Abstract
Anthracnose caused by Colletotrichum species is an important disease of mango (Mangifera indica L.) affecting leaf, flowers and fruits in mango orchards worldwide. The fungus Colletotrichum sp. usually invade the fruits during its developmental stage but remains quiescent until fruit ripening culminating in devastating anthracnose disease, especially during post-harvest stage. In contrary, new kind of pre-harvest anthracnose symptoms were observed on green unripe mangoes with varying level of incidence at field level in the state of Odisha, situated in Eastern India. This study attempted to characterize the Colletotrichum species affecting mango cultivars and causing new kind of symptoms under field condition on maturing green mangoes in comparison to post-harvest phase using morphological methods and molecular tools as well as by pathogenicity tests on intact green as well as on detached mature fruits. Eight Colletotrichum isolates from pre-harvest phase and two from post-harvest phase isolated from different mango varieties were studied for their diversity through morphological examination as well as sequence analysis of internal transcribed spacer (ITS), chitin synthase (CHS-1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-tubulin (TUB2) and ApMat genomic regions. Multigene phylogeny of all ten isolates revealed the identification of C. siamense. Pathogenicity assay of all 10 Colletotrichum isolates on green intact fruits in field as well as on detached ripening fruits in laboratory resulted in similar anthracnose symptoms on two selected test varieties Arka Anmol and Mallika. Results confirmed the association of C. siamense with both pre as well as post-harvest anthracnose symptoms of mango. Accurate pathogen identification provides a reliable basis for devising disease management schedules against anthracnose occurring at different phenological stages of the mangoes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mango is one of the most sought-after seasonal fruits growing in tropical and subtropical parts of the world. India is the home of thousands of varieties of mangoes which come in various sizes, shapes, and colors with a wide variety of flavors and tastes, with a definite eco-geographical need for optimum growth and yield. India is the world’s largest producer of mango with a production of 24 million tons of mango in 2020 (FAO-STAT 2021). However, mango production is constrained by diseases, pests, and poor post-harvest handling of fruit. Anthracnose disease on mango fruits caused by Colletotrichum species is a serious problem encountered in many tropical and sub-tropical countries which not only reduces the fruit quality drastically, but also adversely affects shelf life and marketability of mature mango fruits in ripening stage. Typically, anthracnose disease result in fruit spots which start as oval to round, black, sunken spots on fruit skin when mangoes start ripening. Colletotrichum species also causes leaf spot and blossom blight in mango plants at different growth stages. In tropical conditions, fruit spoilage due to anthracnose disease caused by Colletotrichum species at pre and/ or post-harvest stages ranges from slight loss in quality, to total spoilage of the fruits, resulting in reduced sales while impacting domestic and export marketing (FAOSTAT 2021; Hindorf 2000).
In our experimental farm located in the state of Odisha, Eastern India, in past few years we observed a new kind of preharvest anthracnose symptoms (without tear stains or pepper spots) on few varieties of mango. The symptoms generally start as shiny, dark brown to black circular, sub-circular to irregular necrotic patches which enlarge to 1.0–2.5 inches diameter covering the major portion of the fruits, even though the symptoms varied with varieties. The infected skin became dry, flaky, with or without yellow halo, which sometimes manifested as big cracks in fruits as the disease advanced. The symptoms of preharvest of anthracnose were noticed in other varieties of mango during 2017–2018. According to our investigation, the incidence of preharvest anthracnose reached as high as 45% in highly susceptible varieties seriously affecting the fruit quality. It is known that once the spores of Colletotrichum land on peel of unripe fruits under field condition, spores germinate by producing germ tubes which will then form melanized appressoria that penetrate the cuticle directly. At this stage, this pathogen is noted for its ability to maintain itself in an extended dormant state until fruit ripening (Giblin et al. 2010) except on young fruits (Estrada et al. 2000). Once the fruits start ripening the pathogen embarks on necrotrophic infection and decay symptoms develop on the peel resulting in anthracnose disease symptoms (Dodd 1991; Akem 2006). In preliminary investigations, we observed that even though the fruiting bodies produced on the diseased green fruits indicated the association of Colletotrichum species, the peculiar difference in symptomatology from the post-harvest anthracnose necessitated the systematic investigation of the pathogen population with pre as well as post-harvest phases of mango.
Due to the economic relevance of mango in India, it was decided to undertake comprehensive work to determine the species composition of the Colletotrichum population involved in causing anthracnose in the pre-harvest phase of mango, under coastal Eastern India conditions. As the taxonomic intricacy among Colletotrichum species is well known further morpho-taxonomic identification alone potentially can be misleading. Although internal transcribed spacer (ITS) gene sequence data in many Colletotrichum species complexes show modest phylogenetic resolution (Willingham et al. 2012), the polyphasic method is advised for their correct identification. This study presents the symptomatology, pre-harvest anthracnose incidence on different mango varieties, taxonomy, and phylogenetic relationships of Colletotrichum isolates associated with pre-harvest anthracnose along with post-harvest anthracnose of mango in the state of Odisha, Eastern India through morphological, molecular, and pathogenicity analyses.
Materials and methods
Sampling and isolation of causal agents
Immature green mangoes showing dry anthracnose symptom were collected from eight different cultivars of mango viz., Arka Anmol (Alphonso x Janardhan Pasand), Arka Neelkiran (Alphonso x Neelum), Manjeera (Rumani x Neelum), Mahmood Bahar, Sai Sugandh (Totapuri x Kesar), Sindhu (Ratna x Alphonso), Totapuri from our experimental farm of ICAR-IIHR- Central Horticultural Experiment Station and Mallika farmer’s field (Fig. 1). Ripened fruits of the above varieties showing post-harvest fruit rot symptoms were collected from the local market and var. Mallika and Amrapali used for comparison in this study (Fig. 2). Isolation of the pathogen was done as per Sharma et al. (2017). Small pieces (0.5 cm2) of infected tissue sections partially consisting of healthy portions from the affected fruits were dissected and surface-sterilized with 1.0% sodium hypochlorite for 30–60 s and subsequently rinsed with sterile distilled water thrice. These tissues were blot dried on a sterile paper towel and placed aseptically on Potato Dextrose Agar medium (PDA, HiMedia™ Laboratories Pvt Ltd ) amended with streptomycin sulphate (0.1 mg mL−1) and incubated at 25–27 °C for 5 days. Pure culture of the fungi was obtained by single hyphal tip method and all isolates were subjected to morphological characterization and phylogenetic study. The eight Colletotrichum isolates resulted from preharvest anthracnose symptoms and two Colletotrichum isolates from ripe anthracnose symptoms were cultured at 25 °C for 7 days, PDA media. Subsequently, the cultures were subjected to morphological characterization (colony morphology, conidial measurements), and growth rate as well as assessed for color of the conidial masses (Than et al. 2008). Conidia were mounted in 100% lactic acid and digital images were recorded with an Olympus BX53 microscope (Olympus Co., Tokyo, Japan). The length and width of 30 conidia per isolate were measured with the Q capture Pro image analyzer. Each morphological feature was compared with C. siamense-type strains (Weir et al. 2012). After seven days, the colony’s diameter was measured to calculate its growth rate (mm/day).
Fungal genomic DNA extraction and PCR amplification
All 10 fungal isolates were cultured in potato dextrose broth (PDB, HiMedia™ Laboratories Pvt Ltd) and incubated at 28 ± 2 °C for seven days under a static condition. Then the mycelia were harvested, dried on sterile filter paper on laminar hood, and homogenized into a fine powder using liquid nitrogen. One hundred milligrams of powdered mycelia were subjected to DNA extraction using the using the Fungal DNA Purification Kit (HiPurATM; HiMedia, Maharashtra, India) according to the manufacturer’s instructions. The Apn2/Mat1-2-1 intergenic spacer (ApMat), beta-tubulin (TUB2) gene, chitin synthase (CHS-1), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and inter transcribed spacer (ITS) region were amplified using the primers as given in Table 1. The isolated genomic DNA was subjected to PCR using 25 µl 2X PCR Master Mix (Emerald Amp® MAX, DSS Takara Bio Inc.), 1 µl of each primer (10mM), 2 µl of DNA template, and the final volume were adjusted to 50 µl using sterile distilled water (nuclease-free). The PCR amplification cycle followed is shown in Table 1. PCR products were separated by gel electrophoresis in an agarose gel (1.2%) stained with ethidium bromide (EtBr), viewed and photographed in the Gel Documentation system (Vilber, Marne-la-Vallée, France). The target amplicon was eluted from gel using Gel Extraction Kit (QIAquick from Qiagen India Pvt Ltd, New Delhi, India) following the manufacturer’s instructions. Subsequently sequencing for amplified regions of respective genes were performed with 10 picomoles of each primer in both directions for the identification of isolates by Sanger sequencing method (Medauxin sequencing services). The resultant sequences were edited, and assembled with the Bio Edit software V.7.0.9.0) (Hall 1999) and the obtained sequences were subjected to nBLAST analysis. The forward and reverse sequences were assembled and final consensus sequences thus obtained were submitted in NCBI database and the (http://www.ncbi.nlm.nih.gov) accession numbers were obtained. Table 2 provides the gene sequences of list of type strains that belong to the Colletotrichum species complex used for phylogenetic analysis.
Single locus and multi-locus phylogenetic analysis
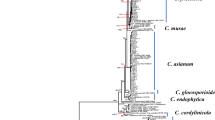
For each of the five loci, respective sequences of reference strains of Colletotrichum species were retrieved from the NCBI GenBank database via Basic Local Alignment Search Tool (BLAST) (Altschul 1990) and included in this study for phylogenetic analysis (Table 2). First phylogenetic trees were constructed for individual gene sequences. Subsequently, the concatenated gene sequences for all 5 genes viz., ApMat, TUB2, CHS-1, GAPDH, and ITS, sequenced of present 10 isolates as well as other selected references sequences given in Table 2 were generated using software Sequence Matrix version 1.8 (Vaidya et al. 2011). The concatenated sequences of the present 10 isolates as well as other reference sequences were aligned using software MEGA X (Kumar et al. 2018). All the regions that were unambiguously aligned were removed from the analysis and gaps were regarded as missing data. To get the maximum parsimonious tree, analysis was performed through the software PAUP 4.0 (Phylogenetic Analysis using Parsimony v. 4.0b10) (Swofford 2002). The bootstrap replication was kept at 1000 and all the tree statistics viz., tree length, CI (consistency index), RI (retention index), RC (rescaled consistency index), and HI (homoplasy index) were calculated. The phylogenetic tree was constructed using the neighbour joining method and was rooted through Colletotrichum boninense CBS123755.
Pathogenicity evaluation on intact green mangoes under field condition
Field experimentation was commenced in March 2018 at CHES farm on 15-year-old Arka Anmol and Mallika trees. Fruits of cricket ball-sized stage (6–10 cm) on were selected. One representative isolates CHES2020-CS-AA from pre-harvest anthracnose symptoms of Arka Anmol and RP6 from post-harvest anthracnose symptoms collected from the local market taken for pathogenicity study. No chemical treatment was applied to the fruit during the entire season. Ten trees were selected for study and 10 fruit per tree were selected randomly, ensuring that mangoes were free of any apparent marks or disease and were tagged. On each tree, a control fruit was tagged, giving a total of 10 control fruit. Fruits were pinpricked on the shoulder area and an 8 mm diameter mycelial plug of each isolate was placed on the wounds covered with moist sterile absorbent cotton and sealed with parafilm to ensure that the plug should not fall as the fruits were hanging on the tree. Each fungal isolate was inoculated onto randomly chosen five fruits on all 10 trees. Control fruits were inoculated with a sterile PDA plug. Fruit was enclosed for 48 h in a polypropylene bag and plastic bag with both ends tied. After 48 h the bottom end was untied however the bags were not removed till the experimentation. Fruits were assessed for visible lesions, and the lesion was measured after a week of inoculation and at weekly intervals up to 6 weeks of inoculation. Fungi were re-isolated from the lesions and reidentified to verify the Koch’s postulates. The experiment was repeated twice.
Pathogenicity evaluation on detached (ripening) mango fruits under laboratory condition
Physiologically matured, unblemished fruits, de-sapped mango fruits were collected from the experimental orchard and ensured that these mangoes were not sprayed with any fungicides during maturation. The fruits were washed under running tap water for 2 mins followed by surface sterilization with 70% ethanol and rinsed with sterile distilled water to inoculation. The same representative isolates CHES2020-CS-AA and RP6 were taken for a pathogenicity study on detached ripening fruits to determine the pathogenicity. The samples were inoculated using the wound inoculation method (Lin et al. 2002) which included a 3 mm depth pin-prick using a sterile needle on the mid portion of fruit including the control fruits. Then the fruits were inoculated at the wounded portion with a 5 mm mycelial disc taken from the actively growing region of seven-day-old culture. Fruits inoculated with sterile agar plug served as the negative control. The inoculated set of fruits were placed in plastic containers lined with paper towels wetted with sterile distilled water to maintain a high humidity necessary for infection and arrangements were made to avoid direct contact of fruits with water and were removed out of containers after 48 h of inoculation and maintained at room temperature (28 ± 2 °C) and lesion development was monitored periodically. The experiments were arranged in a completely randomized design with three replications containing four fruits for each fungal isolate. The experiment was repeated twice. Disease assessments were done at 7 DAI depending on the presence of visible anthracnose lesions and by measuring the lesion size. Fungi were re-isolated from the lesions and reidentified to verify the Koch’s postulates.
Results
Symptomatology and incidence of pre-harvest anthracnose on different mango varieties
New kind of anthracnose symptoms were observed on green intact mango fruits on the trees in contrast to usual post-harvest anthracnose wherein anthracnose spots develop on fruits during its ripening phase. Even though disease incidence varies with varieties, these dry anthracnose symptoms were observed in several varieties grown in our germplasm block, which includes many commercial varieties. In a few susceptible varieties, symptoms appear in all stages of fruit development starting from the pea stage, lemon stage, cricket ball stage, and mature fruits with varied levels of incidence and severity. Pre-harvest dry anthracnose caused 1 mm to several centimeter wide black, big elliptical, round to irregular patches on the skin leading to the typical spreading lesion and eventual cracking of infected fruits. Lesions on fruits produced orange, conspicuous, spore masses in a concentric ring pattern with gum-like exudates in dry conditions. The lesions were mostly confined to the rind only with 1–3 mm brownish lesion touching the resin canal however not extending to a pulp. However, when fruits were harvested and kept in room condition, the lesions exhibited during the field stage started extending to pulp to cause a maximum of up to 1.0–3.0 cm deep lesions but not further. The preharvest dry anthracnose spots are raised, flaky, and dry in contrast to sunken, wet with slightly raised edges of lesions on ripe fruits. Nonetheless, even superficial disease development resulted in serious aesthetic damage and rejection of fruit along the marketing chain. Different stages of pre-harvest dry anthracnose in the highly susceptible var. Arka Anmol and Mahmood Bahar occurred at natural field conditions has been elaborated in Table 3 and depicted in Fig. 1. The incidence and susceptibility levels of various mango cultivars grown in our experimental farm are listed in Table 4 and depicted in Fig. 2. During our study, the incidence of pre-harvest anthracnose symptoms was observed only on a few non-commercial varieties during 2013 with the incidence of up to 45% in highly susceptible cultivars, but slowly it was observed on noted commercial varieties such as Deshahari, Langra and Totapuri (Banglora) up to 5–10% incidence (Table 4).
Fungal isolation and morphological characterization
A total of 8 fungal isolates were isolated from symptomatic green fruits belonging to different varieties showing pre-harvest anthracnose (7 isolates from our experimental orchard, one from a farmer’s field) and two isolates from ripe fruits showing post-harvest anthracnose symptoms under market condition (var. Amrapali and Kesar) were taken for study. The uniform colony emerged out of infected anthracnose spots from pre-harvest dry anthracnose is depicted in Fig. 3a, b. The isolates showed little difference according to the colony characteristics like colony color texture, and pattern of sporulation. Colonies produced from isolates varied in color from off-white to dull pink with dense, cottony mycelium, and salmon orange conidial masses around the inoculation point, black acervuli were observed to be randomly distributed on culture plates. On average, the growth rates were varying from 9.61 to 13.05 mm per day. Length and width of conidia were recorded and the details are presented in Table 5. Conidia were all hyaline, cylindrical, one-celled, guttulate, and fusiform with obtuse ends with an average length of 14.74–18.44 μm and width of 4.53–5.91 μm (Fig. 3c). Zhang et al. (2020) studied the morphological characteristics of C. siamense infecting straw berry and reported the average length and width of conidia as 13.9 to 19.1 μm and 4.7 to 6.5 μm respectively wherein the conidial morphology is almost in agreement with our present study.
Single and multigene-based phylogenetic analysis
All the 5 genes viz., ITS, GAPDH, CHS-1 TUB2 and ApMat of present 10 isolates were successfully amplified and sequenced. All 5 regions when used individually to construct the phylogenetic tree resulted in topographically different trees. Moreover, individual genes did not result in a robust phylogenetic tree with reliable species delimitation. Different isolates of the same Colletotrichum species clustered in different clades. The BLAST analysis of individual genes indicated that all the present isolates belong to the C. gloeosporoides species complex. ITS sequences of these isolates showed more than 99% identity with the available sequences of C. aenigma, C. siamense, C. gloeosporoides, C. fructicola, and C. queenslandicum; CHS-1 sequences showed 98–100% similarity with the sequences of C. fructicola and C. siamense; GAPDH, TUB2 and ApMat sequences showed 99–100% identity with the sequences of C. siamense. The single gene phylogenetic trees are shown in Figs. S1–S5.
Multi-locus phylogenetic analysis was conducted among 51 strains including 10 present isolates and C. boninense as out-group (C. boninense CBS123755) (Table 1). The concatenated sequences for all 5 genes included 3141 positions. The gene boundaries in the multigene sequence dataset included ApMat: 1-1061; BTUB: 1062-1854; CHS: 1855-2163; GADPH: 2164-2460; and ITS: 2461-3141. Of all the characters, 1157 characters were constant, 440 variable characters were parsimony uninformative, and 1106 characters were parsimony informative. This parsimony analysis resulted in the most parsimonious tree presented in Fig. 4 (TL: 2762, CI: 0.730, HI: 0.270, RI: 0.938, RC: 0.685). All the present isolates were phylogenetically similar and clustered with C. siamense isolates viz., GM473, MU1, GM172, and GM390 derived from Mango, and thus were identified as C. siamense. The phylogenetic tree depicted in Fig. 1 differentiated the species of Colletotrichum genus very clearly which were included in the analysis. According to the morphological characterizations and phylogenetic analyses, the causal agent of pre-harvest as well as post-harvest anthracnose infecting Mangifera indica from Eastern India was identified as C. siamense.
Pathogenicity evaluation on intact green mangoes under field condition
Both tested isolates could infect intact green mangoes inoculated under field conditions as well as detached ripe mangoes inoculated under laboratory conditions. Similarly, both the isolates successfully produced dark, prominent, black lesions on all three inoculated fruits which were on the tree (Fig. 5). The fruits began to show obvious symptoms after a week of inoculation under field conditions. On and near the inoculation point, a near-circular blackish necrotic lesion was observed on the skin (Fig. 5). The rind of wounded points was rotted severely, followed by spread to the surrounding area were also rapidly infected and became necrotic black brown. The lesion became sub-circular to irregular measuring 1–2-inch diameter and taking different shape during the phase of infection (Fig. 6). However, the fruits did not fall from the tree. The fruits inoculated with non-colonized sterile agar plugs showed no symptoms. Koch’s postulates were verified by reisolating the same fungal isolates from the symptomatic fruits of mango and confirming their identification through morphological characteristics.
Pathogenicity evaluation on detached ripening mango fruits under laboratory condition
Two representative isolates namely CHES2020-CS-AA and RP6 were inoculated on detached mature mangoes of Var. Arka Anmol and Mallika. The dark, sunken, and black lesions started appearing at the site of inoculation five days after inoculation, it progressed further and developed as big lesions of 2–5-inch diameter in var. Arka Anmol as well as Mallika. The fruits of Arka Anmol inoculated with isolates CHES2020-CS-AA and RP6 have been depicted in the picture (Fig. 7). The fruits of control groups inoculated with non-colonized sterile agar plugs showed no symptoms. The morphological characteristics of fungus obtained from the lesions on inoculated fruits of mango matched those of the inoculated fungal isolate, thusconfirming Koch’s postulates.
Discussion
Anthracnose stands as a significant threat to the production of quality mango fruits in almost all mango-producing nations globally. Even though, on fruits, the post-harvest phase of anthracnose is much more prevalent, the pre-harvest anthracnose phase has not been reported as a common phenomenon. Nelson (2008), documented the occurrence of “tear stain” symptoms on fruits intact on the tree in Hawaii leading to the “alligator skin” effect and even causing fruits to develop wide, deep cracks in the epidermis that extend into the pulp. In Australia, the occurrence of tear stains on green mango referred to as pepper spots, caused by C. gloeosporioides has been described (Giblin et al. 2010). Apart from this, there were no reports available in the Web of Science about the occurrence of anthracnose on green maturing mangoes before harvest. Instead of pepper spots or tear stain symptoms as described by earlier workers. In our study, on single green fruit, we observed either one single large circular anthracnose lesions of several centimeters in size with concentrically arranged salmon orange-colored fruiting bodies of the fungi or otherwise many isolated smaller individual lesions with or without fungal fruiting bodies. Further, these anthracnose lesions were dry, hard, flaky confined to the rind, and not able to reach the fruit pulp until the fruit started ripening. As per the general perception of scientists, on green mango fruits, conidia of Colletorichum often remain latent and imperceptible until ripening, however, this fact has to be re-visited in days to come. This above scenario necessitated us to conduct a detailed investigation on the etiology of pre-harvest anthracnose occurring on green fruits of different mango varieties in comparison with a known post-harvest phase of anthracnose.
Across the world, the involvement of several Colletotrichum species causing anthracnose on ripened mango fruits has been documented by several researchers. Colletotrichum isolates causing anthracnose disease generally consist of pathogenically and genetically different populations of C. gloeosporioides which were studied by many researchers across the world (Hodson et al. 1993; Alahakoon et al. 1994; Hayden et al. 1994). Johnston et al. (2010) reported the cryptic nature of the Colletotrichum species complex in which the species are morphologically similar but genetically different. In India, studies based on either morphology and or ITS gene-sequence data reported C. gloeosporioides as a causal agent of mango anthracnose (Kumar et al. 2017; Sangeetha and Rawal 2009; Gupta et al. 2010; Lakshmi et al. 2011). Chowdappa and Mohan Kumar (2012) from India reported that as C. gloeosporioides isolates infecting mango varied in their level of virulence during pathogenicity testing, hence authors opined that the disease may be caused by more than one species. Worldwide most records post-2008 related to mango anthracnose pathogen were based on morphological identification or similarity with the non-type sequences submitted to GenBank (Cai et al. 2011). The necessity to re-examine the occurrence of C. gloeosporioides sensu lato on tropical fruits was emphasized by Phoulivong et al. (2010). Hence, several studies which re-examined the Colletotrichum species associated with mango anthracnose and other crops during the past decade were based on multigene phylogeny given the resolution of C. gloeosporioides sensu lato into several cryptic species (Weir et al. 2012; Jayawardena et al. 2016).
Our investigation led to the identification of C. siamense as a causative agent for both pre as well as post-harvest anthracnose phases of mango fruits based on morphological characteristics of the pathogen, pathogenicity study as well as multigene phylogenetic analysis. It ruled out which ruled out our curiosity about the involvement of additional new species of Colletotrichum which might have had the ability to cause the pre-harvest phase of anthracnose. However, the association of C. siamense on mango has been reported in mango-growing countries of the world including India (Sharma et al. 2013), China (Liu et al. 2017; Qin et al. 2017), Mexico (Tovar-Pedraza et al. 2020), Taiwan (Wu et al. 2020) and Thailand (Rattanakreetakul et al. 2023). In India, four known species of Colletotrichum namely C. frgariae sensu stricto, C. fructicola, C. jasmine-sambac and C. melanocaulon and five Colletotrichum lineages (with no species names) were found to be associated with mango tissues/ anthracnose (Sharma et al. 2013). Involvement of several other Colletotrichum species namely, C. asianum, C. alienum, C. cliviicola, C. cordylinicola, C. endophytica, C. fructicola, C. fioriniae, C. gloeosporioides, C. grossum, C. gigasporum, C. karsti, C. liaoningense, C. plurivorum, C. musae, C. queenslandicum, C. scovillei, C. simmondsii, C. tropicale and C. theobromicola has also been reported to be associated with mango anthracnose by number researchers around the world (Liu et al. 2013; Lima et al. 2013; Ismail et al. 2015; Pardo-De la Hoz et al. 2016; Qin et al. 2019; Manzano León et al. 2018; Li et al. 2019; Vitale et al. 2020; Tovar-Pedraza et al. 2020; Alvarez et al. 2020).
Apart from mango, C. siamense has been reported to infect many hosts growing in tropical and sub-tropical regions of the world (Prihastuti et al. 2009; Phoulivong et al. 2010, Weir et al. 2012, Liu et al. 2013), even though it was first described on Coffea arabica. (Prihastuti et al. 2009. In India, C. siamense has been reported to be associated with other host crops namely Chilli (Sharma and Shenoy 2013), Cliff Banana (Kumar et al. 2017), Pongamia pinnata (Dwarka et al. 2016), Cotton (Salunkhe et al. 2020) and elephant foot yam (Prasad et al. 2017).
Our pathogenicity study at the field and laboratory demonstrated that C. siamense isolated from the pre-harvest anthracnose phase of mango has the potential to induce both pre-harvest as well as post-harvest anthracnose symptoms on green maturing as well as ripened mango respectively. Similarly, C. siamense isolated from the post-harvest anthracnose phase of mango has the potential to induce both post-harvest as well as pre-harvest anthracnose symptoms on ripened mango as well as on green maturing mangoes respectively. This result necessitated us to re-verify the claim of the latent phase of Colletotrichum on green maturing mangoes.
As scientific community believes that the conidia of Colletotrichum remain dormant in green mangoes till fruits enter to ripening phase, as green fruits have a constitutive defence barrier, known as dienes (Prusky et al. 1982), antifungal resorcinols in fruit peel and latex (Hassan et al. 2007) and elaborate constitutive defence system comprising antifungal gallotannins, resorcinols in latex, and the enzyme chitinases (Karunanayake et al. 2011). However, as evident from our current study, C. siamense causing pre-harvest anthracnose symptoms on the peel of mango must have happened by breaking the defense barrier as listed by earlier researchers which shows the unparallel adaptation of C. siamense on green mango fruits by breaking the concept of preformed defense barriers. Additional studies are warranted for a better understanding of biochemical and physiological mechanisms that led to the establishment of preharvest anthracnose on green mangoes to tackle the emerging pre-harvest phase of mango anthracnose.
Conclusion
The identity of Colletotrichum associated especially with pre-harvest anthracnose rot of mango was confirmed by morphology, multigene phylogeny as well as by pathogenicity study conducted on intact green mango fruits at field level as well as on detached ripening mango fruits. Supplementary studies are warranted for a better understanding of the physiological machineries leading to pre-harvest anthracnose on green fruits. Further, with the advances in genomics and transcriptomics, there would be more new vistas in the exploration of the molecular mechanism of anthracnose occurring on green mango fruits where fungi could break the constitutive defense barrier.
References
Akem CN (2006) Mango anthracnose disease: present status and future research priorities. Plant Pathol J 5(3):266–273. https://doi.org/10.3923/ppj.2006.266.273
Alahakoon PW, Brown AE, Sreenivasaprasad S (1994) Cross-infection potential of genetic groups of Colletotrichum gloeosporioides on tropical fruit. Physiol Mol Plant Pathol 44:93–103. https://doi.org/10.1016/S0885-5765(05)80104-3
Altschul S (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1006/jmbi.1990.9999
Alvarez LV, Yukako H, Chester CD, Carmelita PM, Arcibel BB, Mark JBC, Kauske N, Shunsuke K, Keiichi M, Chiharu N (2020) Colletotrichum asianum causes anthracnose in Philippine mango cv. Carabao. Australas Plant Disease Notes 15:1–5. https://doi.org/10.1007/s13314-020-00384-x
Cai L, Udayanga D, Manamgoda DS, Maharachchikumbura SSN, McKenzie EHC, Guo LD, Liu XZ, Bahkali AH, Hyde KD (2011) The need to carry out re-inventory of plant pathogenic fungi. Trop Plant Pathol 36:205–213
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3):553–556
Chowdappa P, Mohan Kumar SP (2012) Existence of two genetically distinct populations of Colletotrichum gloeosporioides Penz in mango from India. Pest Manag Hort Ecosyst 18:161–170
Dodd JC (1991) The effect of climatic factors on Colletotrichum gloeosporioides, causal agent of mango anthracnose, in the Philippines. Plant Pathol 40(4):568–575
Dwarka DJ, Sharma G, Rajasab AH (2016) Colletotrichum siamense causes anthracnose on the fruits of Pongamia pinnata in India. Mycosphere 7(4):92–498. https://doi.org/10.5943/mycosphere/7/4/8
Estrada AB, Dodd JC, Jeffries P (2000) Effect of humidity and temperature on conidial germination and appressorium development of two Philippine isolates of the mango anthracnose pathogen Colletotrichum gloeosporioides. Plant Pathol 49:608–618. https://doi.org/10.1046/j.1365-3059.2000.00492.x
FAOSTAT (2021) Food and Agriculture Organisation of the United Nations Statistical Database, Statistical Division. FAO, Rome, Italy. http://www.fao.org/statistics/en/
Giblin FR, Coates LM, Irwin JAG (2010) Pathogenic diversity of avocado and mango isolates of Colletotrichum gloeosporioides causing anthracnose and pepper spot in Australia. Australas Plant Pathol 39:50–62
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61(4):1323–1330
Gupta VK, Pandey A, Kumar P, Pandey BK, Gaur RK, Bajpai V, Sharma N, Sharma S (2010) Genetic characterization of mango anthracnose pathogen Colletotrichum gloeosporioides Penz. By random amplified polymorphic DNA analysis. Afr J Biotechnol 9(26):4009–4013
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hassan MK, Dann EK, Irving DE, Coates LM (2007) Concentrations of constitutive alk (en) ylresorcinols in peel of commercial mango varieties and resistance to postharvest anthracnose. Physiol Mol Plant Pathol 71(4–6):158–165
Hayden HL, Pegg KP, Aitken EAB, Irwin JAG (1994) Genetic relationships as assessed by molecular markers and cross-infection among strains of Colletotrichum gloeosporioides. Australian J Bot 42:9–18. https://doi.org/10.1071/BT9940009
Hindorf H (2000) Colletotrichum species causing anthracnose of tropical crops. Plant Pathol 39:343–366
Hodson A, Mills PR, Brown AE (1993) Ribosomal and mitochondrial DNA polymorphisms in Colletotrichum gloeosporioides isolated from tropical fruit. Mycol Res 97:329–335
Ismail AM, Cirvilleri G, Yaseen T, Epifani F, Perrone G, Polizzi G (2015) Characterisation of Colletotrichum species causing anthracnose disease of mango in Italy. J Plant Pathology 97(1):167–171
Jayawardena RS, Hyde KD, Damm U, Cai L, Liu M, Li XH, Zhang W, Zhao WS, Yan JY (2016) Notes on currently accepted species of Colletotrichum. Mycosphere 7:1192–1260
Johnston AR, Gillespie TR, Rwego IB, Tranby McLachlan TL, Kent AD, Goldberg TL (2010) Molecular epidemiology of cross-species Giardia Duodenalis transmission in western Uganda. PLOS Negl Trop Dis 4(5):683
Karunanayake CA, Nimal K, Mallika B, Ratnayake A (2011) Role of antifungal gallotannins, resorcinols and chitinases in the constitutive defence of immature mango (Mangifera indica L.) against Colletotrichum gloeosporioides. J Phytopathol 159:657–664. https://doi.org/10.1111/j.1439-0434.2011.01818.x
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547
Kumar VS, Nair BA, Nair PVR, Annamalai A, Jaishanker R, Umamaheswaran K, Sooraj NP, Peethambaran CK (2017) First report of Colletotrichum siamense causing anthracnose of cliff Banana in India. Plant Dis 101:390
Lakshmi BK, Reddy PN, Prasad RD (2011) Cross-infection potential of Colletotrichum gloeosporioides Penz. Isolates causing anthracnose in subtropical fruit crops. Trop Agricultural Res. https://doi.org/10.4038/tar.v22i2.2827
Li Q, Bu J, Shu J, Yu Z, Tang L, Huang S, Guo T, Mo J, Luo S, Solangi GS, Hsiang T (2019) Colletotrichum species associated with mango in southern China. Sci Rep 9:18891. https://doi.org/10.1038/s41598-019-54809-4
Lima NB, Batista MVA, Morais Junior MA, Barbosa MAG, Michereff SJ, Hyde KD, Câmara MPS (2013) Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers 61:75–88
Lin Y, Wu X, Deng Z, Wang J, Zhou S, Vrijmoed LL, Jones EG (2002) The metabolites of the mangrove fungus Verruculina Enalia 2606 from a salt lake in the Bahamas. Phytochem 59(4):469–471
Liu F, Damm U, Cai L, Crous PW (2013) Species of the Colletotrichum gloeosporioides complex associated with anthracnose diseases of Proteaceae. Fungal Divers 61:89–105
Liu LP, Shu J, Zhang L, Hu R, Chen CQ, Yang LN, Lu BH, Liu YN, Yu L, Wang X, Li Y (2017) First report of post-harvest anthracnose on mango (Mangifera indica) caused by Colletotrichum siamense in China. Plant Dis 101(5):833
Manzano León AM, Serra Hernández W, García Pérez L, Crespo K, Guarnaccia V (2018) First report of leaf anthracnose caused by Colletotrichum grossum on mango (Mangifera indica) in Cuba. J Plant Pathol 100(2):329
Nelson SC (2008) Mango anthracnose (Colletotrichum gloeosporiodes). University of Hawaii, Honolulu (HI), p 9. Plant Disease PD-48
Pardo-De la Hoz CJ, Calderón C, Rincón AM, Cárdenas M, Danies G, López‐Kleine L, Restrepo S, Jiménez P (2016) Species from the Colletotrichum acutatum, Colletotrichum boninense and Colletotrichum gloeosporioides species complexes associated with tree tomato and mango crops in Colombia. Plant Pathol 65(2):227–237
Phoulivong S, Cai L, Chen H, McKenzie EHC, Abdelsalam K, Chukeatirote E, Hyde KD (2010) Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers 44:33–43
Prasad L, Javeria S, Kumar B, Sharma P (2017) First report of anthracnose of elephant foot yam caused by Colletotrichum siamense in India. New Dis Rep 36(21):2044–0588
Prihastuti H, Cai L, Chan H, McKenzie EHC, Hyde KD (2009) Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers 39:89–109
Prusky D, Keen NT, Sims JJ, Midland SL (1982) Possible involvement of an antifungal diene in the latency of Colletotrichum gloeosporioides on unripe avocado fruits. Phytopathology 72(12):1578–1582
Qin LP, Huang SL, Lin SH, Lin CH (2017) First report of anthracnose of Mangifera indica caused by Colletotrichum siamense in Sanya city in China. Plant Dis 101(6):1038
Qin LP, Zhang Y, Su Q, Chen YL, Nong Q, Xie L, Yu GM, Huang SL (2019) First report of anthracnose of Mangifera indica caused by Colletotrichum scovillei in China. Plant Dis 103(5):1043
Rattanakreetakul C, Keawmanee P, Bincader S, Mongkolporn O, Phuntumart V, Chiba S, Pongpisutta R (2023) Two newly identified Colletotrichum species associated with mango anthracnose in Central Thailand. Plants 12:1130. https://doi.org/10.3390/plants12051130
Salunkhe VN, Gawande SP, Gokte-Narkhedkar N, Nagrale DT, Hiremani NS, Waghmare VN (2020) First report of Colletotrichum siamense causing leaf anthracnose on cotton in India. Plant Dis 104(7):2025
Sangeetha CG, Rawal RD (2009) Temperature requirement of different isolates of Colletotrichum gloeosporioides isolated from mango. Am Eurasian J Sci Res 4(1):20–25
Sharma G, Kumar N, Weir BS, Hyde KD, Shenoy BD (2013) The ApMat marker can resolve Colletotrichum species: a case study with Mangifera indica. Fungal Divers 61:117–138
Sharma G, Maymon M, Freeman S (2017) Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Sci Rep 7(1):15839
Sharma G, Shenoy BD (2013) Multigene sequence-based identification of Colletotrichum Cymbidiicola, C. Karstii and C. Phyllanthi from India. Czech Mycol 65(1):79–88
Silva DN, Talhinas P, Várzea V, Cai L, Paulo OS, Batista D (2012) Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: an example from coffee (Coffea spp.) hosts. Mycologia 104:396–409
Swofford DL (2002) PAUP: phylogenetic analysis using parsimony (version 4.0 b10)
Templeton MD, Rikkerink EH, Solon SL, Crowhurst RN (1992) Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus glomerella cingulate. Gene 122(1):225–230
Than PP, Jeewon R, Hyde KD, Pongsupasamit S, Mongkolporn O, Taylor PW (2008) Characterization and pathogenicity of Colletotrichum species associated with anthracnose on Chilli (Capsicum spp.) in Thailand. Plant Pathol 57(3):562–572
Tovar-Pedraza JM, Mora-Aguilera JA, Nava-Diaz C, Lima NB, Michereff SJ, Sandoval-Islas JS, Camara MP, Téliz-Ortiz D, Leyva-Mir SG (2020) Distribution and pathogenicity of Colletotrichum species associated with mango anthracnose in Mexico. Plant Dis 104(1):137–146
Vaidya G, Lohman DJ, Meier R (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27(2):171–180
Vitale A, Alfenas AC, de Siqueira DL, Magistà D, Perrone G, Polizzi G (2020) Cultivar resistance against Colletotrichum Asianum in the world collection of mango germplasm in southeastern Brazil. Plants 9(2):182
Weir BS, Johnston PR, Damm U (2012) The Colletotrichum gloeosporioides species complex. Stud Mycol 73:115–180. https://doi.org/10.3114/sim0011
White TJ, Bruns T, Lee SJ, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 18(1):315–322
Willingham S, Cooke A, Coates L, Pegg KG (2012) Pepper spot: a new preharvest Colletotrichum disease of avocado cv. Hass Australas Plant Pathol 29:151–151. https://doi.org/10.1071/AP00025
Wu C, Chen H, Ni H (2020) Identification and characterization of Colletotrichum species associated with mango anthracnose in Taiwan. Eur J Plant Pathol 157:1–15. https://doi.org/10.1007/s10658-020-01964-4
Zhang L, Song L, Xu X, Zou X, Duan K, Gao Q (2020) Characterization and fungicide sensitivity of Colletotrichum species causing strawberry anthracnose in eastern China. Plant Dis 104(7):1960–1968
Acknowledgements
The authors extend sincere thanks to the Director, ICAR-IIHR, Bengaluru, India, for the facilities provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies involving human participants or animals (vertebrates) performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ganesan, S., Kumari, N., Sahu, S. et al. Characterization of Colletotrichum species causing new pre-harvest anthracnose symptoms on mango in Eastern India. Australasian Plant Pathol. 53, 239–252 (2024). https://doi.org/10.1007/s13313-024-00973-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-024-00973-9