B, et al. Identification of novel bisbenzimidazole derivatives as anticancer vacuolar (H+)-ATPase inhibitors. Molecules [Internet] 2017;22:1–14. Available from: http://www.smartscitech.com/index.php/CCM/article/view/807
Stadie WC, O’Brien H. The catalysis of the hydration of carbon dioxide and dehydration of carbonic acid by an enzyme isolated from red blood cells. J Biol Chem [Internet]. 1933 [cited 2018 Apr 25];103:521–9. Available from: http://www.jbc.org/
Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res [Internet]. 2004;64:6160–5. https://doi.org/10.1158/0008-5472.CAN-03-2224.
Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–68.
Luong-Player A, Liu H, Wang HL, Lin F. Immunohistochemical reevaluation of carbonic anhydrase IX (CA IX) expression in tumors and normal tissues. Am J Clin Pathol [Internet]. 2014 [cited 2018 Apr 20];141:219–25. Available from: https://academic.oup.com/ajcp/article-lookup/doi/10.1309/AJCPVJDS28KNYZLD
Parkkila S, Parkkila A-K, Saarnio J, Kivelä J, Karttunen TJ, Kaunisto K, et al. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem [Internet]. 2000 [cited 2018 Apr 20];48:1601–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11101628.
Gieling RG, Parker CA, De Costa LA, Robertson N, Harris AL, Stratford IJ, et al. Inhibition of carbonic anhydrase activity modifies the toxicity of doxorubicin and melphalan in tumour cells in vitro. J Enzyme Inhib Med Chem [Internet]. 2013;28:360–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23163664.
McDonald PC, Sanghera J, Singh M, Lou Y, Vallejo M, Supuran CT, et al. Abstract 841: Therapeutic targeting of cancer cells in the hypoxic microenvironment using an orally bioavailable small molecule inhibitor of carbonic anhydrase IX. Cancer Res [Internet]. 2014 [cited 2018 Apr 27];74:841–841. Available from: http://www.ingentaconnect.com/content/10.3727/096504017X14965111926391
Riemann A, Güttler A, Haupt V, Wichmann H, Reime S, Bache M, et al. Inhibition of carbonic anhydrase IX by ureidosulfonamide inhibitor U104 reduces prostate cancer cell growth, but does not modulate daunorubicin or cisplatin cytotoxicity. Oncol Res Featur Preclin Clin Cancer Ther [Internet]. 2017 [cited 2018 may 28];26:191–200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28631600.
Janoniene A, Liu Z, Baranauskiene L, Mäkilä E, Ma M, Salonen J, et al. A versatile carbonic anhydrase IX targeting ligand-functionalized porous silicon nanoplatform for dual hypoxia cancer therapy and imaging. ACS Appl Mater Interfaces. 2017;9:13976–87.
Wallert MA, Hammes D, Nguyen T, Kiefer L, Berthelsen N, Kern A, et al. RhoA Kinase (Rock) and p90 Ribosomal S6 Kinase (p90Rsk) phosphorylation of the sodium hydrogen exchanger (NHE1) is required for lysophosphatidic acid-induced transport, cytoskeletal organization and migration. Cell Signal [Internet]. NIH Public Access; 2015 [cited 2018 May 3];27:498–509. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25578862.
Amith SR, Fliegel L. Regulation of the Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res [Internet]. American Association for Cancer Research; 2013 [cited 2018 may 3];73:1259–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23393197.
Xie R, Wang H, Jin H, Wen G, Tuo B, Xu J. NHE1 is upregulated in gastric cancer and regulates gastric cancer cell proliferation, migration and invasion. Oncol Rep [Internet]. 2017 [cited 2018 May 2];37:1451–60. Available from: https://www.spandidos-publications.com/10.3892/or.2017.5386
Yang X, Wang D, Dong W, Song Z, Dou K. Expression and modulation of Na+/H+ exchanger 1 gene in hepatocellular carcinoma: a potential therapeutic target. J Gastroenterol Hepatol [Internet]. 2011 [cited 2018 May 3];26:364–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21261728.
Cong D, Zhu W, Shi Y, Pointer KB, Clark PA, Shen H, et al. Upregulation of NHE1 protein expression enables glioblastoma cells to escape TMZ-mediated toxicity via increased H+ extrusion, cell migration and survival. Carcinogenesis [Internet]. Oxford University Press; 2014 [cited 2018 May 3];35:2014–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24717311.
Altaf E, Huang X, Xiong J, Yang X, Deng X, Xiong M, et al. NHE1 has a notable role in metastasis and drug resistance of T-cell acute lymphoblastic leukemia. Oncol Lett. 2017;14:4256–62.
Cragoe EJ, Woltersdorf OW, Ricking JB, Kwoxg SF, Jones JH. Pyrazine Diuretics. II. N-Amidino-3-Amino-5-Substituted 6-Halopyrazinecarboxamides. J Med Chem [Internet]. 1967 [cited 2018 May 3];10:66–75. Available from: http://pubs.acs.org/doi/abs/10.1021/jm00313a014
Pannocchia A, Revelli S, Tamponi G, Giorgianni A, Todde R, Bosia A, et al. Reversal of doxorubicin resistance by the amiloride analogue EIPA in multidrug resistant human colon carcinoma cells. Cell Biochem Funct. 1996;14:11–8.
Miraglia E, Viarisio D, Riganti C, Costamagna C, Ghigo D, Bosia A. Na+/H+ exchanger activity is increased in doxorubicin-resistant human colon cancer cells and its modulation modifies the sensitivity of the cells to doxorubicin. Int J Cancer. 2005;115:924–9.
Rupprecht HJ, Vom DJ, Terres W, Seyfarth KM, Richardt G, Schultheibeta HP, et al. Cardioprotective effects of the Na(+)/H(+) exchange inhibitor cariporide in patients with acute anterior myocardial infarction undergoing direct PTCA. Circulation [Internet]. American Heart Association, Inc.; 2000 [cited 2018 may 3];101:2902–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8205693.
Zeymer U, Suryapranata H, Monassier JP, Opolski G, Davies J, Rasmanis G, et al. The Na+/H+exchange inhibitor eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction: results of the evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction (ESCAMI) trial. J Am Coll Cardiol [Internet]. Elsevier; 2001 [cited 2018 May 3];38:E1644–50. Available from: https://www.sciencedirect.com/science/article/pii/S0735109701016084
Kathawala RJ, Gupta P, Ashby CR, Chen Z-S. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat [Internet]. Elsevier Ltd. 2015;18:1–17. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1368764614000788
Kosztyu P, Bukvova R, Dolezel P, Mlejnek P. Resistance to daunorubicin, imatinib, or nilotinib depends on expression levels of ABCB1 and ABCG2 in human leukemia cells. Chem Biol Interact [Internet]. Elsevier; 2014 [cited 2018 Apr 6];219:203–10. Available from: https://www.sciencedirect.com/science/article/pii/S0009279714001884?via%3Dihub
Chauvier D, Kegelaer G, Morjani H, Manfait M. Reversal of multidrug resistance-associated protein-mediated daunorubicin resistance by camptothecin. J Pharm Sci [Internet]. 2002 [cited 2018 Apr 6];91:1765–75. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022354916310619
Singhal SS, Singhal J, Nair MP, Lacko AG, Awasthi YC, Awasthi S. Doxorubicin transport by RALBP1 and ABCG2 in lung and breast cancer. Int J Oncol [Internet]. 2007 [cited 2018 Apr 6];30:717–25. Available from: http://www.spandidos-publications.com/10.3892/ijo.30.3.717
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science [Internet]. 1992 [cited 2018 Jun 8];258:1650–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1360704.
Shen F, Chu S, Bence AK, Bailey B, Xue X, Erickson PA, et al. Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. J Pharmacol Exp Ther. 2008;324:95–102.
Xia CQ, Yang JJ, Gan L-S. Breast cancer resistance protein in pharmacokinetics and drug–drug interactions. Expert Opin Drug Metab Toxicol [Internet]. 2005 [cited 2018 Apr 6];1:595–611. Available from: http://www.tandfonline.com/doi/full/10.1517/17425255.1.4.595
Iwakiri T, Okumura M, Hidaka M, Kumagai Y, Ichihara E, Kawano Y, et al. Inhibition of carrier-mediated uptake of epirubicin reduces cytotoxicity in primary culture of rat hepatocytes. J Appl Toxicol [Internet]. 2008 [cited 2018 Apr 6];28:329–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17604344.
Tagen M, Zhuang Y, Zhang F, Harstead KE, Shen J, Schaiquevich P, et al. P-glycoprotein, but not multidrug resistance protein 4, plays a role in the systemic clearance of irinotecan and SN-38 in mice. Drug Metab Lett [Internet]. 2010 [cited 2018 Apr 6];4:195–201. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20583968.
Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res [Internet]. American Association for Cancer Research; 2001 [cited 2018 Apr 6];61:3458–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10485464.
Kruijtzer CMF, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol [Internet]. 2002 [cited 2018 Apr 6];20:2943–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12089223.
Allen JD, Van Dort SC, Buitelaar M, van Tellingen O, Schinkel AH. Mouse breast cancer resistance protein (Bcrp1/Abcg2) mediates etoposide resistance and transport, but etoposide oral availability is limited primarily by P-glycoprotein. Cancer Res [Internet]. 2003 [cited 2018 Apr 6];63:1339–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12649196.
Benyahia B, Huguet S, Declèves X, Mokhtari K, Crinière E, Bernaudin JF, et al. Multidrug resistance-associated protein MRP1 expression in human gliomas: chemosensitization to vincristine and etoposide by indomethacin in human glioma cell lines overexpressing MRP1. J Neurooncol [Internet]. 2004 [cited 2018 Apr 6];66:65–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15015771.
Zeng H, Chen ZS, Belinsky MG, Rea PA, Kruh GD. Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: effect of polyglutamylation on MTX transport. Cancer Res [Internet]. 2001 [cited 2018 Apr 6];61:7225–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11585759.
de Graaf D, Sharma RC, Mechetner EB, Schimke RT, Roninson IB. P-glycoprotein confers methotrexate resistance in 3T6 cells with deficient carrier-mediated methotrexate uptake. Proc Natl Acad Sci U S A [Internet]. 1996 [cited 2018 Apr 6];93:1238–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8577747.
Kosztyu P, Dolezel P, Mlejnek P. Can P-glycoprotein mediate resistance to nilotinib in human leukaemia cells? Pharmacol Res [Internet]. 2013 [cited 2018 Apr 6];67:79–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23103446.
Czyzewski K, Styczynski J. Imatinib is a substrate for various multidrug resistance proteins. Neoplasma [Internet]. 2009 [cited 2018 Apr 6];56:202–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19309222.
Shukla S, Kouanda A, Silverton L, Talele TT, Ambudkar SV. Pharmacophore modeling of nilotinib as an inhibitor of ATP-binding cassette drug transporters and BCR-ABL kinase using a three-dimensional quantitative structure-activity relationship approach. Mol Pharm [Internet]. American Chemical Society; 2014 [cited 2018 Apr 6];11:2313–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24865254.
Jang SH, Wientjes MG, Au JL. Kinetics of P-glycoprotein-mediated efflux of paclitaxel. J Pharmacol Exp Ther [Internet]. 2001 [cited 2018 Apr 6];298:1236–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11504826.
Miettinen S, Grènman S, Ylikomi T. Inhibition of P-glycoprotein-mediated docetaxel efflux sensitizes ovarian cancer cells to concomitant docetaxel and SN-38 exposure. Anticancer Drugs [Internet]. 2009 [cited 2018 Apr 6];20:267–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19262372.
Tseng E, Kamath A, Morris ME. Effect of organic isothiocyanates on the P-glycoprotein- and MRP1-mediated transport of daunomycin and vinblastine. Pharm Res [Internet]. Kluwer Academic Publishers-Plenum Publishers; 2002 [cited 2018 Apr 6];19:1509–15. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1023/A:1020460700877
Ushigome F, Takanaga H, Matsuo H, Yanai S, Tsukimori K, Nakano H, et al. Human placental transport of vinblastine, vincristine, digoxin and progesterone: contribution of P-glycoprotein. Eur J Pharmacol [Internet]. 2000 [cited 2018 Apr 6];408:1–10. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0014299900007433
Huls M, Russel FGM, Masereeuw R. The role of ATP binding cassette transporters in tissue defense and organ regeneration. J Pharmacol Exp Ther [Internet]. American Society for Pharmacology and Experimental Therapeutics; 2009 [cited 2018 mar 16];328:3–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18791064.
Park S, Shimizu C, Shimoyama T, Takeda M, Ando M, Kohno T, et al. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat [Internet]. Springer US; 2006 [cited 2018 Mar 16];99:9–17. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s10549-006-9175-2
Liu Y, Peng H, Zhang J-T. Expression profiling of ABC transporters in a drug-resistant breast cancer cell line using AmpArray. Mol Pharmacol [Internet]. American Society for Pharmacology and Experimental Therapeutics; 2005 [cited 2018 mar 16];68:430–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15901850.
Bao L, Hazari S, Mehra S, Kaushal D, Moroz K, Dash S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am J Pathol [Internet]. Elsevier; 2012 [cited 2018 May 22];180:2490–503. Available from: https://www.sciencedirect.com/science/article/pii/S0002944012002477
Legrand O, Simonin G, Beauchamp-Nicoud A, Zittoun R, Marie JP. Simultaneous activity of MRP1 and Pgp is correlated with in vitro resistance to daunorubicin and with in vivo resistance in adult acute myeloid leukemia. Blood [Internet]. 1999 [cited 2018 May 22];94:1046–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10419897.
Burgio DE, Gosl MP, PJ MN. Effects of P-glycoprotein modulators on etoposide elimination and central nervous system distribution. J Pharmacol Exp Ther. 1998;287
Shalinsky DR, Jekunen AP, Alcaraz JE, Christen RD, Kim S, Khatibi S, et al. Regulation of initial vinblastine influx by P-glycoprotein. Br J Cancer [Internet]. 1993 [cited 2018 may 22];67:30–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8094005.
Yusa K, Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989;49:5002–6.
Qadir M, O’Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–6.
Wishart GC, Bissett D, Paul J, Jodrell D, Harnett A, Habeshaw T, et al. Quinidine as a resistance modulator of epirubicin in advanced breast cancer: mature results of a placebo-controlled randomized trial. J Clin Oncol [Internet]. 1994 [cited 2018 mar 13];12:1771–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8083699.
Desai AA, Kindler HL, Taber D, Agamah E, Mani S, Wade-Oliver K, et al. Modulation of irinotecan with cyclosporine: a phase II trial in advanced colorectal cancer. Cancer Chemother Pharmacol. 2005;56:421–6.
Ozols RF, Cunnion RE, Klecker RW, Hamilton TC, Ostchega Y, Parrillo JE, et al. Verapamil and adriamycin in the treatment of drug-resistant ovarian cancer patients. J Clin Oncol [Internet] 1987;5:641–7. Available from: http://ascopubs.org/doi/10.1200/JCO.1987.5.4.641
Echizen H, Brecht T, Niedergesäss S, Vogelgesang B, Eichelbaum M. The effect of dextro-, levo-, and racemic verapamil on atrioventricular conduction in humans. Am Heart J. 1985;109:210–7.
Raderer M, Maca T, Kastner J, Kornek G, Weinlaender G, Hejna M, et al. The effect of dextro-, levo-, and racemic verapamil on atrioventricular conduction in humans. Oncol Res Treat. 1995;18:462–7.
Plumb JA, Milroy R, Kaye SB. The activity of verapamil as a resistance modifier in vitro in drug resistant human tumour cell lines is not stereospecific. Biochem Pharmacol. 1990;39:787–92.
Advani R, Fisher GA, Lum BL, Hausdorff J, Halsey J, Litchman M, et al. A phase I trial of doxorubicin, paclitaxel, and valspodar (PSC 833), a modulator of multidrug resistance. Clin Cancer Res. 2001;7:1221–9.
Bates SE, Bakke S, Kang M, Robey RW, Zhai S, Thambi P, et al. A phase I/II study of infusional vinblastine with the P-glycoprotein antagonist valspodar (PSC 833) in renal cell carcinoma. Clin Cancer Res. 2004;10:4724–33.
Wandel C, Richard BK, Kajiji S, Guengerich FP, Wilkinson GR, Wood AJJ. P-glycoprotein and cytochrome P-450 3A inhibition: dissociation of inhibitory potencies. Cancer Res. 1999;59:3944–8.
Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics: I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66:1207–18.
Martin C, Berridge G, Mistry P, Higgins C, Charlton P, Callaghan R. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol. 1999;128:403–11.
Fox E, Widemann BC, Pastakia D, Chen CC, Yang SX, Cole D, et al. Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors. Cancer Chemother Pharmacol. Springer Berlin Heidelberg; 2015;76:1273–83.
Peck RA, Hewett J, Harding MW, Wang YM, Chaturvedi PR, Bhatnagar A, et al. Phase I and pharmacokinetic study of the novel MDR1 and MRP1 inhibitor biricodar administered alone and in combination with doxorubicin. J Clin Oncol [Internet]. 2001 [cited 2018 Feb 8];19:3130–41. Available from: http://ascopubs.org/doi/10.1200/JCO.2001.19.12.3130
Gandhi L, Harding MW, Neubauer M, Langer CJ, Moore M, Ross HJ, et al. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer [Internet]. Wiley Subscription Services, Inc., A Wiley Company; 2007 [cited 2018 Feb 8];109:924–32. Available from: http://doi.wiley.com/10.1002/cncr.22492
Soldner A, Christians U, Susanto M, Wacher VJ, Silverman JA, Benet LZ. Grapefruit juice activates P-glycoprotein-mediated drug transport. Pharm Res [Internet]. Kluwer Academic Publishers-Plenum Publishers; 1999 [cited 2018 Feb 9];16:478–85. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1023/A:1011902625609
Zhang RUI, Lu M, Zhang Z, Tian X, Wang S, Lv D. Resveratrol reverses P-glycoprotein-mediated multidrug resistance of U2OS / ADR cells by suppressing the activation of the NF- κ B and p38 MAPK signaling pathways. 2016;4147–54. Available from: p-gp
Ma W, Feng S, Yao X, Yuan Z, Liu L, Xie Y. Nobiletin enhances the efficacy of chemotherapeutic agents in ABCB1 overexpression cancer cells. Sci Rep [Internet]. 2016 [cited 2018 Mar 13];5:18789. Available from: https://www.nature.com/articles/srep18789.pdf?origin=ppub
Zhang SZ, Yang XN, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol [Internet]. 2004 [cited 2018 Mar 16];65:1208–16. Available from: http://molpharm.aspetjournals.org
Pennock GD, Dalton WS, Roeske WR, Appleton CP, Mosley K, Plezia P, et al. Systemic toxic effects associated with high-dose verapamil infusion and chemotherapy administration. J Natl Cancer Inst [Internet]. 1991 [cited 2018 Apr 9];83:105–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1988684.
Bartlett NL, Lum BL, Fisher GA, Brophy NA, Ehsan MN, Halsey J, et al. Phase I trial of doxorubicin with cyclosporine as a modulator of multidrug resistance. J Clin Oncol. 1994;12:835–42.
Hamilton M, Dahut W, Brawley O, Davis P, Wells-Jones T, Kohler D, et al. A Phase I/II study of high-dose tamoxifen in combination with vinblastine in patients with androgen- independent prostate cancer. Acta Oncol (Madr) [Internet]. 2003 [cited 2018 Apr 9];42:195–201. Available from: https://www.tandfonline.com/doi/pdf/10.1080/02841860310010718
Wilson WH, Jamis-Dow C, Bryant G, Balis FM, Klecker RW, Bates SE, et al. Phase I and pharmacokinetic study of the multidrug resistance modulator dexverapamil with EPOCH chemotherapy. J Clin Oncol [Internet]. 1995 [cited 2018 Apr 9];13:1985–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7636539.
Tidefelt U, Liliemark J, Gruber A, Liliemark E, Sundman-Engberg B, Juliusson G, et al. P-glycoprotein inhibitor valspodar (PSC 833) increases the intracellular concentrations of daunorubicin in vivo in patients with p-glycoprotein–positive acute myeloid leukemia. J Clin Oncol [Internet]. 2000 [cited 2018 Apr 9];18:1837–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10784624.
Cripe LD, Uno H, Paietta EM, Litzow MR, Ketterling RP, Bennett JM, et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood [Internet]. American Society of Hematology; 2010 [cited 2018 Apr 8];116:4077–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20716770.
Chen H, Shien K, Suzawa K, Tsukuda K, Tomida S, Sato H, et al. Elacridar, a third-generation ABCB1 inhibitor, overcomes resistance to docetaxel in non-small cell lung cancer. Oncol Lett [Internet]. Spandidos Publications; 2017 [cited 2018 Apr 8];14:4349–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28959367.
Seiden M V., Swenerton KD, Matulonis U, Campos S, Rose P, Batist G, et al. A phase II study of the MDR inhibitor biricodar (INCEL, VX-710) and paclitaxel in women with advanced ovarian cancer refractory to paclitaxel therapy. Gynecol Oncol [Internet]. Academic Press; 2002 [cited 2018 Apr 8];86:302–10. Available from: https://www.sciencedirect.com/science/article/pii/S0090825802967624
Chi KN, Chia SK, Dixon R, Newman MJ, Wacher VJ, Sikic B, et al. A phase I pharmacokinetic study of the P-glycoprotein inhibitor, ONT-093, in combination with paclitaxel in patients with advanced cancer. Invest New Drugs [Internet]. 2005 [cited 2018 Apr 8];23:311–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16012790.
Pusztai L, Wagner P, Ibrahim N, Rivera E, Theriault R, Booser D, et al. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer. 2005;104:682–91.
Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar S V, Pike VW, et al. The inhibitor Ko143 is not specific for ABCG2. J Pharmacol Exp Ther J Pharmacol Exp Ther [Internet]. 2015 [cited 2018 Mar 24];354:384–93. Available from: https://doi.org/10.1124/jpet.115.225482
Liu T, Liu X, Li W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget [Internet]. 2016 [cited 2018 Apr 8];7:40800–15. Available from: www.impactjournals.com/oncotarget
Ravikumar Reddy D, Khurana A, Bale S, Ravirala R, Samba Siva Reddy V, Mohankumar M, et al. Natural flavonoids silymarin and quercetin improve the brain distribution of co-administered P-gp substrate drugs. Springerplus [Internet]. Nature Publishing Group; 2016 [cited 2018 Apr 8];5:1618. Available from: http://springerplus.springeropen.com/articles/10.1186/s40064-016-3267-1
Quiney C, Billard C, Faussat A-M, Salanoubat C, Kolb J-P. Hyperforin inhibits P-gp and BCRP activities in chronic lymphocytic leukaemia cells and myeloid cells. Leuk Lymphoma [Internet]. 2007 [cited 2018 Apr 8];48:1587–99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17701591.
Szafraniec MJ, Szczygieł M, Urbanska K, Fiedor L. Determinants of the activity and substrate recognition of breast cancer resistance protein (ABCG2). Drug Metab Rev [Internet]. 2014 [cited 2018 Mar 21];46:459–74. Available from: http://www.tandfonline.com/doi/full/10.3109/03602532.2014.942037
Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res [Internet]. American Association for Cancer Research; 2000 [cited 2018 Mar 22];60:47–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10070958.
Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, et al. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res [Internet]. 1998;58:5850–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9865745.
Allen JD, van Loevezijn A, Lakhai JM, van Der V, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. MolCancer Ther. 2002;1:417–25.
Tivnan A, Zakaria Z, O’Leary C, Kögel D, Pokorny JL, Sarkaria JN, et al. Inhibition of multidrug resistance protein 1 (MRP1) improves chemotherapy drug response in primary and recurrent glioblastoma multiforme. Front Neurosci [Internet]. Frontiers; 2015 [cited 2018 Mar 30];9:218. Available from: http://journal.frontiersin.org/Article/10.3389/fnins.2015.00218/abstract
Kuss BJ, Corbo M, Lau WM, Fennell DA, Dean NM, Cotter FE. In vitro and in vivo downregulation of MRP1 by antisense oligonucleotides: A potential role in neuroblastoma therapy. Int J Cancer [Internet]. Wiley-Blackwell; 2002 [cited 2018 Mar 30];98:128–33. Available from: http://doi.wiley.com/10.1002/ijc.10159
Li X, Wang H, Wang J, Chen Y, Yin X, Shi G, et al. Emodin enhances cisplatin-induced cytotoxicity in human bladder cancer cells through ROS elevation and MRP1 downregulation. BMC Cancer [Internet]. BioMed Central; 2016 [cited 2018 Mar 30];16:578. Available from: http://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2640-3
Burkhart CA, Watt F, Murray J, Pajic M, Prokvolit A, Xue C, et al. Small-molecule multidrug resistance–associated protein 1 inhibitor reversan increases the therapeutic index of chemotherapy in mouse models of neuroblastoma. Cancer Res [Internet]. 2009 [cited 2018 Apr 6];69:6573–80. Available from: http://cancerres.aacrjournals.org/content/canres/early/2008/12/31/0008-5472.CAN-09-1075.full.pdf
Shung KK. Diagnostic ultrasound: past, present, and future. J Med Biol Eng [Internet]. 2010 [cited 2018 May 22];31:371–4. Available from: http://www.jmbe.org.tw/files/917/public/917-2752-1-PB.pdf
Escoffre JM, Piron J, Novell A, Bouakaz A. Doxorubicin delivery into tumor cells with ultrasound and microbubbles. Mol Pharm. 2011;8:799–806.
Piron J, Kaddur K, Bouakaz A. Enhancement of doxorubicin effect on cancer cell mortality with ultrasound and microbubbles. 2009 IEEE Int Ultrason Symp [Internet]. IEEE; 2009 [cited 2018 Feb 23]. p. 1803–4. Available from: http://ieeexplore.ieee.org/document/5441897/
Grainger SJ, Serna JV, Sunny S, Zhou Y, Deng CX, El-Sayed MEH. Pulsed ultrasound enhances nanoparticle penetration into breast cancer spheroids. Mol Pharm [Internet]. 2010;7:2006–19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20957996.
Marmottant P, Bouakaz A, Jong N de, Quilliet C. Buckling resistance of solid shell bubbles under ultrasound. J Acoust Soc Am [Internet]. 2011 [cited 2018 Feb 23];129:1231–9. Available from: http://asa.scitation.org/doi/10.1121/1.3543943
van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, et al. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006;112:149–55.
Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet [Internet]. 1999 [cited 2018 Feb 23];353:1409. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0140673699012441
Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Plasma membrane poration induced by ultrasound exposure: implication for drug delivery. J Control Release [Internet]. 2005 [cited 2018 Feb 23];104:213–22. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0168365905000313
Park J, Fan Z, Deng CX. Effects of shear stress cultivation on cell membrane disruption and intracellular calcium concentration in sonoporation of endothelial cells. J Biomech [Internet]. 2011 [cited 2018 Feb 23];44:164–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3003778/pdf/nihms-237233.pdf
Wang ZB, Wu F, Wang ZL, Zhang Z, Zou JZ, Liu C, et al. Targeted damage effects of high intensity focused ultrasound (HIFU) on liver tissues of Guizhou province miniswine. Ultrason Sonochem [Internet]. Elsevier; 1997 [cited 2018 May 22];4:181–2. Available from: https://www.sciencedirect.com/science/article/pii/S135041779700028X
Dimcevski G, Kotopoulis S, Bjånes T, Hoem D, Schjøt J, Gjertsen BT, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release [Internet]. The Authors; 2016;243:172–81. Available from: https://doi.org/10.1016/j.jconrel.2016.10.007
Sadiq AA, Zaltum MAM, Mamman HB, Adon MN, Othman NB, Dalimin MN, et al. An overview: investigation of electroporation and sonoporation techniques. Proc - 2015 2nd Int Conf Biomed Eng ICoBE 2015 [Internet]. IEEE; 2015 [cited 2018 Jul 27]. p. 1–6. Available from: http://ieeexplore.ieee.org/document/7235876/
Saulis G, Saule R. Size of the pores created by an electric pulse: microsecond vs millisecond pulses. Biochim Biophys Acta - Biomembr [Internet]. 2012. p. 3032–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0005273612002167
Shankayi Z, Firoozabadi SM. Antitumor efficiency of electrochemotherapy by high and low frequencies and repetitive therapy in the treatment of invasive ductal carcinoma in balb/c mice. Cell J [Internet]. Royan Institute; 2012 [cited 2018 Aug 6];14:110–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23508227.
Ueki TI, Uemura H, Nagashima Y, Ohta S, Ishiguro H, Kubota Y. Antitumour effect of electrochemotherapy with bleomycin on human prostate cancer xenograft. BJU Int [Internet]. 2008 [cited 2018 Aug 6];102:1467–71. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1464-410X.2008.07793.x
Kuriyama S, Matsumoto M, Mitoro A, Tsujinoue H, Nakatani T, Fukui H, et al. Electrochemotherapy for colorectal cancer with commonly used chemotherapeutic agents in a mouse model. Dig Dis Sci [Internet]. Kluwer Academic Publishers-Plenum Publishers; 2000 [cited 2018 Aug 6];45:1568–77. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1023/A:1005565027969
Ogihara M, Yamaguchi O. Potentiation of effects of anticancer agents by local electric pulses in murine bladder cancer. Urol Res [Internet]. Springer-Verlag; 2000 [cited 2018 Aug 6];28:391–7. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s002400000140
Sundararajan R, Raman V, Masterson V, Madhivanan S, Raakesh M, Camarillo IG. Gemcitabine + cisplatin combination electrochemotherapy for triple negative breast cancers: an in vitro Model Study. Springer, Singapore; 2016 [cited 2018 Aug 6]. p. 280–4. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/978-981-287-817-5_62
Girelli R, Prejanò S, Cataldo I, Corbo V, Martini L, Scarpa A, et al. Feasibility and safety of electrochemotherapy (ECT) in the pancreas: a pre-clinical investigation. Radiol Oncol [Internet]. De Gruyter Open; 2015 [cited 2018 Aug 6];49:147–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26029026.
Campana LG, Mocellin S, Basso M, Puccetti O, De Salvo GL, Chiarion-Sileni V, et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution’s experience with 52 patients. Ann Surg Oncol [Internet]. 2009 [cited 2018 Aug 6];16:191–9. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1245/s10434-008-0204-8
NICE. Interventional procedure overview of electrochemotherapy for metastases of the skin from tunours of non-skin origin (IP984) [Internet]. 2012. Available from: https://www.nice.org.uk/guidance/gid-ip1041/documents/electrochemotherapy-for-metastases-in-the-skin-of-nonskin-origin-and-melanoma-overview2
NICE. Electrochemotherapy for primary basal cell carcinoma and primary squamous cell carcinoma | Guidance and guidelines | NICE. 2014 [cited 2018 Aug 6]; Available from: https://www.nice.org.uk/terms-and-.
NICE. Electrochemotherapy for metastases in the skin from tumours of non-skin origin and melanoma. Interv Proced Guid [Internet]. 2013 [cited 2018 Aug 6];1–7. Available from: https://www.nice.org.uk/terms-and-.
Vásquez JL, Ibsen P, Lindberg H, Gehl J. In vitro and in vivo experiments on electrochemotherapy for bladder cancer. J Urol [Internet]. 2015 [cited 2018 Aug 6];193:1009–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25245485.
Egeland C, Baeksgaard L, Johannesen H, Löfgren J, Plaschke C, Svendsen L, Gehl J, Achiam M Endoscopic electrochemotherapy for esophageal cancer: a phase I clinical study. Endosc Int Open [Internet] 2018;6:E727–34. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/a-0590-4053
Hossen S, Hossain MK, Rahman MT, Uddin MJ. Smart nanocarrier based drug delivery systems for cancer therapy and toxicity study: a review. J Adv Res [Internet]. 2018 [cited 2018 Aug 6]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S2090123218300791
Wakaskar RR. Passive and active targeting in tumor microenvironment. Int J Drug Dev Res [Internet]. International Journal of Drug Development & Research; 2017 [cited 2018 Aug 15];9:37–41. Available from: http://www.ijddr.in/drug-development/passive-and-active-targeting-in-tumor-microenvironment.php?aid=19478#20
Wang M, Thanou M. Targeting nanoparticles to cancer [Internet]. Pharmacol. Res. 2010 [cited 2018 Aug 15]. p. 90–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1043661810000794
Park KM, Baek K, Ko YH, Shrinidhi A, Murray J, Jang WH, et al. Mono-allyloxylated Cucurbit[7]uril acts as an unconventional amphiphile to form light-responsive vesicles. Angew Chemie - Int Ed [Internet]. 2018 [cited 2018 Aug 6];57:3132–6. Available from: http://doi.wiley.com/10.1002/ange.201713059
Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, et al. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and Pgp siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539–50.
Abdalkader R, Kawakami S, Unga J, Higuchi Y, Suzuki R, Maruyama K, et al. The development of mechanically formed stable nanobubbles intended for sonoporation-mediated gene transfection. Drug Deliv [Internet]. 2017 [cited 2018 Aug 16];24:320–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28165819.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Paškevičiūtė, M., Petrikaitė, V. Overcoming transporter-mediated multidrug resistance in cancer: failures and achievements of the last decades. Drug Deliv. and Transl. Res. 9, 379–393 (2019). https://doi.org/10.1007/s13346-018-0584-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0584-7