Abstract
Mango is widely grown in Taiwan and anthracnose is one of the most important diseases of this crop. The aim of this study was to investigate Colletotrichum species associated with mango and the pathogenicity of these fungal species. From 2006 to 2017, mango tissue from 33 mango orchards were collected. Eighty-seven isolates associated with mango were analyzed preliminarily by comparing partial glyceraldehyde-3-phosphate dehydrogenase sequences. Four species belonging to C. gloeosporioides complex were preliminarily identified, namely C. asianum (68 isolates), C. fructicola (four isolates), C. siamense (eight isolates) and C. tropicale (two isolates). The other five isolates were identified as belonging to the C. acutatum complex. Ten isolates, belonging to different Colletotrichum species according to glyceraldehyde-3-phosphate dehydrogenase sequences prediction, were used for further morphology and multi-gene phylogenetic analysis. Five species were identified, namely C. asianum, C. fructicola, C. siamense, C. tropicale and C. scovillei. All five species showed pathogenicity on fruit, and C. asianum isolates C-1076 and C-1646 as well as C. siamense isolate C-526 caused larger lesions than the other isolates. On mango leaves, C. asianum, C. fructicola, C. siamense and C. scovillei isolates were pathogenic, while C. tropicale isolates, C-141 and C-303, failed to cause significant foliar lesions. In addition, C. siamense isolates C-526 and C-848 caused significantly larger lesions on leaves than other isolates. This study reports the identification and pathogenicity of Colletotrichum species related to mango anthracnose in Taiwan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mango (Mangifera indica) is an important fruit crop in Taiwan. According to the Agricultural Statistics Yearbook 2018 (https://agrstat.coa.gov.tw/sdweb/public/book/Book.aspx), the total planted area in Taiwan in 2018 was 16,109 ha, and 146,672 metric tons of mango fruits were produced, with the value of over 243 million US dollars. The main mango-planting areas are Tainan, Pingtung, Kaohsiung, and Chiayi Counties.
The main cultivar planted in Taiwan, Irwin, is very sensitive to anthracnose, which is caused by Colletotrichum species. The disease attacks the leaves, flowers and fruits of Irwin. On young plant tissues, small red spots appear first, and gradually the spots expand and become larger black necrotic lesions. In addition, post-harvest outbreaks of anthracnose symptoms substantially shorten the shelf life of mature mango fruits (Arauz 2000).
In the past, morphological characteristics were relied upon to identify Colletotrichum species. However, since the shapes and sizes of spores are similar among Colletotrichum species and colony diversity is observed under different growth conditions, it is hard to distinguish Colletotrichum species solely by morphology. Therefore, multiple-gene phylogenetic analysis has been introduced to separate Colletotrichum species. For example, Weir et al. (2012) used a set of nuclear gene regions, actin (ACT), calmodulin (CAL), chitin synthase (CHS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the ribosomal internal transcribed spacer (ITS) to distinguish species within the C. gloeosporioides complex. On other research, ACT, CHS, GAPDH, histone H3, ITS and beta-tubulin (TUB) were used to analyze species within the C. acutatum complex (Damm et al. 2012).
In the past, mango anthracnose was considered to be caused by C. gloeosporioides and C. acutatum (Dodd et al. 1997; Freeman et al. 1998; Jayasinghe and Fernando 2009), and these species were also reported as the causal agents of mango anthracnose in Taiwan (Ann 1995; Weng and Chuang 1995). However, with the development of phylogenetic analysis, recent studies have demonstrated that mango anthracnose is caused by multiple Colletotrichum species. For instance, in northeastern Brazil, there are five species, C. asianum, C. fructicola, C. tropicale, C. karstii and C. dianesei, responsible for mango anthracnose (Lima et al. 2013). In Guangxi, China, C. asianum, C. fructicola, C. siamense and C. scovillei were reported to cause anthracnose (Mo et al. 2018; Qin et al. 2019). In Taiwan, C. asianum, C. fructicola, C. tropicale and C. siamense have also been indicated as pathogens of mango anthracnose (Lin 2018).
Anthracnose pathogens used in this study were collected from 33 orchards located in main mango producing areas in Taiwan from 2006 to 2017. There were two main objectives in this study. The first objective was to identify the fungal species associated with mango anthracnose in Taiwan by using multi-gene phylogenetic analysis methods, and the second was to evaluate the pathogenicity of different fungal species on leaves and fruits.
Materials and methods
Fungal isolation
Mango fruits, stems and pedicels from diseased and healthy plants were collected from 33 orchards in different areas in Taiwan in 2006–2017, Chiayi, Tainan, Kaohsiung and Pingtung, and Colletotrichum species were isolated from these plant tissues. For surface disinfection, fruits with anthracnose symptoms were rinsed with 75% ethanol and air-dried. Stems and pedicels were cut into 1-cm-long fragments, soaked for 30 s in 75% ethanol and 30 s in 0.6% NaOCl, and then rinsed twice with sterilized water. After disinfection, the pieces of stem and pedicels were plated onto acidified potato dextrose agar [APDA, 300 mL potato dextrose agar (PDA) with 750 μL 50% (v/v) lactic acid] and incubated for 7 d under 25 °C. The margins of colonies were transferred to PDA. After sporulation, for each isolate, an ooze of spores was mixed with a drop of sterilized water and spread on water agar using a wire loop. A germinating spore was cut from water agar to obtain a single colony.
DNA extraction, PCR and DNA sequencing
For DNA extraction, the mycelium of each isolate was collected from the surface of PDA and ground in 0.5 N NaOH with an electronic grinder. After centrifugation, the supernatant was mixed with 0.1 M Tris buffer (pH 8.0) in a ratio of 1:9 (v/v) and used as the DNA template for PCR (White et al. 1990).
PCR amplification of the ACT, CAL, CHS, GAPDH, ITS and TUB genes was carried out using the primer pairs ACT-512F and ACT-783R (Carbone and Kohn 1999), CL1C and CL2C (Weir et al. 2012), CHS-79F and CHS-345R (Carbone and Kohn 1999), GDF and GDR (Templeton et al. 1992), ITS-1F (Gardes and Bruns 1993) and ITS-4 (White et al. 1990), and T1 (O’Donnell and Cigelnik 1997) and Bt2b (Glass and Donaldson 1995), respectively. The PCR mixtures contained 11.9 μL of double-distilled sterilized water, 4 μL of 5× Phusion HF buffer, 0.4 μL of dNTPs (2.5 mM each dNTP), 1 μL of each primer (10 μM), 0.2 μL (2 U) of Phusion High-fidelity DNA polymerase (ThermoFisher, USA), and 1.5 μL of DNA template. The reactions were performed in a ThermoFisher SensoQuest Labcycler thermal cycler. The PCR program for all genes was 98 °C for 30 s, followed by 35 cycles of 98 °C for 10 s, 52 °C for 30 s, 72 °C for 30 s, and 72 °C for 10 min. The amplification products were sent to Tri-I Biotech Incorporation Company for purification and sequencing, and the sequences for each isolate in this study were deposited in GenBank (Table 1).
Phylogenetic analysis
Bayesian inference was used to construct phylogenetic trees. The ACT, CAL, CHS, GAPDH and ITS nuclear gene regions were used in C. gloeosporioides species complex analysis, and ACT, ITS and TUB were used in C. acutatum species complex analysis. Sequences of Colletotrichum-type species from the GenBank database were included in the analysis (Table 2). Multiple sequence alignment of each gene was conducted using ClustalX v. 2.1 (Larkin et al. 2007), and these alignments were concatenated using SequenceMatrix v. 1.7.8 (Vaidya et al. 2011). jModeltest (Posada 2008) was used to choose the best-fit DNA substitution model under the Bayesian information criterion (BIC) (Table 3). Phylogenetic tree construction was conducted with MrBayes v. 3.2.6 (Ronquist et al. 2012). The analysis was run twice for 5 × 107 generations, and samples were taken from the posterior every 10,000 generations. The first 25% of generations were discarded as burn-in. Convergence of all parameters was checked using the Tracer program (Rambaut and Drummond 2007).
Morphology
For colony observation, isolates were grown on PDA (Merck, Germany) for 7 days at 25 °C under near-UV fluorescent tubes (12 h light/12 h dark). The lengths and widths of 50 conidia from each isolate grown on PDA were observed and measured. To measure the growth rate, five plates of each isolate were incubated at 25 °C in darkness, and the diameters of colonies were measured after 5 and 7 days of incubation. These values were used to calculate the growth rate.
Pathogenicity tests
Pathogenicity tests were conducted on detached young leaves and mature fruits of mango (cv. Irwin).
For the leaf inoculation assay, detached, asymptomatic young leaves were disinfected with 75% ethanol and air-dried. The inoculum was grown on water agar (WA, 2% agar) plates for 5 days in darkness. Two stab wounds were made on both sides of a leaf using a sterilized needle, and one hyphal disc 5 mm in diameter was cut from the WA plate and placed on each wound. Five leaves were inoculated for each isolate. WA discs without fungi were used as a negative control. Inoculated leaves were placed on flat shelves and incubated in sealed plastic boxes with water on the bottom at 25b°C for 5 days, and the diameters of necrotic areas were measured.
On fruits, isolates were incubated on PDA for 5 days at 25 °C, and 5-mm-diameter agar discs were cut from the margins of the colonies for inoculation. For surface disinfection, half-ripe fruits were first soaked in warm water (58 °C) for 2 min, and then soaked immediately in water at room temperature to avoid heat damage. After air-drying, fruits were sealed in paper boxes with 450 g of solid calcium carbide (CaC2) for 2 days to ripen the fruits. Fruits were rinsed with 75% ethanol and air-dried before inoculation. A stab wound was made on each fruit with a sterilized needle, and a hyphal disc was placed on the wound. Ten fruits were inoculated for each isolate, and PDA discs were used as a negative control. The inoculated fruits were sealed in plastic bags with open petri dishes contained water for 2 days. The lesion diameter was measured at 7 days post-inoculation to assess the aggressiveness of different isolates. Differences in aggressiveness caused by Colletotrichum species were examined by one-way ANOVA, and means were compared using Fisher’s LSD test. An F value with P < 0.05 was considered significant. For each isolate, the margin of the lesion was cut and incubated on APDA agar to complete Koch’s postulate.
Results
Fungal isolation
A total of 682 isolates from 560 samples were obtained. Eighty-seven isolates were chosen arbitrarily for initial analysis based on partial sequences of the GAPDH gene. By performing BLASTn searches of these sequences against the GenBank database, the 87 isolates were preliminarily classified into five Colletotrichum species. Four species belonged to the C. gloeosporioides species complex: C. asianum (68 isolates), C. fructicola (four isolates), C. siamense (eight isolates), and C. tropicale (two isolates). In addition, there were five isolates belonging to the C. acutatum species complex.
Phylogenetic analysis
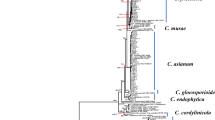
Ten isolates (two each of five different Colletotrichum species) based on GAPDH classification were chosen for multiple-gene phylogenetic analysis. For the C. gloeosporioides species complex analysis, the aligned genes boundaries in the alignment were: ACT: 1–309, CAL: 310–1107, CHS: 1108–1406, GAPDH: 1407–1715, ITS: 1716–2349. For C. acutatum species complex analysis, the aligned genes boundaries were: ACT: 1–255, ITS: 256–822, TUB: 823–1604. The classification of species belonging to the C. gloeosporioides species complex based on the multi-gene phylogenetic tree shown in Fig. 1a was consistent with that based on GAPDH sequences. C-1076 and C-1646 belonged to C. asianum, and C-517 and C-557 belonged to C. fructicola. C-141 and C-303 were identified as C. tropicale, and C-526 and C-848 were identified as C. siamense. C-212 and C-1428 were grouped into the same clade as C. scovillei in the multi-gene phylogenetic tree (Fig. 1b).
Bayesian phylogenetic trees of Colletotrichum isolates from mango in Taiwan and from GenBank. aC. goloeosporioides species complex tree was built using concatenated sequences of partial ACT, CAL, CHS, GAPDH and ITS genes. C. hippeastri and C. boninense were used as outgroups. bC. acutatum species complex tree was built using concatenated sequences of partial ACT, ITS and TUB genes. Two C. orchidophilum strains were used as outgroups. The scale bars indicate the number of expected changes per site. Isolates from this study are emphasized in bold
Morphology
Colonies of all isolates were white in color during the first two or three days of cultivation, after which some of them turned gray or salmon in color at the upper or lower surface. Colonies were mainly separated into four groups based on their appearance, but diversity in colony morphology was evident even within the same species (data not shown). Among the 10 isolates investigated, conidial sizes and shapes were similar among the four species belonging to C. gloeosporioides species complex (Fig. 2). Conidia of these species were aseptate, hyaline and cylindrical, and size ranged from 13.6 to 16.5 μm long × 5.3 to 6.0 μm wide. Conidia of C. scovillei isolates C-212 and C-1428 were hyaline, unicellular and cylindrical to slightly fusiform with both ends acute, and size ranged from 12.4 to 12.8 μm long × 4.6–4.8 μm wide.
Morphological characteristics of colonies and conidia of mango Colletotrichum isolates on PDA at 7 days after incubation. Upper (a) and reverse (b) sides of PDA plate and conidia (c) of C. asianum isolate C-1646. Upper (d) and reverse (e) sides of PDA plate and conidia (f) of C. fructicola isolate C-517. Upper (g) and reverse (h) sides of PDA plate and conidia (i) of C. siamense isolate C-848. Upper (j) and reverse (k) sides of PDA plate and conidia (l) of C. tropicale isolate C-303. Upper (m) and reverse (n) sides of PDA plate and conidia (o) of C. scovillei isolate C-1428. Bars: 10 μm
Pathogenicity test
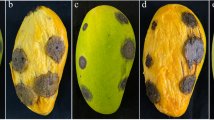
All five Colletotrichum species were pathogenic on wounded mango fruits with the level of aggressiveness varying among the 10 Colletotrichum isolates (Table 4). Typical symptoms were sunken and dark lesions expanding from the inoculation sites. The lesion diameters ranged from 4.9 mm to 21.5 mm. Colletotrichum asianum isolates C-1076 and C-1646 and C. siamense isolate C-526 caused significantly larger lesions on mango fruits than other isolates, and C. tropicale isolates C-141 and C-303 showed relatively weak ability to cause necrosis on fruits. No lesions were observed in the negative control.
On leaves, all Colletotrichum species were pathogenic except C. tropicale. Small, needle-like spots emerged on leaves near the fungal agar disc 5 days after inoculation with C. tropicale. Larger, round lesions appeared 6 days after inoculation, but C. tropicale was not isolated from the margins of the round lesions. Therefore, the pathogenicity of C. tropicale on mango leaves was uncertain. In contrast, the other four Colletotrichum species caused black lesions with clear edges on leaves 5 days post-inoculation, and pathogens were re-isolated from the edges of the lesions, completing Koch’s postulates. Agar discs used as a negative control did not cause lesions on leaves.
Discussion
This is the latest large-scale survey of diversity of Colletotrichum pathogens associated with mango anthracnose in Taiwan. A recent study by Lin (2018) also surveyed diversity of Colletotrichum pathogens associated with mango anthracnose in Taiwan; however, only the abstract of this research is accessible by internet search engines. In this study, five species of Colletotrichum (C. asianum, C. fructicola, C. scovillei, C. siamense and C. tropicale) were found to be associated with mango anthracnose disease, and C. asianum was the most commonly isolated species. This result is different from that of an earlier investigation in Taiwan, in which only two species, C. gloeosporioides (about 82%) and C. acutatum (about 18%), were determined to be the causal agents of mango anthracnose in Taiwan (Weng and Chuang 1995). However, in this study, the 87 strains analyzed were chosen arbitrarily, which raised the possibility that some Colletotrichum species are not identified.
The species mentioned in this paper were also described from other countries or areas. In Brazil, of the five species identified as being pathogens of mango anthracnose (C. asianum, C. fructicola, C. tropicale, C. karstii and C. diansei) (Lima et al. 2013), three were also found to be associated with mango anthracnose in the current study. In Guangxi, China, C. asianum, C. fructicola, C. siamense and C. scovillei are responsible for mango anthracnose disease, and the aggressiveness differed significantly among isolates of the same species (Mo et al. 2018; Qin et al. 2019). C. scovillei was reported to cause mango disease only in China and this report currently, which suggests this mango-pathogenic C. scovillei might be endemic or restricted in some areas of Asia. In India, the pathogenicity of C. asianum, C. fructicola and C. siamense on mango was documented (Sharma et al. 2013). In addition to the countries mentioned above, C. asianum, the most frequently isolated species in this paper, was also identified as a cause of mango anthracnose in South Africa, Sri Lanka and Malaysia (Krishnapillai and Wilson Wijeratnam 2014; Latiffah et al. 2015; Sharma et al. 2015). Colletotrichum tropicale, on the contrary, is much less reported, which suggests that this species is not commonly found in mango crops or less aggressive.
Wound inoculation is a common method for evaluating pathogenicity of Colletotrichum spp. causing mango anthracnose (Lima et al. 2013; Mo et al. 2018; Sharma et al. 2013). In our study, a pretest of fungal disc inoculation with no wounding was conducted on leaves; C. siamense isolate C-848 caused lesions on five of six inoculation points 7 days post inoculation, and C. asianum isolate C-1076 caused lesions on all six inoculation points. However, results from wound inoculation were more consistent than from non-wounded inoculations. In nature, wounding on plant surface caused by strong winds or insects is common, and it facilitates the invasion of pathogens. Nevertheless, removing host surface defense may lead to overestimation of pathogen aggressiveness in the field. Further studies should be conducted to determine the aggressiveness of pathogens under non-wounded condition.
All species identified in this study have all been demonstrated to cause anthracnose on other crops. For example, C. scovillei was reported as the causal agent of pepper anthracnose in Brazil and China (Caires et al. 2014; Zhao et al. 2016). C. asianum and C. fructicola were also able to cause lesions on peppers (Phoulivong et al. 2012), and. C. tropicale was pathogenic on cassava in Brazil (Oliveira et al. 2019). Phoulivong et al. (2012) found that C. siamense isolated from coffee also caused lesions on wounded papaya and orange. Giblin et al. (2018) reported that C. siamense infected both mango and avocado without wounding. In Taiwan, C. siamense has only been reported to cause pepper spot disease on lychee (Ni et al. 2017) and crown rot disease on strawberry (Chung et al. 2019). However, in other countries, C. siamense also infects banana (Kumar et al. 2017), citrus (Cheng et al. 2013), dragon fruit (Meetum et al. 2015) and pear (Fu et al. 2019). All of the fruits mentioned above are important crops in Taiwan and are affected by anthracnose, suggesting that C. siamense might be a potential causal agent of anthracnose disease on these fruits in Taiwan. Phoulivong et al. (2012) also found that C. asianum and C. fructicola were able to cause cross infection by the same isolates among different hosts.
The sensitivity of Colletotrichum species on mango to fungicide treatment has been investigated in Taiwan, and resistance has emerged in some species and areas. A previous study based on restriction fragment length polymorphism analysis indicated that fungicide resistance against benzimidazoles occurred in Colletotrichum species isolated from mango in Tainan (Lou et al. 2010). A recent study demonstrated that most Colletotrichum species on mango show resistance to thiophanate-methyl except C. tropicale (Lin 2018). In addition, our investigation of lychee anthracnose also indicated that thiophanate-methyl resistance is present in Colletotrichum species collected from post-harvest lychee fruits in Tainan, and single nucleotide polymorphisms related to fungicide resistance in these strains were confirmed (unpublished data). These results and the cross-infection ability of Colletotrichum species suggests the possibility that the hazard of fungicide resistance may shift among crops, and this should be taken into consideration by growers when choosing a fungicide for one crop when resistance has been detected in Colletotrichum species on other crops.
In conclusion, this study characterized the identity and pathogenicity of the species causing mango anthracnose in Taiwan. These results are the basis of future studies such as diversity of fungicide sensitivity or host range among these anthracnose species. The information from this and future studies will help pathologists and producers enact plant protection management programs.
References
Ann, P. J. (1995). The sexual stage (Glomerella cingulara) of Colletotrichum gloeosporioides from mango, and effect of temperature and light on its reproduction. Plant Pathology Bulletin, 4, 173–179.
Arauz, L. F. (2000). Mango anthracnose: Economic impact and current options for integrated management. Plant Disease, 84, 600–611.
Caires, N., Pinho, D., Souza, J., Silva, M., Lisboa, D., Pereira, O., & Furtado, G. (2014). First report of anthracnose on pepper fruit caused by Colletotrichum scovillei in Brazil. Plant Disease, 98, 1437. https://doi.org/10.1094/PDIS-04-14-0426-PDN.
Carbone, I., & Kohn, L. M. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia, 91, 553–556.
Cheng, B. P., Huang, Y. H., Song, X. B., Peng, A. T., Ling, J. F., & Chen, X. (2013). First report of Colletotrichum siamense causing leaf drop and fruit spot of Citrus reticulata Blanco cv. Shiyue Ju in China. Plant Diasease, 97, 1508.
Chung, P. C., Wu, H. Y., Ariyawansa, H. A., Tzean, S. S., & Chung, C. L. (2019). First report of anthracnose crown rot of strawberry caused by Colletotrichum siamense in Taiwan. Plant Disease. https://doi.org/10.1094/PDIS-12-18-2167-PDN.
Damm, U., Cannon, P. F., Woudenberg, J. H., & Crous, P. W. (2012). The Colletotrichum acutatum species complex. Studies in Mycology, 73, 37–113. https://doi.org/10.3114/sim0010.
Dodd, J. C., Prusky, D., & Jeffries, P. (1997). Fruit diseases. In R. E. Litz (Ed.), The Mango: Botany, Production and Uses (pp. 257–280). Slough: CAB international publication.
Freeman, S., Katan, T., & Shabi, E. (1998). Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Disease, 82, 596–605.
Fu, M., Crous, P. W., Bai, Q., Zhang, P. F., Xiang, J., Guo, Y. S., Zhao, F. F., Yang, M. M., Hong, N., Xu, W. X., & Wang, G. P. (2019). Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia, 42, 1–35.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes – Application to the identification of mycorrhizae and rusts. Molecular Ecology, 2, 113–118.
Giblin, F. R., Tan, Y. P., Mitchell, R., Coates, L. M., Irwin, J. A. G., & Shivas, R. G. (2018). Colletotrichum species associated with pre- and post-harvest diseases of avocado and mango in eastern Australia. Australasian Plant Pathology, 47, 269–276.
Glass, N. L., & Donaldson, G. C. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology, 61, 1323–1330.
Jayasinghe, C. K., & Fernando, T. H. P. S. (2009). First report of Colletotrichum acutatum on Mangifera indica in Sri Lanka. Ceylon Journal of Science (Biological Sciences), 38, 31–34.
Krishnapillai, N., & Wilson Wijeratnam, R. S. (2014). First report of Colletotrichum asianum causing anthracnose on Willard mangoes in Sri Lanka. New Disease Reports, 29, 1.
Kumar, V. S., Nair, B. A., Nair, P. V. R., Annamalai, A., Jaishanker, R., Umamaheswaran, K., Sooraj, N. P., & Peethambaran, C. K. (2017). First report of Colletotrichum siamense causing anthracnose of cliff banana in India. Plant Disease, 101, 390.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., & McGettigan, P. A. (2007). Clustal W and Clustal X v. 2.0. Bioinformatics, 23, 2947–2948.
Latiffah, Z., Nurul, Z. J., Suzianti, I. V., & Intan, S. M. A. (2015). Molecular characterization of Colletotrichum isolates associated with anthracnose of mango fruit. Sains Malaysiana, 44, 651–656.
Lima, N. B., de A. batista, M. V., De Morais, M. A., Barbosa, M. A. G., Michereff, S. J., Hyde, K. D., & Câmara, M. P. S. (2013). Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Diversity, 61(1), 75–88.
Lin, W. L. (2018). Phylogenetic species and biological characteristics of mango anthracnose pathogens in Taiwan. M.S. thesis. National Chung Hsing University.
Lou, P. S., Huang, Y. J., Chung, W. C., Cheng, A. S., & Chung, W. H. (2010). Application of PCR-RFLP in detecting benzimidazoles-resistant isolates of Colletotrichum gloeoeporioides from mango in Tainan area. Plant Pathology Bulletin, 19, 255–260.
Meetum, P., Leksomboon, C., & Kanjanamaneesathian, M. (2015). First report of Colletotrichum aenigma and C. siamense, the causal agents of anthracnose disease of dragon fruit in Thailand. Journal of Plant Pathology, 97, 402.
Mo, J., Zhao, G., Li, Q., Solangi, G. S., Tang, L., Guo, T., Huang, S., & Hsiang, T. (2018). Identification and characterization of Colletotrichum species associated with mango anthracnose in Guangxi, China. Plant Disease, 102, 1283–1289.
Ni, H. F., Huang, C. W., Wu, C. J., Yang, H. R., Lin, C. Y., Chang, J. Y., & Chang, J. W. (2017). First report of pepper spot disease of lychee caused by Colletotrichum siamense in Taiwan. Journal of Plant Pathology, 99, 808.
O’Donnell, K., & Cigelnik, E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution, 7, 103–116.
Oliveira, S. A. S., Bragança, C. A. D., & Silva, L. L. (2019). First report of Colletotrichum tropicale causing anthracnose on the wild cassava species Manihot dichotoma and M. epruinosa in Brazil. Plant Disease, 100, 2171.
Phoulivong, S., Mckenzie, E., & Hyde, K. D. (2012). Cross infection of Colletotrichum species: A case study with tropical fruits. Current Research in Environmental & Applied Mycology, 2, 99–11.
Posada, D. (2008). jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution, 25, 1253–1256.
Qin, L. P., Yu, G. M., Zhang, Y., Su, Q., Nong, Q., Huang, S. L., & Xie, L. (2019). First report of anthracnose of Mangifera indica caused by Colletotrichum scovillei in China. Plant Disease. https://doi.org/10.1094/PDIS-11-18-1980-PDN.
Rambaut, A. & Drummond, A. J. (2007). Tracer v. 1.4, Available from http://beast.bio.ed.ac.uk/Tracer
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D., & Darling, A. (2012). MrBayes v. 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Sharma, G., Kumar, N., Weir, B. S., Hyde, K. D., & Shenoy, B. D. (2013). The ApMat marker can resolve Colletotrichum species: A case study with Mangifera indica. Fungal Diversity, 61(1), 117–138.
Sharma, G., Gryzenhout, M., Hyde, K. D., Pinnaka, K., & Shenoy, D. (2015). First report of Colletotrichum asianum causing mango anthracnose in South Africa. Plant Disease, 99, 725.
Templeton, M. D., Rikkerink, E. H. A., Solon, S. L., & Crowhurst, R. N. (1992). Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene, 122, 225–230.
Vaidya, G., Lohman, D. J., & Meier, R. (2011). SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics, 27, 171–180.
Weir, B. S., Johnston, P. R., & Damm, U. (2012). The Colletotrichum gloeosporioides species complex. Studies in Mycology, 73, 115–180. https://doi.org/10.3114/sim0011.
Weng, F. Y., & Chuang, T. Y. (1995). Grouping of mango anthracnose fungus in Taiwan. Plant Protection Bulletin, 37, 295–309.
White, T. J., Bruns, T., Lee, S., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR Protocols: A Guide to Methods and Applications (pp. 315–322). New York: Academic Press.
Zhao, W., Wang, T., Chen, Q. Q., Chi, Y. K., Swe, T. M., & Qi, R. D. (2016). First report of Colletotrichum scovillei causing anthracnose fruit rot on pepper in Anhui Province, China. Plant Disease, 100, 2168.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no potential conflicts of interest.
This research is not involving human participants and/or animals
Therefore, there is no informed consent needed.
Rights and permissions
About this article
Cite this article
Wu, CJ., Chen, HK. & Ni, HF. Identification and characterization of Colletotrichum species associated with mango anthracnose in Taiwan. Eur J Plant Pathol 157, 1–15 (2020). https://doi.org/10.1007/s10658-020-01964-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-01964-4