Abstract
The development of resistance to a variety of chemotherapeutic agents, also known as multidrug resistance (MDR), is a main impediment to the success of cancer chemotherapy, which refers to many factors such as increased efflux, blocked apoptosis, decreased drug influx, and altered cell cycle regulation. Considerable efforts have been devoted to develop chemosensitizers to conquer drug resistance, while their safety and unwanted pharmacokinetic drug interaction hindered their clinical applicability. Nano-sized drug carriers have great superiority in overcoming drug resistance due to the improved therapeutic index of drugs, enhanced drug targeting in tumor sites, and success in escaping from recognition of ABC transporter-mediated drug efflux. This chapter summarizes the most recent developments in the field of nanotherapeutics toward overcoming drug resistance by drug-targeted delivery, increased intracellular availability, changed subcellular localization, and combination of drug delivery with the agents that regulate intracellular pH, energy delivery, and apoptotic threshold.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Polymeric Micelle
- Triphenyl Phosphonium
- Overcome Drug Resistance
- Major Vault Protein
- Lung Resistance Protein
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Cancer is one of the major causes of death worldwide. Multidrug resistance (MDR), which is classically defined as a state of resilience against structurally and/or functionally unrelated drugs, is the main obstacle in cancer therapy. Generally, MDR can be divided into two types: intrinsic MDR and acquired MDR. Intrinsic MDR can be favored by the selection pressures in the tumor microenvironment, whereas acquired MDR can be induced by the traditional chemotherapy in common dose. Many factors can contribute to MDR, such as increased efflux, decreased drug influx, mutated cell cycle regulation, and blocked apoptosis.
The intracellular concentration of drugs and therapeutic efficiency can be increased using nanovehicles, which can escape from the recognition of efflux pumps and thus be endocytosed by tumor cells. Moreover, drug resistance gene and protein inhibitors can also be loaded in vehicles to modulate cell apoptosis and intensify drug efficacy.
Tumor Microenvironment and Cancer-Initiating (Stem) Cells
The microenvironment of a tumor cell contributes to the development of MDR and determines cell response to chemotherapy. The microenvironmental selection pressures that contribute to the development of MDR also always make the cells hypersensitive to growth stimulation.
Meanwhile, complex phenotype transformations can occur in cancer cells under hypoxic conditions, which are necessary for cell survival under such conditions. This kind of survival is a cascade initiated by the translocation of the alpha subunit of hypoxia-inducible factor (HIF) from the cytoplasm to the nucleus. The translocation is followed by abnormal tumor vasculature, hypoxia, decreased pH, increased interstitial fluid pressure, and alterations in the expression of tumor suppressors and oncogenes. Among them, abnormal (i.e., leaky and unorganized) tumor vasculature and the occurrence of hypoxic regions (transient and/or chronic), which are both common to all solid tumors, have been confirmed to play the most critical roles. Other hallmarks of the tumor microenvironment include the upregulation of oncogenes and DNA repair mechanisms as well as the downregulation of tumor suppressors and cell cycle regulation. Besides, the introduction of growth factor receptors and nutrient importers result in the complexation with the beta subunit of HIF to form an active transcription factor. This HIF complex binds to hypoxia-responsive elements on target genes that are always relevant to invasion, proliferation, metabolism, and drug resistance (Fig. 15.1).
Schematic description of the selection pressures in the tumor microenvironment that leads to the development of multidrug resistance. Selection pressures such as hypoxia (A), genetic mutations in regulatory genes, and altered regulation of apoptotic factors (H) can lead to cellular adaptation and aggressive MDR characteristics. Such characteristics include increased expression of growth factor receptors (E), increased expression of drug efflux pumps (F), reversion to anaerobic metabolism (G), decreased pH (D), and increased interstitial fluid pressure (D). The abnormal vasculature in the microenvironment of tumors (B) and (C) contributes to hypoxia (selection pressure) as well as to invasion and metastasis (from [1])
Tumor-initiating cells, commonly called cancer stem cells (CSCs), represent a small proportion of cancer cells possessing the common properties of normal stem cells (SCs). CSCs can initiate new tumors after injection into animal models, which is different in other cancer cells. The small proportion of cancer cells have the function of drug resistance modulating the metastasis of cancer cells, thus resulting in the relapse of cancers by acting as an obstacle in cancer therapy. Tumor drug resistance is reported to be closely associated with CSCs because of their intrinsic or acquired properties, including the following: quiescence, specific morphology, ability to repair DNA, ability to enhance the expression of antiapoptotic proteins and drug efflux transporters, as well as ability to detoxify enzymes. Currently available radio- and chemotherapies can kill the majority of cancer cells but are usually unable to eliminate the initiating CSCs that are protected by specific resistance mechanisms. Surviving CSCs promote the growth of new tumors and metastases, resulting in cancer relapse. The recurrent tumors become even more malignant and spread more quickly. Moreover, they become resistant to previously used radiotherapy and drugs, making them more difficult to treat and leading to increased patient suffering. Different signaling pathways and genes are involved in the maintenance of CSCs in the tumor microenvironment, which refers to a range of signaling pathways and genes. Based on the relevant phenotypes, CSCs can be characterized as a small subpopulation of cancer cells, for example, CD34+/CD38− in leukemia cells, CD44+/CD24− in solid tumors, and CD133+ in other tumors. Therefore, therapy strategies immediately applied after general cancer therapy is the most promising treatment option to achieve the goal of targeting CSCs [2].
Mechanisms of Drug Resistance in Tumors and Modulation of Drug Resistance
The characteristics of MDR include abnormal vasculature, regions of hypoxia, upregulation of ATP-binding cassette (ABC) transporters, aerobic glycolysis, and an elevated apoptotic threshold. The major course of antitumor drug resistance involves five stages: (1) decreased drug influx, (2) increased drug efflux predominantly through ATP-driven extrusion pumps frequently of the ABC superfamily, (3) activation of DNA repair, (4) metabolic modification, and (5) detoxification and inactivation of apoptosis pathways with parallel activation of antiapoptotic cellular defense modalities. Members of the ABC superfamily, such as P-glycoprotein (Pgp/ABCB1), multidrug resistance proteins (MRPs/ABCC), and breast cancer resistance protein (BCRP/ABCG2), can act as ATP-driven drug efflux transporters by forming a unique barrier against chemotherapeutics as well as numerous endotoxins and exotoxins (Fig. 15.2).
(a) MDR characteristics and treatment strategy. HIF-1α is located in the cytoplasm and associated with a complex of regulatory proteins under normoxic conditions. Under hypoxic conditions and cell stress, HIF-1α translocates into the nucleus, complexes with HIF-1β, and then binds to hypoxia-responsive elements on target genes that increase transcription and subsequent translation (e.g., EGFR). Current treatment strategies utilize a nanocarrier modified with EGFR-specific peptides to capitalize the overexpression. This receptor targeting allows facilitated uptake of the formulation, followed by the release of active agents. Receptor targeting of this nanocarrier system to the EGFR receptor should decrease residual toxicity associated with traditional chemotherapy, whereas the combination of paclitaxel with lonidamine offers a unique strategy for terminating the energy supply of MDR cancer and induce apoptosis. (b) Hexokinase 2 (HXK2) and lonidamine. This figure depicts the association of HXK2 with the components of the mitochondrial permeability transition pore complex (mtPTP) and coupling of the components to mitochondrial ATP synthase. ATP exits the mitochondria bypassing ATP synthase to the adenine nucleotide translocator (also located in the inner mitochondrial membrane), to the voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane. Association of HXK2 with VDAC prevents binding of pro-apoptotic Bcl-2 family member proteins to the mtPTP (from [3])
Although these mechanisms are independent of each other and can work separately, their functions are constantly interconnected and synergistic. Cancer cells that are adapted to the reduction/loss/alteration of specific drug target, enhanced drug metabolism, and enhanced cellular repair are often resistant to a group of drugs that are similar in either structure or function. For cancer cells that are adapted to reduced drug uptake, enhanced drug efflux and drug compartmentalization alter drug accumulation within cancer cells, leading to resistance to a variety of drugs that are structurally and functionally independent [4].
Drug Delivery in Overcoming the Drug Resistance of Tumors
Various nanovehicles have been specifically designed to overcome the drug resistance of cancer cells. The drug cargo is usually released from the nanovehicle either extracellularly in the tumor or in the tumor microenvironment, i.e., the stroma and vasculature supporting the cancer cells, or intracellularly, typically through cellular uptake by receptor-mediated endocytosis [5].
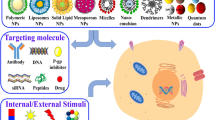
Nanoparticulate systems such as liposomes, polymeric micelles, and polymer–drug conjugates have led to about two dozen clinically approved therapeutic products [6]. Other nanoparticles (NPs) that reportedly deliver therapeutic cargoes in combination include oil nanoemulsions [7], mesoporous silica NPs (MSNPs) [8], and iron oxide NPs [9, 10] (Fig. 15.3 and Table 15.1).
As multicomponent multifunctional systems, nanoparticles can be functionalized with hydrophilic polymers (e.g., PEG), targeting molecules (e.g., antibodies), drugs, and imaging contrast agents. The interior core can be solid (e.g., quantum dots), liquid (e.g., liposomes), or contain an encapsulated drug (from [11])
Using nanocarriers for the treatment of MDR is highly advantageous because they can bypass efflux by ABC transporters. Nanocarriers are internalized by non-specific endocytosis (or facilitated uptake for targeted nanocarriers), which results in higher intracellular accumulation [46]. Nanotechnology-based cancer therapy accomplishes two or more objectives in one therapeutic strategy and can dramatically improve the therapeutic index of an agent. This strategy can enable the reduction of toxicity by increasing the bioavailability, and it also converts an agent with a low therapeutic potential into a drug candidate.
Multifunctional NPs are often engineered to achieve two or more of the following objectives: drug delivery, RNAi/DNA delivery, active targeting, decreased clearance, imaging/tracking, and stimuli-responsive capabilities. To date, NPs combining a cytotoxic drug and an agent for neutralization of a well-defined mechanism of drug resistance have been tested in vivo, but none has reached clinical trials yet.
Nanocarriers for Tumor-Targeted Delivery
Several specific approaches are currently being explored as strategies for future cancer therapy using nanomedicines for the delivery of chemotherapeutic drugs, chemosensitizers targeting drug resistance proteins, or diagnostic aids. Accumulation of nanocarriers at the tumor site is actually enhanced relative to the normal tissue because of the enhanced permeability and retention (EPR) effect [47]. The EPR effect results in higher accumulation of nanocarriers at the tumor site as compared with the control because of the leaky vasculature that allows passage of nanocarriers into the tumor matrix. Meanwhile, receptor targeting is also being extensively explored in experimental and clinical researches. Receptor targeting is aimed at selectively increasing the accumulation of a nanocarrier system at the tumor site by engaging a biological target that is overexpressed in cancer cells. The surface of the nanocarriers is modified with a ligand or antibody for receptor targeting, antigen targeting, or carbohydrate targeting.
The approaches to targeted nanocarrier for overcoming drug resistance are: (1) targeting the proliferating bulk of tumor cells and their intracellular compartments, (2) addressing the crosstalk between tumor cells and their microenvironment in an attempt to minimize the contribution of the stroma and vasculature to tumor cell survival and proliferation as well as to minimize drug resistance, and (3) targeting CSCs or tumor-initiating cells (TICs) [48].
Targeted delivery to the bulk of tumor cells has been extensively studied, and folate, EGFR-2 (or HER2), and transferrin are some of the most commonly used ligands. Similarly, the attachment of anti-HER2 onto NP surfaces also improves the cellular internalization of gelatin/albumin and gold NPs. Transferrin, an iron-binding glycoprotein, is a well-studied ligand for tumor targeting because of the upregulation of its receptors in numerous types of cancer. Meanwhile, various approaches aimed at targeting the microenvironment of tumor cells or the cross talk between tumor cells and their supporting stroma and/or vasculature are being developed.
Hypoxic conditions in many tumors can be potentially selected for the development of nanocarriers with redox-specific labile bonds, which can selectively target the microenvironment as well as increase drug accumulation and efficacy. Moreover, the depletion of oxygen levels in tissue (i.e., hypoxia) has long been considered as a major feature of the tumor microenvironment, which is a potential contributor to the enhanced tumorigenicity of CSCs. Targeting hypoxic factors with small interfering RNA (siRNA) or topoisomerase inhibitors are reportedly effective in overcoming drug resistance in preclinical studies. Therefore, the development of effective, systemic, and therapeutic approaches specifically focused on the tumor microenvironment is highly desirable. HIF-1α is an attractive therapeutic target because it is a key transcription factor in tumor development and only accumulates in hypoxic tumors. Cationic mixed micellar NPs consisting of amphiphilic block copolymers poly(ε-caprolactone)-block-poly(2-aminoethylethylene phosphate) (PCL29-b-PPEEA21) and poly(ε-caprolactone)-block-poly(ethylene glycol) are suitable carriers for HIF-1α siRNA to treat hypoxic tumors. These NPs are an excellent example of a clinical strategy of specific siRNA therapy for cancer treatment aimed at the hypoxic tumor microenvironment [49].
The concept of CSCs has been explored since the late 1930s, and these concepts have been solidified and received considerable attention in recent years. The two main aspects concerning CSCs are as follows: (1) CSCs are regular SCs that uncontrollably grown and caused cancer and (2) CSCs arise from a subpopulation of cancer cells. In many situations, both of these concepts are rational and related to the microenvironment of a tumor. The survival and accumulation of drug-resistant CSCs following chemotherapy or radiotherapy are common explanations for the recurrence of increasingly invasive and malignant tumors. Many novel molecular targets are bound to be developed with continued in-depth research on CSCs, although it can be challenging for cancer therapy. As such, inhibiting the SC factor in MDR cells may increase the effectiveness of treatment by reducing the apoptotic threshold of these cells.
To date, the main directions in the treatment of drug-resistant cancer cells and CSC targeting are associated with four main areas. First is the design of novel gene-targeting therapies (e.g., siRNA, miRNA, and antisense oligos) against the proteins responsible for the intrinsic drug resistance and survival of CSCs, such as drug efflux transporters, antiapoptotic proteins, and members of underlying signaling pathways. Second is the development of novel and efficient small drug molecules and inhibitors, as well as polymeric drug conjugates and nanocarriers, which are able to target to the niche of CSCs. Third is the development of sensitive bio-imaging approaches, including theranostics, for the precise location of CSCs. Fourth is the potential application of physical destruction methods, such as thermoablation, photodynamic therapy (PDT), laser therapy, and surgery.
Multiple transporters have been found in CSCs, including Pgp, BCRP, and MRP. The expression of MRP1 (ABCC1) and the activity of the apoptosis inhibitor β-livin cause a high survival rate for glioblastoma CSCs after etoposide treatment. Cell surface markers expressed by CSCs/TICs are generally shared by normal somatic SCs. However, the differences between the subtle surface antigens as well as signaling pathway and metabolic alterations of CSCs/TICs and normal somatic SCs may be exploited for the selection for targeted delivery of NPs in this field. For example, the overexpression of CD44 in cancer cells is strongly linked to therapeutic drug resistance. Another marker, CD133+, previously found in abundance in the embryonic epithelium, is also expressed in CSCs of many cancers. Therefore, CSC targeting can be potentially applied using surface carboxylic groups. Many CSC-associated surface biomarkers, such as CD44 and CD133, can be utilized for targeting dot in anticancer therapies by vectorized nanocarriers. Recently, Wang et al. [49] designed anti-CD133 mAb-conjugated single-walled carbon nanotubes. They demonstrated that these nanotubes can selectively target CD133+ glioblastoma cells and assist in their photothermal destruction by a NIR laser.
The concept of “a niche” maintenance in CSCs is widely accepted by researchers because of its specific protective microenvironment as one of the intrinsic properties of CSCs. This property potentially allows them to hide in a quiescent state in tissues and avoid the damage of chemotherapy. Various physicochemical methods for the specific destruction of CSCs and the CSC-supporting environment (niche) are currently being investigated. The Notch pathway plays a critical role in the connection between angiogenesis and self-renewal of CSCs and can thus be considered as a potential therapeutic target. The niche is defined as the microenvironment where CSCs are located and where they interact with other types of cells. Evidently, the CSC niche is a dynamic supportive system that contains a variety of cell types, cytokines, and signaling pathways. Several Notch inhibitors are being developed [50]. Mamaeva et al. [51] recently described the application of another type of nanocarriers, namely, MSNPs, for the targeted delivery of γ-secretase inhibitors of Notch signaling, which are potentially effective against CSCs.
Wnt signaling is another well-known pathway that plays a major role in embryogenesis and cancer development. Similarly, blocking the Wnt pathway in CD133+ colon cancer cells results in the reversal of their resistance to 5-fluorouracil [52].
Increasing Intracellular Availability and Changing Subcellular Localization
Reaching the tumor site as well as their intracellular site of action is important for therapeutics. For this phenomenon to occur, the therapeutics must escape the endosomal pathway and subsequent lysosomal degradation. Many strategies have evolved to ensure endosomal escape. A popular strategy is to modify the particles with cell-penetrating peptides that enable cell entry while evading lysosomal degradation. Another method for endosomal escape is to use pH-sensitive nanocarriers such as poly(ethylene glycol) (PEG)-modified dioleoyl phosphatidylethanolamine pH-sensitive liposomes. The pH of the intra-tumor destabilizes the liposomes, causing them to fuse with the endosomal membrane and subsequently release the cargo into the cytoplasm. Similarly, an optimized, pH-sensitive, mixed micelle system conjugated with folic acid is prepared to challenge MDR in cancers. The micelles are composed of poly(histidine-co-phenylalanine)-b-PEG and poly(l-lactic acid)-b-PEG-folate. Doxorubicin (DOX)-loaded micelles effectively kill both wild-type sensitive (A2780) and DOX-resistant ovarian MDR cancer cell lines (A2780/DOX(R)) through an instantaneous high dose of DOX in the cytosol, which results from active internalization, accelerated DOX release triggered by endosomal pH, and endosomal membrane disruption [53].
For polymer micelles, some polymers have effects on the function or expression of some efflux pump proteins. For example, Pluronic 85 (P85) can prevent the development of MDR1 phenotype in leukemia cells in vitro and in vivo as determined by Pgp expression and functional assays of the selected cells. In addition to mdr1, P85 alters the changes in genes implicated in apoptosis, drug metabolism, stress response, molecular transport, and tumorigenesis [54]. Meanwhile, our previous studies demonstrated that liposomes not only increase DOX levels allocated to nuclei but also extended retention in the nuclei of resistant cells [55]. Many clinical first-line anticancer drugs, such as DOX, camptothecin (CPT), and cisplatin, are DNA toxins that destroy DNA or its associated enzymes. Their cytotoxicity is maximized once they are inside the nucleus probably because of the direct damage to DNA. Thus, similar to therapeutic genes, these drugs have to localize in the nucleus to exert their pharmacological effects. For drug-resistant tumor cells, the drug can pump out for the existence of Pgp protein. Thus, encapsulating the drug in the nanocarrier is important to overcome the function of Pgp. A polylactide-surfactant block copolymer poly(l-lactide)-vitamin E TPGS (PLA-TPGS) has been synthesized using bidentate sulfonamide zinc ethyl complex as an efficient catalyst, and its self-assembled NPs are used as carriers of DOX. The activity of Pgp in drug-resistant breast cancer MCF-7/ADR cells is found to decrease after incubation with PLA-TPGS NPs. In addition, the nuclear accumulation and cytotoxicity of DOX are significantly increased by encapsulation of the drug into the NPs [56].
Similar results are obtained using a biodegradable polymer coupled to a photosensitizer, and the resulting photosensitizer NPs are loaded with the chemotherapeutic agent DOX. The combination of photosensitizer and chemotherapeutic agent has a synergistic action on a DOX-resistant breast cancer MCF-7 cell line. This combination of photodynamic activity in a powerful nanocarrier loaded with the chemotherapeutic agent DOX can be used to deliver two types of cancer therapy simultaneously, and the addition of TPGS can further enhance the entry of DOX into the nucleus [57].
As reported, the unique and evolutionary highly conserved major vault protein (MVP) is the main component (more than 70 %) of vaults, which are ribonucleoparticles with a hollow barrel-like structure that still contains two additional proteins and vault RNAs (vRNA). Identification of MVP with human lung resistance protein, together with its upregulation in Pgp-negative chemoresistant cancer cell lines, suggests that vaults play a role in cellular detoxification processes and consequently contribute to MDR by drug sequestration or shuttling drugs from the nucleus to cytoplasmic vesicles [58]. Thus, polyamidoamine (PAMAM) dendrimers are functionalized by a polysaccharide hyaluronic acid (HA) to effectively deliver DOX as well as MVP-targeted siRNA to improve DOX chemotherapy in MCF-7/ADR cells by downregulating MVP expression. As a result, co-delivery of siRNA and DOX by PAMAM-HA exhibits satisfactory gene silencing effect as well as enhanced stability and efficient intracellular delivery of siRNA. This phenomenon allows DOX to enter into the nucleus efficiently and induce more subsequent cytotoxicity than when siRNA is absent as a result of MVP knockdown [29].
For the cell interior, the mitochondrion is the major organelle implicated in the cellular bioenergetic and biosynthetic changes accompanying cancer. These bioenergetic modifications contribute to the invasive, metastatic, and adaptive properties typical in most tumors. Moreover, mitochondrial DNA mutations are linked to the bioenergetic changes in cancer. Targeting to tumor cell metabolism or mitochondria has been proposed as a novel strategy for the treatment of tumor. The most important aspect in the physiology of cancer is the role of mitochondria in energy metabolism and cell cycle regulation. Strong evidence supports the rationale for the development of anticancer strategies based on mitochondrial targets. Mitochondria play a key role in the complex apoptotic mechanism and trigger cell death through several mechanisms, such as disrupting electron transport and energy metabolism, releasing or activating proteins that mediate apoptosis, and altering the cellular redox potential.
Nanotechnology, which encompasses materials and methods at the nanoscale, is an attractive approach to designing mitochondrial therapeutics that either target or avoid mitochondria. Nanosystems that target mitochondria can enhance efficacy in treating mitochondrial diseases, whereas those that avoid mitochondria may help reduce mitochondrial toxicity. The surface modification of nanocarriers can also be tailored to achieve subcellular localization such as mitochondrial targeting, which is often achieved using mitochondrial leader sequences or the negative membrane potential of mitochondria through the use of “mitochondriotropics.” Mitochondriotropics are molecules that have delocalized positive charges such as triphenyl phosphonium [59].
For mitochondrial targeting, the selective accumulation of Au NPs in the mitochondria of cancer cells has been reported [60]. Their long-term retention decreases the mitochondrial membrane potential and increases the reactive oxygen species level that enhances the likelihood of cell death. Taking advantage of the development of SV30, a new analog of the pro-apoptotic molecule HA14-1, 57 nm organic solvent-free lipid nanocapsules loaded with SV30 (SV30-LNCs) are formulated using an inversion phase process. Encapsulated SV30 is found to improve mitochondrial targeting, which may elicit considerable interest toward the development of mitochondrion-targeted nanomedicines [61]. In addition, the known mitochondriotropic ligand triphenyl phosphonium (TPP) has been conjugated on the surface of a dendrimer. A fraction of the cationic surface charge of G(5)-D is neutralized by partial acetylation of the primary amine groups. The newly developed TPP-anchored dendrimer (G(5)-D-Ac-TPP) is efficiently consumed by the cells and demonstrates good mitochondrial targeting [62].
Combination Therapy Toward Overcoming Drug Resistance
Many combinatorial NP formulations have been successful in reversing MDR in vitro and in vivo of cancer models by co-delivering chemosensitizing agents and chemotherapy agents. Among many cellular mutations that diminish the effectiveness of anticancer drugs, the overexpression of multidrug transporters and altered apoptosis are the two underlying mechanisms by which cancer cells acquire resistance to multiple structurally and mechanistically unrelated drugs. NPs of 10–200 nm in diameter have shown more favorable antitumor pharmacokinetic profiles than small-molecule drugs. These drug-loaded NPs exhibit prolonged systemic circulation lifetime, sustained drug release kinetics, and advanced tumor accumulation [6]. Various NP platforms such as liposomes, polymeric micelles, dendrimers, nanoemulsion, and mesoporous silica particles have been used to carry broad classes of therapeutics, including cytotoxic agents, chemosensitizers, siRNA, and antiangiogenic agents (Fig. 15.4 and Table 15.2).
Schematic of nanoscale drug carriers used for combinatorial drug delivery: (a) liposome, (b) polymeric micelle, (c) polymer–drug conjugate, (d) dendrimer, (e) oil nanoemulsion, (f) mesoporous silica nanoparticle, and (g) iron oxide nanoparticle (from [63])
Combination of Drug Delivery and Drug Efflux Modulation
Drug resistance is considered to be the main reason for therapeutic failure in advanced cancer treatment. In many cases, drug transporter proteins (e.g., Pgp and MRP) that can pump out the intracellular drug are always overexpressed in most drug-resistant cancer cell lines. These drug transporter proteins are some of the most extensively characterized barriers to chemotherapy. Accordingly, a number of nanocarriers have been designed to sensitize drug-resistance tumor cells because they can aid in drug escape from the transporters, inhibit ATPase activity, or indirectly deplete cellular ATP, thereby leading to enhanced intracellular accumulation of therapeutic agents [68].
During chemotherapy, one of several ABC drug transporters, such as Pgp, MRP1, or ABCG2, becomes upregulated in some cancer cells. This phenomenon causes insensitivity to drugs and, subsequently, drug resistance. To date, the genes for 48 ABC proteins have been identified in the human genome and subdivided into seven families (ABC A–G) based on structural and sequential similarities. The decrease in intracellular drug accumulation is always caused by an undetermined energy-dependent, carrier-mediated mechanism. Not until 1976 was a 170 kDa cell membrane glycoprotein named Pgp discovered, and its link to the MDR phenotype was confirmed by Juliano and Ling [88]. In addition to Pgp (ABCB1), MRP1 (ABCC1), and ABCG2, at least 12 other ABC transporters are currently linked to MDR or can cause reduced intracellular drug accumulation.
Attempts on inhibitor-based chemosensitization and on the identification of new inhibitors of ABC transporters are currently ongoing. A large number of cancer-treating drugs have been identified as substrates of Pgp, including Vinca alkaloids, taxanes, etoposide, teniposide, colchicines, actinomycin D, CPTs, imatinib mesylate, saquinavir, methotrexate, and mitoxantrone. An appealing approach to overcoming MDR is the co-administration of a chemotherapeutic agent and a Pgp inhibitor. Nanocarrier systems containing a combination of cytotoxic drugs and efflux pump inhibitors, such as cyclosporine, verapamil, and tariquidar, have been used to suppress the MDR effect. The first attempt to co-deliver a chemosensitizer with chemotherapeutics in a single nanocarrier was a polyalkylcyanoacrylate NP system loaded with Pgp inhibitor cyclosporin A (CyA) and DOX [89]. Against a DOX-resistant leukemia cell line (P388), the co-encapsulation of CyA and DOX induces nearly a twofold increase in toxicity compared with DOX-only NPs. The enhanced efficacy is not observed when free CyA is applied with the DOX-only NPs. This finding suggests that the NP-coordinated delivery of two bioactive agents is essential for their cooperative activity. Various drug delivery nanovehicles are engineered to evade or overcome drug extrusion by drug efflux transporters, thereby resulting in enhanced chemotherapeutic drug accumulation in the cytosol and/or the nucleus of cancer cells and consequent elimination of tumor cells. These nanovehicles include oil nanoemulsions, polymeric micelles, liposomes, copolymeric NPs conjugated to quantum dots, and metallic NPs.
Permanent elimination or deactivation of any ABC transporter is unrealistic and unreasonable because of their important physiological and pharmacological roles in the human body. However, MDR in cancer caused by the overexpression of ABC drug transporters can be transiently modulated by various means, including direct inhibition, gene silencing, transcriptional regulation, and drug encapsulation. However, no clinically applicable inhibitor of ABC transporters exists to date. The reason for the unsuccessful clinical trials is complex but may be predominantly due to the unfavorable toxicity of inhibitors. MDR in cancer is apparently caused by multiple mechanisms that operate either independently or in unison. Overexpression of drug transporters is just one of the many reasons that cancer cells have adapted to survive the diversity of agents used in cancer chemotherapy. Over 30 years has passed since the discovery of Pgp in 1976, yet no simple and feasible solution to overcoming MDR in cancer has been discovered. The complexity and identification of new MDR-linked ABC transporters produce more challenges. Nevertheless, based on the new discoveries and advancements made on the identification, biological characterization, and structural analysis of MDR-linked ABC transporters over the years, we are one step closer to understanding clinical MDR in cancer.
Combination Drug Delivery and Modulation of Apoptotic Threshold
Cell apoptosis requires a minimum cellular threshold to be overcome. In cancer cells, this threshold is elevated to the extent that extracellular and intracellular insults sufficient in inducing apoptosis in normal cells have no effect. MDR cells have developed various mechanisms for increasing their apoptotic threshold. Decreased ceramide levels and the Warburg effect are the two major mechanisms that MDR cells utilize to increase their apoptotic threshold. The response to MDR is associated with alterations in the apoptosis pathways. Therapeutic NPs have been developed to co-encapsulate compounds that repair the dysfunctional apoptotic signaling. One example of such pro-apoptotic compound is ceramide, which is produced by cells under environmental stress and serves as a key messenger in programmed cell death.
An increasing number of studies have implicated ceramide, sphingosine-1-phosphate, as well as the genes involved in their biosynthesis, catabolism, and signaling, in various aspects of oncogenesis, cancer progression, as well as anticancer drug resistance and radiation resistance. Based on these findings, several research groups have used the strategy of inducing elevated levels of ceramide to decrease the threshold of apoptotic signaling in MDR cells while simultaneously delivering a cytotoxic drug (e.g., paclitaxel) using polymeric NPs.
A polymeric micelle formulation based on poly(ethylene oxide)-poly(epsilon-caprolactone) (PEO-PCL) for co-delivering exogenous ceramide and paclitaxel to address ceramide metabolism has been developed [90]. Against a paclitaxel-resistant ovarian cancer cell line (SKOV-3TR), the combinatorial formulation is found to increase the paclitaxel sensitivity of MDR cells to the same level as non-MDR cells. Combination with ceramide shows a 100-fold increase in efficacy compared with paclitaxel-only NPs. In another study, polymeric blend NPs have been prepared for the co-encapsulation of paclitaxel and C6-ceramide (CER), a synthetic analog of ceramide [75]. In vivo studies indicate that combination therapy with NPs harboring both paclitaxel and CER can enhance apoptotic signaling and reduce the tumor volume at least twofold compared with traditional standard paclitaxel monotherapy [75]. Yet another approach to increasing intracellular ceramide is the use of siRNA to silence glucosylceramide synthase. This strategy decreases the expression of Pgp in MDR cells, verifying the significance of ceramide in apoptotic modulation [91].
Combination Drug Delivery and Intracellular pH Modulation
The decreased pH associated with MDR cells has been utilized in many strategies for overcoming MDR. Some strategies are aimed at altering intracellular pH; others make use of pH-sensitive constituents to control the release of drugs. Novel pH-responsive polymers such as poly(β-amino ester), soluble below pH 6.5, are incorporated into NP formulations to localize the release of therapeutic agents in the acidic cellular environment of tumors and subcellular endosomal/lysosomal compartments.
Drugs encapsulated in pH-sensitive polymeric micelles have also been developed to target MDR cancer. Zwitterionic oligopeptide liposomes (HHG2C(18)-L) containing a smart lipid (1,5-dioctadecyl-l-glutamyl 2-histidyl-hexahydrobenzoic acid, HHG2C(18)) have been developed to overcome the barriers faced by anticancer drugs on the route from the site of injection into the body to the final antitumor target within transport steps with multiple physiological and biological barriers. HHG2C(18)-L shows a multistage pH response to the tumor cell (the mitochondria in this case). Their multistage pH response leads to more effective entry of anticancer agents into the tumor cell, improved escape from the endolysosomes, and accumulation in the mitochondria [92].
Nanocarriers for Combination Drug and Energy
Any organ heated to temperatures between 41 and 46 °C is defined as hyperthermia. Hyperthermia leads to reversible cell damage; however, when used as an adjunct treatment, it can help increase the efficacy of chemotherapy and enhance radiation-induced tumor damage. Hyperthermia has been utilized to change the morphology of a tumor to enhance the delivery of polymeric and liposomal NPs by increasing the blood flow to the tumor. It has also been successfully combined with DOX-loaded liposomes that target the folate receptor of tumor cells [93]. These temperature-sensitive systems can be designed to release drug payloads in the presence of specific temperature triggers.
In addition, clinical improvements to ultrasound focusing are being developed to improve the control and precise targeting of ultrasonic waves [94]. Combining localized ultrasound with nanocarrier therapies can exert a dramatic effect on the reduction of the residual toxicity associated with chemotherapy. Meanwhile, PDT is a form of cancer treatment that involves the use of photosensitizers as therapeutic agents. Under light irradiation, photosensitizers enter a triplet state of excitation. This triplet state of energy is easily transferred to oxygen molecules, which are subsequently converted into reactive oxygen species that are capable of damaging cells [95]. This method of treatment has high selectivity because only the cells exposed to both light and photosensitizer are affected.
RNA Interference to Overcome MDR
The clinical applications of small-molecule drugs that inhibit Pgp are not all successful. Hence, therapeutic strategies using RNA interference technology to overcome MDR are actively being explored. siRNA is a short double-stranded RNA that shows specific and effective gene silencing activity by the sequence-specific downregulation of a complementary messenger RNA. Therapeutic applications of siRNA have been limited because of their rapid enzymatic degradation by ribonuclease activity in serum and poor cellular uptake by passive diffusion [96]. The reversibility of the MDR phenotype of human cancer cells through the activation of the RNAi pathway by knocking down the MDR1/Pgp encoding mRNA was first reported in 2003 [97].
Drug efflux transporter genes that are being targeted include ABCB1 (MDR1/Pgp) and ABCC1 (MRP1), and these genes have been studied for decades. Gene silencing may be achieved at the mRNA level using siRNA constructs or antisense oligodeoxynucleotides (asODNs), which results in decreased MDR1 expression. Various drug delivery carriers for the targeted silencing of drug resistance genes have been described, including liposomes and different polymers, typically of cationic nature such as chitosan and its derivatives. A micellar system consisting of degradable poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) block copolymers with functional groups on both blocks has been prepared. The functional group on the PCL block is used to incorporate short polyamines for complexation with siRNA or to chemically conjugate DOX using a pH-sensitive hydrazone linkage. This system is used to improve the efficacy of DOX in multidrug-resistant MDA-MB-435 human tumor models that overexpress Pgp. The improvement is carried out by the simultaneous intracellular accumulation of DOX and siRNA against Pgp expression [98].
Targeting MDR1 gene transcripts has also been developed by harnessing bacterium-derived minicells encapsulating specific siRNA duplexes and chemotherapeutics [99]. Minicells targeted by specific antibodies to surface receptors of tumor cells are then used to deliver synergistic cargoes to tumor xenografts with high specificity.
Among the mechanisms of drug resistance independent of drug efflux pumps that have been targeted with an NP approach, some modalities are related to the Bcl2 and HIF1α genes. Bcl2 family proteins are regulators of programmed cell death (particularly apoptosis), and the HIF1α gene encodes for a transcription factor that plays a key role in the cellular response to hypoxia. Gene silencing is performed using siRNA or as ODNs. MSNs are utilized for the simultaneous delivery of Dox and Bcl2 siRNA [8]. Dox-loaded MSNs modified with amine-terminated PAMAM dendrimers facilitate conjugation with Bcl2 siRNA. Moreover, the simultaneous delivery of Bcl2 siRNA significantly suppresses Bcl2 mRNA and efficiently overcomes the MDR phenotype presumably using an inhibitory activity that these PAMAM dendrimer-based NPs exert on Pgp-mediated drug efflux [8].
A chemotherapeutic agent (DOX) and Pgp siRNA can be co-encapsulated by MSNPs and transported to a drug-resistant cancer cell line (KB-V1 cells), subsequently accomplishing cell killing in an additive or synergistic fashion [85]. Although a number of research have reported the RNAi modulation of cancer MDR in vivo, the lack of an efficient delivery strategy for administering shRNA to cancer patients is the major drawback. Various strategies have been explored but with no successful results. These studies demonstrate that a more efficient mode of delivery and nanocarriers, which are a promising platform for the efficient delivery of RNAi, is important in the clinical application of RNAi.
Conclusion
The unsatisfactory therapeutic effect of chemotherapy in treating solid tumors is multifactorial, and the occurrence of clinical tumor drug resistance is usually caused by a complex and unknown mechanism. Moreover, solid tumors are heterogeneous, structurally complex, and contain different kinds of cell. To the best of our knowledge, although various nanocarrier platforms for targeted delivery of anticancer drugs have already undergone in vivo testing in animal models and clinical evaluation in humans, no reports exist on NPs for the delivery of drug combinations aimed at overcoming drug resistance. The development of appropriate combinations of chemotherapies and nanotherapies, including novel gene-silencing, drug efflux-inhibiting, and CSC-targeting strategies, are the most effective methods of treating drug-resistant and aggressive tumors.
References
Jabr-Milane LS, van Vlerken LE, Yadav S, Amiji MM (2008) Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev 34(7):592–602
Vinogradov S, Wei X (2012) Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine (Lond) 7(4):597–615
Milane L, Duan Z, Amiji M (2011) Development of egfr-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol Pharm 8(1):185–203
Wu CP, Hsieh CH, Wu YS (2011) The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol Pharm 8(6):1996–2011
Xia W, Low PS (2010) Folate-targeted therapies for cancer. J Med Chem 53(19):6811–6824
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83(5):761–769
Ganta S, Amiji M (2009) Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm 6(3):928–939
Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H (2009) Co-delivery of doxorubicin and bcl-2 sirna by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small 5(23):2673–2677
Dilnawaz F, Singh A, Mohanty C, Sahoo SK (2010) Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials 31(13):3694–3706
Singh A, Dilnawaz F, Mewar S, Sharma U, Jagannathan NR, Sahoo SK (2011) Composite polymeric magnetic nanoparticles for co-delivery of hydrophobic and hydrophilic anticancer drugs and mri imaging for cancer therapy. ACS Appl Mater Interfaces 3(3):842–856
Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE (2007) Characterization of nanoparticles for therapeutics. Nanomedicine (Lond) 2(6):789–803
Fan Y, Li C, Cao H, Li F, Chen D (2012) The intranuclear release of a potential anticancer drug from small nanoparticles that are derived from intracellular dissociation of large nanoparticles. Biomaterials 33(16):4220–4228
Lei T, Srinivasan S, Tang Y, Manchanda R, Nagesetti A, Fernandez-Fernandez A, McGoron AJ (2011) Comparing cellular uptake and cytotoxicity of targeted drug carriers in cancer cell lines with different drug resistance mechanisms. Nanomedicine 7(3):324–332
Susa M, Iyer AK, Ryu K, Hornicek FJ, Mankin H, Amiji MM, Duan Z (2009) Doxorubicin loaded polymeric nanoparticulate delivery system to overcome drug resistance in osteosarcoma. BMC Cancer 9:399
Wang X, Li J, Wang Y, Cho KJ, Kim G, Gjyrezi A, Koenig L, Giannakakou P, Shin HJ, Tighiouart M, Nie S, Chen ZG, Shin DM (2009) Hft-t, a targeting nanoparticle, enhances specific delivery of paclitaxel to folate receptor-positive tumors. ACS Nano 3(10):3165–3174
Liu XQ, Xiong MH, Shu XT, Tang RZ, Wang J (2012) Therapeutic delivery of sirna silencing hif-1 alpha with micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharm 9(10):2863–2874
Wang X, Li J, Wang Y, Koenig L, Gjyrezi A, Giannakakou P, Shin EH, Tighiouart M, Chen ZG, Nie S, Shin DM (2011) A folate receptor-targeting nanoparticle minimizes drug resistance in a human cancer model. ACS Nano 5(8):6184–6194
Shalviri A, Raval G, Prasad P, Chan C, Liu Q, Heerklotz H, Rauth AM, Wu XY (2012) Ph-dependent doxorubicin release from terpolymer of starch, polymethacrylic acid and polysorbate 80 nanoparticles for overcoming multi-drug resistance in human breast cancer cells. Eur J Pharm Biopharm 82(3):587–597
Susa M, Iyer AK, Ryu K, Choy E, Hornicek FJ, Mankin H, Milane L, Amiji MM, Duan Z (2010) Inhibition of abcb1 (mdr1) expression by an sirna nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One 5(5):e10764
Wang Z, Yu Y, Ma J, Zhang H, Wang X, Wang J, Zhang X, Zhang Q (2012) Lyp-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol Pharm 9(9):2646–2657
Han M, Diao YY, Jiang HL, Ying XY, Chen DW, Liang WQ, Gao JQ (2011) Molecular mechanism study of chemosensitization of doxorubicin-resistant human myelogenous leukemia cells induced by a composite polymer micelle. Int J Pharm 420(2):404–411
Qiu L, Zhang L, Zheng C, Wang R (2011) Improving physicochemical properties and doxorubicin cytotoxicity of novel polymeric micelles by poly(epsilon-caprolactone) segments. J Pharm Sci 100(6):2430–2442
Xiao L, Xiong X, Sun X, Zhu Y, Yang H, Chen H, Gan L, Xu H, Yang X (2011) Role of cellular uptake in the reversal of multidrug resistance by peg-b-pla polymeric micelles. Biomaterials 32(22):5148–5157
Xu YY, Du YZ, Yuan H, Liu LN, Niu YP, Hu FQ (2011) Improved cytotoxicity and multidrug resistance reversal of chitosan based polymeric micelles encapsulating oxaliplatin. J Drug Target 19(5):344–353
Pinzon-Daza M, Garzon R, Couraud P, Romero I, Weksler B, Ghigo D, Bosia A, Riganti C (2012) The association of statins plus ldl receptor-targeted liposome-encapsulated doxorubicin increases in vitro drug delivery across blood-brain barrier cells. Br J Pharmacol 167(7):1431–1447
Zhao YZ, Dai DD, Lu CT, Chen LJ, Lin M, Shen XT, Li XK, Zhang M, Jiang X, Jin RR, Li X, Lv HF, Cai L, Huang PT (2013) Epirubicin loaded with propylene glycol liposomes significantly overcomes multidrug resistance in breast cancer. Cancer Lett 330(1):74–83
Padhye SS, Guin S, Yao HP, Zhou YQ, Zhang R, Wang MH (2011) Sustained expression of the ron receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody-directed chemotherapeutics. Mol Pharm 8(6):2310–2319
Kono K, Kojima C, Hayashi N, Nishisaka E, Kiura K, Watarai S, Harada A (2008) Preparation and cytotoxic activity of poly(ethylene glycol)-modified poly(amidoamine) dendrimers bearing adriamycin. Biomaterials 29(11):1664–1675
Han M, Lv Q, Tang XJ, Hu YL, Xu DH, Li FZ, Liang WQ, Gao JQ (2012) Overcoming drug resistance of mcf-7/adr cells by altering intracellular distribution of doxorubicin via mvp knockdown with a novel sirna polyamidoamine-hyaluronic acid complex. J Control Release 163(2):136–144
Dhanikula RS, Argaw A, Bouchard JF, Hildgen P (2008) Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: enhanced efficacy and intratumoral transport capability. Mol Pharm 5(1):105–116
Najlah M, Freeman S, Attwood D, D’Emanuele A (2007) Synthesis and assessment of first-generation polyamidoamine dendrimer prodrugs to enhance the cellular permeability of p-gp substrates. Bioconjug Chem 18(3):937–946
Gurdag S, Khandare J, Stapels S, Matherly LH, Kannan RM (2006) Activity of dendrimer-methotrexate conjugates on methotrexate-sensitive and -resistant cell lines. Bioconjug Chem 17(2):275–283
Qiu L, Zheng C, Zhao Q (2012) Mechanisms of drug resistance reversal in dox-resistant mcf-7 cells by ph-responsive amphiphilic polyphosphazene containing diisopropylamino side groups. Mol Pharm 9(5):1109–1117
Kunjachan S, Blauz A, Mockel D, Theek B, Kiessling F, Etrych T, Ulbrich K, van Bloois L, Storm G, Bartosz G, Rychlik B, Lammers T (2012) Overcoming cellular multidrug resistance using classical nanomedicine formulations. Eur J Pharm Sci 45(4):421–428
Yang YP, Chien Y, Chiou GY, Cherng JY, Wang ML, Lo WL, Chang YL, Huang PI, Chen YW, Shih YH, Chen MT, Chiou SH (2012) Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microrna145 with cationic polyurethane-short branch pei. Biomaterials 33(5):1462–1476
He Q, Gao Y, Zhang L, Zhang Z, Gao F, Ji X, Li Y, Shi J (2011) A ph-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials 32(30):7711–7720
Huang IP, Sun SP, Cheng SH, Lee CH, Wu CY, Yang CS, Lo LW, Lai YK (2011) Enhanced chemotherapy of cancer using ph-sensitive mesoporous silica nanoparticles to antagonize p-glycoprotein-mediated drug resistance. Mol Cancer Ther 10(5):761–769
Shen J, He Q, Gao Y, Shi J, Li Y (2011) Mesoporous silica nanoparticles loading doxorubicin reverse multidrug resistance: performance and mechanism. Nanoscale 3(10):4314–4322
Lukianova-Hleb EY, Belyanin A, Kashinath S, Wu X, Lapotko DO (2012) Plasmonic nanobubble-enhanced endosomal escape processes for selective and guided intracellular delivery of chemotherapy to drug-resistant cancer cells. Biomaterials 33(6):1821–1826
Zhang XG, Miao J, Dai YQ, Du YZ, Yuan H, Hu FQ (2008) Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int J Pharm 361(1–2):239–244
Min Y, Mao CQ, Chen S, Ma G, Wang J, Liu Y (2012) Combating the drug resistance of cisplatin using a platinum prodrug based delivery system. Angew Chem Int Ed Engl 51(27):6742–6747
Yang L, Meng L, Zhang X, Chen Y, Zhu G, Liu H, Xiong X, Sefah K, Tan W (2011) Engineering polymeric aptamers for selective cytotoxicity. J Am Chem Soc 133(34):13380–13386
Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68(16):6652–6660
Liu Y, Huang L, Liu F (2010) Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol Pharm 7(3):863–869
Lin YL, Liu YK, Tsai NM, Hsieh JH, Chen CH, Lin CM, Liao KW (2012) A lipo-peg-pei complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomedicine 8(3):318–327
Davis ME, Chen ZG, Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7(9):771–782
Maeda H, Fang J, Inutsuka T, Kitamoto Y (2003) Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol 3(3):319–328
Shapira A, Livney YD, Broxterman HJ, Assaraf YG (2011) Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat 14(3):150–163
Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA (2011) Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with cd133 monoclonal antibody. Nanomedicine 7(1):69–79
Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, Miele L (2010) Targeting notch to target cancer stem cells. Clin Cancer Res 16(12):3141–3152
Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A, Fortelius LE, Landor S, Toivola DM, Linden M, Sahlgren C (2011) Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of notch signaling in cancer. Mol Ther 19(8):1538–1546
Deng YH, Pu XX, Huang MJ, Xiao J, Zhou JM, Lin TY, Lin EH (2010) 5-fluorouracil upregulates the activity of wnt signaling pathway in cd133-positive colon cancer stem-like cells. Chin J Cancer 29(9):810–815
Kim D, Lee ES, Oh KT, Gao ZG, Bae YH (2008) Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal ph. Small 4(11):2043–2050
Sharma AK, Zhang L, Li S, Kelly DL, Alakhov VY, Batrakova EV, Kabanov AV (2008) Prevention of mdr development in leukemia cells by micelle-forming polymeric surfactant. J Control Release 131(3):220–227
Xu DH, Gao JQ, Liang WQ (2008) Liposome-based intracellular kinetics of doxorubicin in k562/dox cells. Pharmazie 63(9):646–649
Li PY, Lai PS, Hung WC, Syu WJ (2010) Poly(l-lactide)-vitamin e tpgs nanoparticles enhanced the cytotoxicity of doxorubicin in drug-resistant mcf-7 breast cancer cells. Biomacromolecules 11(10):2576–2582
Shieh MJ, Hsu CY, Huang LY, Chen HY, Huang FH, Lai PS (2011) Reversal of doxorubicin-resistance by multifunctional nanoparticles in mcf-7/adr cells. J Control Release 152(3):418–425
Ryu SJ, An HJ, Oh YS, Choi HR, Ha MK, Park SC (2008) On the role of major vault protein in the resistance of senescent human diploid fibroblasts to apoptosis. Cell Death Differ 15(11):1673–1680
Durazo SA, Kompella UB (2012) Functionalized nanosystems for targeted mitochondrial delivery. Mitochondrion 12(2):190–201
Wang L, Liu Y, Li W, Jiang X, Ji Y, Wu X, Xu L, Qiu Y, Zhao K, Wei T, Li Y, Zhao Y, Chen C (2011) Selective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapy. Nano Lett 11(2):772–780
Weyland M, Manero F, Paillard A, Gree D, Viault G, Jarnet D, Menei P, Juin P, Chourpa I, Benoit JP, Gree R, Garcion E (2011) Mitochondrial targeting by use of lipid nanocapsules loaded with sv30, an analogue of the small-molecule bcl-2 inhibitor ha14-1. J Control Release 151(1):74–82
Biswas S, Dodwadkar NS, Piroyan A, Torchilin VP (2012) Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials 33(18):4773–4782
Hu CM, Zhang L (2012) Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol 83(8):1104–1111
Misra R, Sahoo SK (2011) Coformulation of doxorubicin and curcumin in poly(d, l-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in k562 cells. Mol Pharm 8(3):852–866
Koganti S, Jagani HV, Palanimuthu VR, Mathew JA, Rao MC, Rao JV (2012) In vitro and in vivo evaluation of the efficacy of nanoformulation of sirna as an adjuvant to improve the anticancer potential of cisplatin. Exp Mol Pathol 94(1):137–147
Khdair A, Handa H, Mao G, Panyam J (2009) Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro. Eur J Pharm Biopharm 71(2):214–222
Patil YB, Sadhukha T, Ma L, Panyam J (2009) Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release 136(1):21–29
Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J (2010) The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials 31(2):358–365
Shen J, Yin Q, Chen L, Zhang Z, Li Y (2012) Co-delivery of paclitaxel and survivin shrna by pluronic p85-pei/tpgs complex nanoparticles to overcome drug resistance in lung cancer. Biomaterials 33(33):8613–8624
Das M, Sahoo SK (2012) Folate decorated dual drug loaded nanoparticle: role of curcumin in enhancing therapeutic potential of nutlin-3a by reversing multidrug resistance. PLoS One 7(3):e32920
Ma P, Dong X, Swadley CL, Gupte A, Leggas M, Ledebur HC, Mumper RJ (2009) Development of idarubicin and doxorubicin solid lipid nanoparticles to overcome pgp-mediated multiple drug resistance in leukemia. J Biomed Nanotechnol 5(2):151–161
Yadav S, van Vlerken LE, Little SR, Amiji MM (2009) Evaluations of combination mdr-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol 63(4):711–722
Prasad P, Shuhendler A, Cai P, Rauth AM, Wu XY (2013) Doxorubicin and mitomycin C co-loaded polymer-lipid hybrid nanoparticles inhibit growth of sensitive and multidrug resistant human mammary tumor xenografts. Cancer Lett 334(2):263–273
Wang J, Yin C, Tang G, Lin X, Wu Q (2012) Glucose-functionalized multidrug-conjugating nanoparticles based on amphiphilic terpolymer with enhanced anti-tumorous cell cytotoxicity. Int J Pharm 441(1–2):291–298
Devalapally H, Duan Z, Seiden MV, Amiji MM (2007) Paclitaxel and ceramide co-administration in biodegradable polymeric nanoparticulate delivery system to overcome drug resistance in ovarian cancer. Int J Cancer 121(8):1830–1838
Devalapally H, Duan Z, Seiden MV, Amiji MM (2008) Modulation of drug resistance in ovarian adenocarcinoma by enhancing intracellular ceramide using tamoxifen-loaded biodegradable polymeric nanoparticles. Clin Cancer Res 14(10):3193–3203
Song XR, Cai Z, Zheng Y, He G, Cui FY, Gong DQ, Hou SX, Xiong SJ, Lei XJ, Wei YQ (2009) Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in plga nanoparticles. Eur J Pharm Sci 37(3–4):300–305
Saad M, Garbuzenko OB, Minko T (2008) Co-delivery of sirna and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond) 3(6):761–776
Li X, Lu WL, Liang GW, Ruan GR, Hong HY, Long C, Zhang YT, Liu Y, Wang JC, Zhang X, Zhang Q (2006) Effect of stealthy liposomal topotecan plus amlodipine on the multidrug-resistant leukaemia cells in vitro and xenograft in mice. Eur J Clin Invest 36(6):409–418
Liang GW, Lu WL, Wu JW, Zhao JH, Hong HY, Long C, Li T, Zhang YT, Zhang H, Wang JC, Zhang X, Zhang Q (2008) Enhanced therapeutic effects on the multi-drug resistant human leukemia cells in vitro and xenograft in mice using the stealthy liposomal vincristine plus quinacrine. Fundam Clin Pharmacol 22(4):429–437
Patel NR, Rathi A, Mongayt D, Torchilin VP (2011) Reversal of multidrug resistance by co-delivery of tariquidar (xr9576) and paclitaxel using long-circulating liposomes. Int J Pharm 416(1):296–299
Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ (2007) Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci 10(3):350–357
Lu HL, Syu WJ, Nishiyama N, Kataoka K, Lai PS (2011) Dendrimer phthalocyanine-encapsulated polymeric micelle-mediated photochemical internalization extends the efficacy of photodynamic therapy and overcomes drug-resistance in vivo. J Control Release 155(3):458–464
Wang J, Qu H, Jin L, Zeng W, Qin L, Zhang F, Wei X, Lu W, Zhang C, Liang W (2011) Pegylated phosphotidylethanolamine inhibiting p-glycoprotein expression and enhancing retention of doxorubicin in mcf7/adr cells. J Pharm Sci 100(6):2267–2277
Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE (2010) Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and p-glycoprotein sirna to overcome drug resistance in a cancer cell line. ACS Nano 4(8):4539–4550
Taratula O, Garbuzenko OB, Chen AM, Minko T (2011) Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and sirna. J Drug Target 19(10):900–914
Xiao Y, Jaskula-Sztul R, Javadi A, Xu W, Eide J, Dammalapati A, Kunnimalaiyaan M, Chen H, Gong S (2012) Co-delivery of doxorubicin and sirna using octreotide-conjugated gold nanorods for targeted neuroendocrine cancer therapy. Nanoscale 4(22):7185–7193
Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in chinese hamster ovary cell mutants. Biochim Biophys Acta 455(1):152–162
Soma CE, Dubernet C, Bentolila D, Benita S, Couvreur P (2000) Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin a in polyalkylcyanoacrylate nanoparticles. Biomaterials 21(1):1–7
van Vlerken LE, Duan Z, Seiden MV, Amiji MM (2007) Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res 67(10):4843–4850
Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC (2005) Glucosylceramide synthase blockade down-regulates p-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res 65(9):3861–3867
Mo R, Sun Q, Xue J, Li N, Li W, Zhang C, Ping Q (2012) Multistage ph-responsive liposomes for mitochondrial-targeted anticancer drug delivery. Adv Mater 24(27):3659–3665
Gaber MH (2002) Modulation of doxorubicin resistance in multidrug-resistance cells by targeted liposomes combined with hyperthermia. J Biochem Mol Biol Biophys 6(5):309–314
Rapoport N (2004) Combined cancer therapy by micellar-encapsulated drug and ultrasound. Int J Pharm 277(1–2):155–162
Samia AC, Chen X, Burda C (2003) Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc 125(51):15736–15737
Reischl D, Zimmer A (2009) Drug delivery of sirna therapeutics: potentials and limits of nanosystems. Nanomedicine 5(1):8–20
Nieth C, Priebsch A, Stege A, Lage H (2003) Modulation of the classical multidrug resistance (mdr) phenotype by rna interference (rnai). FEBS Lett 545(2–3):144–150
Xiong XB, Lavasanifar A (2011) Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of mdr-1 sirna and doxorubicin. ACS Nano 5(6):5202–5213
MacDiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, Sedliarou I, Wetzel S, Kochar K, Brahmbhatt VN, Phillips L, Pattison ST, Petti C, Stillman B, Graham RM, Brahmbhatt H (2009) Sequential treatment of drug-resistant tumors with targeted minicells containing sirna or a cytotoxic drug. Nat Biotechnol 27(7):643–651
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Han, M., Gao, JQ. (2013). Nanotherapeutics in Multidrug Resistance. In: Bae, Y., Mrsny, R., Park, K. (eds) Cancer Targeted Drug Delivery. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7876-8_15
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7876-8_15
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7875-1
Online ISBN: 978-1-4614-7876-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)