Abstract
Background
WS24-3A is a newly bred non-heading Chinese cabbage genic male-sterile line, in which sterility is controlled by a recessive gene, designated as Bra2ms. WS24-3A has been used for hybrid breeding.
Objective
To reveal the underlying molecular mechanisms responsible for the sterility of WS24-3A.
Methods
Cytological observation of the process of sterile/fertile anther development was performed to determine the tissue and stage in which sterility occurs. Phenotyping and transcriptomic analyses were performed to identify differentially expressed genes (DEGs) between sterile and fertile flower buds at different stages.
Results
Cytological analysis revealed no tetrads at stage 7 or at later stages of anther development, and the degradation of callose was delayed. Abnormal meiocytes were surrounded by sustaining callose that degenerated gradually in WS24-3A. Comparative transcript profiling identified 3282 DEGs during three anther developmental stages, namely, pre-meiotic anther, meiotic anther, and anthers with single-celled pollen stage. The difference in DEG percentage between up-regulated and down-regulated at meiotic anther stage was obviously larger than at the other two stages; further, most DEGs are important for male meiosis, callose synthesis and dissolution, and tapetum development. Ten DEGs were found to be involved in anther and pollen development, which were analyzed by quantitative PCR.
Conclusion
Bra2ms affected gene expression in meiocytes and associated with callose synthesis, degradation and tapetum development. Our results provide clues to elucidate the molecular mechanism of genic male sterility in non-heading Chinese cabbage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In nature, male sterility is a common phenomenon in flowering plants and an effective strategy for the utilization of crop heterosis. In many crops, F1 hybrids perform and yield better than their parents (Fu et al. 2014). For this purpose, genic male sterility (GMS) and cytoplasmic male sterility (CMS) have been applied for extensive production of hybrid seeds, as these traits produce seeds of high purity at low cost without intensive labor (Akter et al. 2016; Bohra et al. 2016). The whole process of anther development has been divided into 14 stages, including anther morphogenesis, microsporocyte division and differentiation, tetrad formation, callose and tapetum degradation, pollen grain development and maturation, anther dehiscence, and pollen grains release (Sanders et al. 1999). Anther development initiates with the differentiation of three cell layers, namely, L1, L2, and L3 at stage 1. The three cell layers gradually differentiate into four somatic cell layers, from the surface to the interior, namely epidermis, endothecium, middle layer, and tapetum. Reproductive cells and microspore mother cells develop between stages 2 and 5. Subsequently, microspore mother cells undergo meiosis during stage 6 and generate tetrads of haploid microspores at stage 7. Microspores are released from the tetrads at stage 8 and develop into mature pollen grains with three nucleus between stages 9 and 12 (Sanders et al. 1999). As the innermost somatic cell layer, tapetal cell differentiation and subsequent degradation coincide very well with the development of reproductive cells, and provide various nutrients for microspores, such as enzymes, lipids, and other molecules that are vital for normal microsporogenesis (Zhang and Yang 2014). Tapetal cells are formed at stage 5, they enter into the secretory phase at stage 6, function efficiently at stages 7–9, initiate degeneration via programmed cell death (PCD) at stage 10, and disappear after stage 11 (Sanders et al. 1999). Eventually, tapetal cell remnants are integrated into the pollen wall (Parish and Li 2010).
Anther development is controlled by a complex gene regulatory network that requires the coordinated activity of different tissues and cells derived from both sporophyte and gametophyte (Chang et al. 2011; Feng and Dickinson 2010; Hafidh et al. 2016; Ma 2006, 2013; Zhao 2009; Zhu et al. 2011). In Arabidopsis thaliana, there are approximately 3500 genes specifically expressed during anther development (Goldberg et al. 1993; Scott et al. 2004); additionally, several key genes controlling the differentiation and function of tapetal cells and meiosis of meiocytes have been cloned and elaborated (Cui et al. 2016; Glover et al. 1998; Hird et al. 1993; Ito et al. 2004; Lu et al. 2014; Schiefthaler et al. 1999; Yang et al. 2003b; Zhu et al. 2008, 2015). SPOROCYTELESS/NOZZLE(SPL/NZZ) encoding a putative transcription factor plays a central role in specification of both reproductive cells and somatic cells in early anther development (Schiefthaler et al. 1999). The endothecium, middle layer, tapetum, and meiocytes were absent in spl/nzz mutant anthers. DYSFUNCTIONAL TAPETUM 1 (DYT1) encoding a putative bHLH transcription factor acts downstream of SPL and upstream of Defective in Tapetal Development and Function 1 (TDF1), a transcription factor essential for tapetum development. DYT1, which preferentially expressed in tapetal cells and at a lower level in meiocytes, is a crucial component of a genetic network that regulates tapetum development. The dyt1 mutant showed premature vacuolation of the tapetum and often failed to complete meiotic cytokinesis (Cui et al. 2016; Feng et al. 2012; Li et al. 2017; Zhang et al. 2006). TDF1 encoding a putative R2R3 MYB transcription factor plays a vital role in tapetal cell differentiation and function, and preferentially expresses in both tapetal cells and meiocytes between stage 5 and stage 7. Tapetal cells were unable to change into the secretory tapetum and the tetrads failed to be released into the locule in tdf1 mutant (Zhu et al. 2008). DUET (also known as MALE MEIOCYTE DEATH 1, MMD1) encoding a PHD-finger protein, is expressed preferentially in meiocytes at stage 6 and is required for meiotic chromosome organization and progression during meiosis of meiocytes. The mmd1 mutation disrupted male meiosis and triggered cell death in male meiocytes at meiotic prophase I, finally no tetrads were produced in the mmd1 mutant (Reddy et al. 2003; Wang et al. 2016b; Yang et al. 2003b). As a direct target of DUET, TDM1 encodes a tetratricopeptide repeat protein required for determining the number of meiotic divisions. The tdm1 mutant produced polyads or dyads of spores, depending on the mutation site (Andreuzza et al. 2015; Cifuentes et al. 2016; Glover et al. 1998).
In developing anthers of angiosperms, callose is a linear β-1, 3-glucan molecule. A specialized temporary cell wall consisting of callose was synthesized between the primary cell wall and the plasma membrane in microsporocytes. CALS5 was the most important gene responsible for callose deposition surrounding meiocytes (Dong et al. 2005). A6 is a significant component of the callose mixture secreted by the tapetum. CDM1, encoding a CCCH-type zinc finger protein, regulates callose metabolism in male meiocytes during micriosporogenesis and the cdm1 mutation affects the expression of CALS5 and A6 (Lu et al. 2014). The C3Hs are a large family of zinc finger TFs that may function in RNA processing by binding RNA (Wang et al. 2008).
Non-heading Chinese cabbage (NHCC; Brassica rapa ssp. chinensis, 2n = 2x = 20) is one of the most economically important green leafy-vegetables worldwide, especially in China, with significant economic value. Non-heading Chinese cabbage is a typical cross-pollinated crop with obvious heterosis. In recent years, GMS has been used for breeding of non-heading Chinese cabbage. However, the underlying molecular mechanism for GMS in this species remains unclear. Recently, as the whole genome of Brassica rapa was sequenced and annotated (Wang et al. 2011), RNA-seq was successfully used to identify differentially expressed genes (DEGs) between fertile and sterile buds/anthers in Brassica rapa, which allowed us to gain an insight into the mechanism of sterility (Chang et al. 2016; Chen et al. 2018). In a previous study, we generated the genic male-sterility line WS24-3A of non-heading Chinese cabbage and its maintainer line, WS24-3B. Male sterility of WS24-3A was found to be controlled by one recessive gene locus designated as Bra2ms (Li et al. 2016). In this study, we performed comparative transcript profiling and microsection observation of fertile and sterile buds using the WS24-3A/B lines. The results of this work will contribute to our understanding of the mechanism underlying male sterility in this line and provide helpful information for further gene cloning of Bra2ms in WS24-3A.
Materials and methods
Plant materials
WS24-3A is a newly reported genic male-sterile line, whose sterility is controlled by one recessive gene locus designated as Bra2ms (Li et al. 2016). For this study, we used the recessive genic male-sterile (RGMS) two-type line, WS24-3AB (Bra2msBra2ms/Bra2MsBra2ms), which was sib-mated for five generations. The line was sown and grown at Huazhong Agriculture University (Wuhan, Hubei Province, China). After flowering, male fertile plants, WS24-3B (Bra2MsBra2ms), and male sterile plants, WS24-3A (Bra2msBra2ms), were easily distinguished from one to another (Fig. S1) and flower buds were stripped from at least 10 different plants for transcriptomic analysis. Buds were separately divided into three pools for sterile and fertile plants according to developmental stage (Table 1), snap-frozen in liquid nitrogen, and kept at − 80 °C for total RNA extraction.
Semi-thin sections and light microscopy
Sterile and fertile flower buds were harvested and directly fixed in FAA (10 ml formalin, 3 ml acetic acid, and 87 ml of 50% ethanol) for 24 h at the initial stages of flower development. Then, samples were dehydrated through a series of graded ethanol solutions (50%, 70%, 90%, and 100%), and embedded in resin using a Technovit Embedding Kit (Germany). Semi-thin (2.0 μm) sections were obtained using an automatic microtome (Microm HM 360, Thermo Scientific). Selected sections were stained with 0.1% toluidine blue (Sigma-Aldrich) for 10 s at room temperature and observed with a Nikon Eclipse 80i microscope (Nikon, Japan). Images of the anthers at different developmental stages were captured with a Nikon DS-Ri1 camera (Nikon, Japan). For observation of fluorescence expression of callose, sections were stained with aniline blue and micrographs were taken using a fluorescence microscope (Nikon Eclipse 80i) with the appropriate filter under ultraviolet light.
RNA Isolation, cDNA library construction and illumina sequencing
Total RNA was extracted with the TRIzol kit (Invitrogen, USA) according to manufacturer instructions and purified using an mRNA purification kit (Promega, China) following the protocol suggested by the manufacturer. mRNA was reverse transcribed by Powderscript™ II (Takara, China). Double-stranded cDNA was amplified using a random hexamer, DNA polymerase I and RNase H, and purified using a DNA purification kit (QIAGEN, Germany). Finally, cDNA libraries were prepared from a 300–500 bp size-selected fraction following agarose gel separation and adapter ligation for final Illumina sequencing. The libraries were sequenced using a paired-end read protocol with 125 bp of data collected per run on the Illumina Hiseq 2000 platform.
Analysis of illumina sequencing results
Clean reads were obtained from raw data by filtering adaptor sequences and low-quality sequences as previously described (Wang et al. 2016a). Then, sequence reads were aligned to the reference genome (http://brassicadb.org/brad/) using Tophat (Trapnell et al. 2010). Following alignments, the number of reads mapped to each Brassica rapa gene model was derived and normalized by the FPKM (fragments per kilobase of exon per million fragments mapped) method using Cufflinks (Trapnell et al. 2012).
Identification of differentially expressed genes (DEGs)
Differentially expressed genes between male fertile and male sterile flower buds at different developmental stages were identified with the DESeq package (Wang et al. 2010). A combination of FDR (false discovery rate) value ≤ 0.01 and the absolute value of log2 (fold-change) ≥ 1 were used as threshold to estimate the significance of the difference in gene expression. The orthologs of these DEGs in Arabidopsis thaliana were retrieved from BRAD (http://brassicadb.org/brad/). After data normalization, hierarchical clustering analysis of the expression patterns was performed by MeV software (http://www.tm4.org/mev.html).
GO and KEGG enrichment analysis
To identify putative biological functions and pathways of identified DEGs, Blast2Go (Gotz et al. 2008) and KOBAS (Xie et al. 2011) (http://kobas.cbi.pku.edu.cn/home.do) were used to perform GO and KEGG annotation and enrichment, respectively. WEGO (http://wego.genomics.org.cn/) was used to plot GO annotation (Ye et al. 2006). GO functional enrichment and KEGG pathway enrichment analysis were tested at a significance cutoff of 0.05 FDR.
Real-time PCR (qRT-PCR) validation
For real time quantitative RT-PCR, 1 μg total RNA was used to synthesize the first-strand cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). Gene-specific primers were designed based on the selected DEGs sequences. Reactions were performed with the SYBR Green Realtime PCR Master Mix (TOYOBO, Japan) in a Bio-Rad CFX96 instrument. The RT-PCRs were carried out with following protocol: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 60 s. Three biological replicates for each sample and three technical replicates for each biological replicate were analyzed. Significant differences of gene expression level between male fertile and sterile flower buds were evaluated using a Student’s t-test. BrActin7 (XM_009127097) was used as internal control to normalize gene expression.
Availability of supporting data
Supporting data sets are included in the Supplementary Online Materials. Sequencing raw data have been deposited in an SRA database at the NCBI under accession number SRP095649.
Results
Phenotypic characteristics of fertile and sterile flower buds
No significant differences were observed between fertile and sterile buds at the initial stage of flower bud development. Subsequently, anthers and filaments of sterile buds showed growth arrest. Finally, sterile anthers produced no mature pollen and the filaments were markedly shorter than those in fertile flowers, although the pistil of sterile flowers appeared normal (Fig. S1).
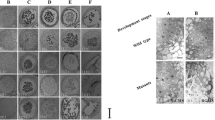
To accurately determine the tissue and stage in which sterility occurs, the process of sterile/fertile anther development was observed by cytological microscopic techniques. As shown in Fig. 1, there was no obvious difference between sterile and fertile anthers at stage 5 of development, at which, microspore mother cells (MMCs) developed normally in both fertile and sterile anthers, surrounded by the tapetum, middle layer, endothecium, and epidermis from the innermost to the outermost layer (Fig. 1a, g). However, entering the meiotic period (stage 6), MMCs in sterile anthers exhibited larger vacuoles and less concentrated nuclei, with less staining than those of fertile anthers (Fig. 1b, h). Tetrads formed in fertile anther at the end of meiosis (stage 7); tapetal cells walls degraded; tapetal cells shrank and were stained deeply (Fig. 1c). Then, with the gradual degradation of callose surrounding the tetrads, separated microspores were released into the locules and further developed into mature pollen grains at stage 11 in fertile anthers (Fig. 1d, e, f). In contrast, no tetrads were observed in sterile anther at that stage or later. This indicated that MMCs were unable to complete meiosis (Fig. 1i, j), with tapetal cell walls remaining intact and visible and tapetal cells containing more vacuoles with less staining at stage 7 (Fig. 1i), which means that tapetal cells failed to transform into secretory cells. Subsequently, these abnormal meiocytes surrounded by callose degenerated gradually, and finally no mature pollen was produced (Fig. 1j, k).
Pollen development in fertile (a–f) and sterile (g–l) Chinese cabbage anthers. Stage 5 (a, g), stage 6 (b, h), stage 7 (c, i), stage 8 (d, j), stage 9 (e, k) and stage 11 (f, l). DM degenerating meiocytes, E epidermis, En endothecium, MC meiotic cell, ML middle layer, MMC microspore mother cell, Msp microspore, PG pollen grain, T tapetum, Tds tetrads. Scale bar 50 μm
In order to further understand the abnormalities of callose dissolution in sterile anthers, fertile and sterile anther sections from different developmental stages were stained with aniline blue and observed under ultraviolet light. At meiosis (stage 6) callose fluorescence was observed in both locules of fertile and sterile anthers (Fig. 2a, e), and the fluorescence signal became clearest at the tetrad stage (Fig. 2b, f), after which, the signal remained to be observed in sterile anthers (Fig. 2g, h), but not in fertile anthers (Fig. 2c, d). These results indicated that degradation of callose was delayed in sterile anthers.
cDNA library construction and overview of RNA sequencing
According to the morphological features of fertile anthers and the corresponding floral bud size, fertile and sterile buds were divided separately into three distinct stages, pre-meiotic anther (PMA), meiotic anther (MA) and anthers with single-celled pollen (SCP). S1, S2 and S3 are samples of stages PMA, MA and SCP for sterile buds, while F1, F2 and F3 are samples of the corresponding stage in fertile buds, respectively (Table 1).
At each stage, fertile and sterile buds were further divided separately into two pools as two biological repeats for constructing cDNA libraries. In all, 12 libraries of fertile and sterile buds were created for RNA-seq. After quality control, approximately 435 million paired-end reads were obtained (Table 2). Of the total clean reads, an average of 75.02% of these reads were mapped to the reference B. rapa genome by Tophat, and a total 41020 predicted genes were annotated.
Global analysis and functional classification of DEGs
One of the basic goals of transcriptome study was to perform a global comparative analysis to obtain DEGs between sterile and fertile flower buds at different stages. In this study, 3282 genes (|log2 (fold-change)| ≥ 1, FDR ≤ 0.001) exhibited expression changes during the three anther developmental stages, PMA, MA and SCP, in sterile compared with fertile buds (Table S1). Among these genes, 1625 were up-regulated and 2055 were down-regulated during the three developmental stages, including 199 genes that showed regulated fluctuations across different stages. There were 25 and 26 DEGs up- and down- regulated, respectively, in all three stages. Moreover, comparative analysis of the number of stage-specific genes, between up- and down- regulated genes clusters, showed that the number of MA-specific genes in the down-regulated gene cluster (463) was significantly larger than the number in up-regulated gene cluster (144). However, there were no such obvious difference observed at the other two stages (Fig. 3a). Statistics of DEGs at different stages revealed that the number of DEGs increased with the anther development. There were 227 DEGs (119 up-regulated and 98 down-regulated), 908 DEGs (282 up-regulated and 626 down-regulated) and 2555 DEGs (1224 up-regulated and 1331 down-regulated) at PMA, MA and SCP stages, respectively. The difference in DEG percentage between up-regulated (282, 31%) and down-regulated (626, 69%) at MA stage was obviously larger than those at the other two stages (Fig. 3b). The heat-map of hierarchical cluster of 3282 DEGs was shown in Fig. 4. The correlations of different samples showed that S1 and F1, S2 and S3 had a closer relationship and F3 was most distant from other samples (Fig. 4). Among these 3282 DEGs, 2522 had corresponding orthologous genes in Arabidopsis thaliana according to the retrieval from BRAD (http://brassicadb.org/brad/) (Table S1).
Hierarchical clustering analysis of differentially expressed genes (DEGs) based on RPKM data. F1, F2 and F3 are samples of anther developmental stage PMA (pre-meiotic anther), MA (meiotic anther), and SCP (anthers with single celled pollen), respectively, for fertile buds, and S1, S2, and S3 are samples of the corresponding stages in sterile buds, respectively. The color key represents RPKM (reads per kilobase per million mapped reads) normalized log2 transformed counts. Red represents high expression and green represents low expression. Each row represents a gene (color figure online)
To explore the information of functional annotation, all DEGs were aligned against the Gene Ontology (GO) (Ashburner et al. 2000) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al. 2008) database. For GO analysis, all DEGs were annotated into three major categories: Cell Component (CC), Molecular Function (MF) and Biological Process (BP), which were further classified into 45 GO subcategories (Fig. 5). The GO patterns of up- and down- regulated genes were very similar, except that the GO: 0015457 (auxiliary transport protein) only occurred in the up-regulated genes and the GO: 0001906 (cell killing) only occurred in the down-regulated genes. KEGG analysis showed that 95 and 117 categories were involved in up- and down- regulated genes, respectively, including metabolic pathways, biosynthesis of secondary metabolites, starch and sucrose metabolism and others. The top 10 KEGG pathway categories in either up- or down- regulated genes (Fig. 6).
GO analysis of differentially expressed genes. The x-axis indicates the sub-categories forming three main categories: cellular component, molecular function and biological process; the right y-axis indicates the number of DEGs in a sub-category and the left y-axis indicates the percentage of a specific category of genes in that main category
Expression analysis of meiosis-related genes preferentially expressed in meiocytes
The observation of microsections showed that no tetrads were formed in sterile anthers at stage 7 (Fig. 1i). This suggested that sterility of WS24-3A might be linked with differential expression of meiosis-related genes, which are preferentially expressed in meiocytes. In Arabidopsis, 296 potential meiosis-related genes were identified by comparing meiocyte transcriptomes to those obtained from root tips and leaves, and further crossing with the set of down-regulated genes in spl mutant anthers (Libeau et al. 2011). Of these, 88 genes corresponding to 112 orthologous genes in Brassica rapa exhibited differential expression during the three anther developmental stages in sterile anther (Table S2). Among these genes, 20 and 2 were up-regulated and down-regulated at the PMA stage, respectively. In contrast, 43 genes were down-regulated, whereas only 1 gene was up-regulated at MA stage in sterile flower buds. At SCP stage, 13 and 48 genes were up-regulated and down-regulated in sterile flower buds, respectively. There was a marked enrichment of meiosis-related genes among down-regulated genes during MA and SCP stages (p(Χ2) < 0.001). Furthermore, of the 43 down-regulated genes at the MA stage (Table 3), three genes were identified, whose orthologous genes were required for meiosis in Arabidopsis. They were Bra004222, Bra035777, and Bra039753, which were orthologous with ASY1 (Wei and Zhang 2010), MSH5 (Higgins et al. 2008) and DUET/MMD1(Andreuzza et al. 2015; Reddy et al. 2003; Wang et al. 2016b; Yang et al. 2003b), respectively. In particular, DUET/MMD1 encoding a PHD finger protein is required for cell cycle transition and meiotic chromosome condensation during male meiosis (Andreuzza et al. 2015; Wang et al. 2016b). Meanwhile, Bra038794, orthologous with TDM1 which is a direct target of DUET/MMD1, also exhibited down regulation at the MA stage (Table S1).
Expression analysis of genes for callose synthesis and dissolution
Callose is a linear β-1, 3-glucan molecule that plays significant roles in anther development. In this study, 17 genes related to callose synthesis were identified, among which, two were found related with callose synthesis and 15 with callose dissolution, which exhibited differential expression during the three stages of development under study in sterile anther (Table 4). Bra028343, homologous with CALS5, was the most important gene responsible for callose deposition surrounding meiocytes (Dong et al. 2005); the gene was significantly down-regulated in sterile flowers at both MAT and SCP stages. There were two, three, and eight genes involved in callose dissolution that were down-regulated at PAM, MAT, and SCP stage, respectively. Most noticeably of all, A6, whose expression level is a significant component of the callose mixture secreted by the tapetum, has 3 orthologous genes in B. rapa, Bra032758, Bra037057, and Bra038969. These 3 BrA6 genes were all down-regulated in sterile flower buds at the SCP stage. In addition, we observed that Bra004288, orthologous with CDM1, showed a 35-fold transcript decline in sterile, compared with fertile flower buds at the MAT stage (Table S1).
Expression analysis of transcription factors (TF)
Transcriptional regulation is a major mechanism controlling differentiation of meiocytes and neighboring somatic tissues including tapetum during anther development (Ma 2006; Zhu et al. 2011).The list of B. rapa TF-genes, including 2502 putative TF genes classified into 57 TF families, were obtained from the Plant and Transcription Factor Database (http://planttfdb.cbi.pku.edu.cn/index.php). Based on RPKM, 252 genes of the 2502 putative TFs, accounting for 10.1% of total TF genes, showed significant differential expression in sterile buds compared with fertile buds during the three stages of anther development, PAM, MAT, and SCP (Table S2). The 252 TF genes came from 38 TF families and the top five TF families were NAC (43), MYB (22), ERF (21), bHLH (18), and C2H2 (18). Of all 252 TFs, there were 220, 28, and 4 accounting for 87.3%, 11.1%, and 1.6%, showing differential expression at one, two, and three anther developmental stage, respectively. Bra023927, the only EIL TF among 252 TFs, was up-regulated during all three stages. Interestingly, MYB-related TFs, Bra004503, Bra030496, and Bra033291 were all down-regulated during all three stages. At MAT stage, 4 MYB TFs, Bra005597, Bra012337, Bra026281, and Bra037828 were all up-regulated. In addition, Bra013519 and Bra025337, orthologous with DYT1, encoding bHLH TF, and TDF1, encoding a putative R2R3 MYB TF, were down-regulated at the MAT stage in sterile buds. Concomitantly, all 7 C3H TFs were down-regulated, including the three genes, Bra004288, Bra030148, and Bra038204, at the MAT stage and 4 genes, Bra006465, Bra020750, Bra025776, and Bra040220, at the SCP stage.
Validation of transcriptomics data by quantitative Real-Time PCR
To verify the reliability of our transcriptome sequencing results, expression analysis of ten sterility-related genes selected randomly from the transcriptome was performed by qPCR (Fig. 7). These ten genes were down-regulated in sterile line of WS24-3A. Two genes of Bra033736 and Bra007457 were down-regualted in sterile line at MAT stage. Four genes of Bra007577, Bra002401, Bra038969 and Bra027246 were down-regualted in sterile line at SCP stage. Bra028343, Bra034793 and Bra013041 were lowly expressed in WS24-3A at PMA stage and SCP stage. Bra028969 was further validated for all the three stages. The expression levels of the ten genes displayed the same trend as the transcriptome sequencing results. These consequences ensured the high reliability of the RNA-seq data obtained in the present study.
Discussion
In this study, abnormal development and differentiation of both meiocyte and tapetal cells were observed in sterile anthers of WS24-3A (Figs. 1, 2). No tetrads were produced during or after stage 7 (Fig. 1i, j, l), and tapetal cells failed to convert to secretory cells, thus leading to delayed degradation of the callose wall in anthers of WS24-3A (Fig. 2g, h). These abnormalities differed from the defective phenotype of reported recessive genic-sterile lines in Brassica crops (Dun et al. 2011; Yi et al. 2010; Zhou et al. 2012). S45A and 7365A were both recessive genic-sterile lines of Brassica napus. Meiocytes of male sterile mutant S45A could complete meiosis to produce tetrads at stage 7, but the pattern of apoptosis in the tapetum was seriously defective, resulting in no pollen exine formation (Yi et al. 2010). Meiocytes of male sterile mutant 7365A completed meiosis to produce tetrads at stage 7, but tapetal cells exhibited abnormal enlargement at anther stage 6 and lost their secretory function during and after stage 7, resulting in abnormal microspore release (Dun et al. 2011; Zhou et al. 2012). Interestingly, a dominant genic-sterile line, FM195A, exhibited arrested meiotic chromosome during meiosis, and failed to produce tetrads. However, the dominant genic-sterile gene encoding FM195A, MS5b, also affected the process of megasporogenesis, thus resulting in severely reduced female fertility, which was remarkably different from WS24-3A (Xin et al. 2016). No significant alterations were observed in the remaining three somatic layers before, during, or after stage 7 in WS24-3A. However, we cannot exclude the possibility that subtle alterations might have been presented or passed undetected. We also observed that the filaments were remarkably shorter in sterile flowers than those in fertile flowers (Fig. S1). This finding suggests that the sterile gene of WS24-3A, Bra2ms, might also play a minor role in flower development.
To obtain helpful information for further cloning of Bra2ms, RNA-seq was employed to perform a global comparative analysis between sterile and fertile flower buds in three stages of anther development, PMA, MA, and SCP. In sterile buds, 3282 DEGs, 2055 down-regulated and 1625 up-regulated, were identified during the three stages of anther development (Table S1). Based on our results of microsectioning, as no tetrads were observed, we focused on DEGs at the MA stage. There were 626 down-regulated DEGs, accounting for 69% of a total of 908 DEGs, whereas only 282 up-regulated DEGs were detected. Among the 626 down-regulated DEGs, we underlined genes required for male meiosis, callose synthesis and dissolution, and tapetum development, according to the annotation of orthologous genes in Arabidopsis thaliana. There were 44 DEGs, corresponding to 38 Arabidopsis genes (Table 3), accounting for 12.8% of a subset of genes preferentially expressed in meiocytes, including three genes, ASY1 (Wei and Zhang 2010), MSH5 (Higgins et al. 2008) and DUET/MMD1 (Andreuzza et al. 2015; Wang et al. 2016b), that proved necessary for male meiosis (Libeau et al. 2011). These results were consistent with the observations of microsections, indicating that Bra2MS might function upstream of the male meiosis-related genes in meiocytes at stage 6, and play an important role in meiosis of meiocytes.
The orthologous genes of Brassica rapa functioning at an early stage and determining tapetal formation, such as SPL/NZZ (Schiefthaler et al. 1999), BAM1 (DeYoung et al. 2006), BAM2 (Hord et al. 2006), EMS1/EXS (Canales et al. 2002), SERK1 (Albrecht et al. 2005), SERK2 (Colcombet et al. 2005), and TPD1 (Cifuentes et al. 2016; Huang et al. 2016; Yang et al. 2003a) did not show differential expression (Table S1), which indicated that the early formation and differentiation of tapetal cells were normal in anthers of WS24-3A. Significantly, Bra013519 and Bra025337, orthologous with DYT1, were both down-regulated at the MA stage. DYT1 acts downstream of SPL and upstream of TDF1, strongly expressed in the tapetum from late stage 5 to early stage 6, and a crucial component of a genetic network that regulates tapetum development. Meiocytes of mutant dyt1 often failed to complete meiotic cytokinesis at stage 7 and the tapetum and middle layer cells gradually formed excess vacuolization during and after stage 6, which were similar and different with those in WS24-3A. We also found 17 related genes involved in callose synthesis and dissolution, including the orthologous genes of CALS5 (Dong et al. 2005), A6 (Hird et al. 1993), and CDM1 (Lu et al. 2014), which showed differential expression during the three stages of anther development studied. Especially, Bra004288, orthologous with CDM1 acting upstream of CALS5 and A6, showed a 35-fold transcript decline in sterile anthers at the MAT stage (Table S1). Together, these results indicate that the early formation and differentiation of tapetal cells were normal before late stage 5, but further development of tapetum in WS24-3A was defective at and after stage 6. Defective tapetum development mainly reflected as a delay of the dissolution of the callose wall surrounding the miocyte after stage 7 (Fig. 2), which indicated that the tapetal cells failed to convert into secretory cells.
The mechanism controlling the specification and development of the tapetum and meiocytes within the anthers of WS24-3A remains a mystery. Although defective in tapetum development and meiocyte meiosis, dyt1 and duet1/mmd1, both failed to produce tetrads, and the somatic cell layers were less affected between anther stage 6 and 10 in the duet1/mmd1mutant, which resembled the mutant phenotype of WS24-3A more closely.
Conclusion
Comparative transcript profiling revealed 3282 DEGs, 2055 down-regulated and 1625 up-regulated, during three stages of anther developmental: pre-meiotic anther, meiotic anther, and anthers with single-celled pollen. At the meiotic anther stage, 626 DEGs were down-regulated, accounting for 69% of DEGs, whereas only 282 were up-regulated at this stage. Furthermore, we identified a series of genes and transcription factors required for male meiosis, callose synthesis and dissolution, and tapetum development among down-regulated DEGs, which included three meiosis-related genes, ASY1, MSH5, and DUET/MMD1; three callose metabolism related genes, CALS5, A6, and CDM1; and two TFs necessary for early tapetum development, DYT1 and TDF1; these observations agreed with the results obtained by microsectioning. Bra2MS might function upstream of the male meiosis-related genes in meiocytes at stage 6 and affect the transition of the tapetum to the secretory cell type in non-heading Chinese cabbage.
Together, our findings contribute to the understanding of the mechanism underlying male sterility and provide helpful information for further gene cloning of Bra2ms in WS24-3A.
References
Akter S, Huq MA, Jung YJ, Cho YG, Kang KK (2016) Application of single nucleotide polymorphism markers for selection of male sterility in crop plants. Plant Breed Biotech 4:379–386
Albrecht C, Russinova E, Hecht V, Baaijens E, de Vries S (2005) The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell 17:3337–3349
Andreuzza S, Nishal B, Singh A, Siddiqi I (2015) The chromatin protein DUET/MMD1 controls expression of the meiotic gene TDM1 during male meiosis in Arabidopsis. PLoS Genet 11:e1005396
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT et al (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25:25–29
Bohra A, Jha UC, Adhimoolam P, Bisht D, Singh NP (2016) Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Rep 35:967–993
Canales C, Bhatt AM, Scott R, Dickinson H (2002) EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol 12:1718–1727
Chang F, Wang Y, Wang S, Ma H (2011) Molecular control of microsporogenesis in Arabidopsis. Curr Opin Plant Biol 14:66–73
Chang L, Liu Z, Li C, Yun Z, Hui F (2016) Comparative transcriptome analysis of fertile and sterile buds from a genetically male sterile line of Chinese cabbage. Vitro Cell Dev Biol Plant 52:130–139
Chen G, Ye X, Zhang S, Zhu S, Yuan L, Hou J, Wang C (2018) Comparative transcriptome analysis between fertile and CMS flower buds in Wucai (Brassica campestris L.). BMC Genom 19:908
Cifuentes M, Jolivet S, Cromer L, Harashima H, Bulankova P, Renne C, Crismani W, Nomura Y, Nakagami H, Sugimoto K et al (2016) TDM1 regulation determines the number of meiotic divisions. Plos Genet 12:e1005856
Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI (2005) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell 17:3350–3361
Cui J, You C, Zhu E, Huang Q, Ma H, Chang F (2016) Feedback regulation of DYT1 by interactions with downstream bHLH factors promotes DYT1 nuclear localization and anther development. Plant Cell 28:1078–1093
DeYoung BJ, Bickle KL, Schrage KJ, Muskett P, Patel K, Clark SE (2006) The CLAVATA1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis. Plant J 45:1–16
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Dun X, Zhou Z, Xia S, Wen J, Yi B, Shen J, Ma C, Tu J, Fu T (2011) BnaC.Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. Plant J 68:532–545
Feng X, Dickinson HG (2010) Cell-cell interactions during patterning of the Arabidopsis anther. Biochem Soc Trans 38:571–576
Feng BM, Lu DH, Ma X, Peng YB, Sun YJ, Ning G, Ma H (2012) Regulation of the Arabidopsis anther transcriptome by DYT1 for pollen development. Plant J 72:612–624
Fu Z, Yu J, Cheng X, Zong X, Xu J, Chen M, Li Z, Zhang D, Liang W (2014) The rice basic helix-loop-helix transcription factor TDR INTERACTING PROTEIN2 is a central switch in early anther development. Plant Cell 26:1512–1524
Glover J, Grelon M, Craig S, Chaudhury A, Dennis E (1998) Cloning and characterization of MS5 from Arabidopsis: a gene critical in male meiosis. Plant J 15:345–356
Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5:1217–1229
Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talon M, Dopazo J, Conesa A (2008) High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res 36:3420–3435
Hafidh S, Fila J, Honys D (2016) Male gametophyte development and function in angiosperms: a general concept. Plant Reprod 29:31–51
Higgins JD, Vignard J, Mercier R, Pugh AG, Franklin FC, Jones GH (2008) AtMSH5 partners AtMSH4 in the class I meiotic crossover pathway in Arabidopsis thaliana, but is not required for synapsis. Plant J 55:28–39
Hird DL, Worrall D, Hodge R, Smartt S, Paul W, Scott R (1993) The anther-specific protein encoded by the Brassica napus and Arabidopsis thaliana A6 gene displays similarity to beta-1,3-glucanases. Plant J 4:1023–1033
Hord CLH, Chen CB, DeYoung BJ, Clark SE, Ma H (2006) The BAM1/BAM2 receptor-like kinases are important regulators of Arabidopsis early anther development. Plant Cell 18:1667–1680
Huang J, Zhang T, Linstroth L, Tillman Z, Otegui MS, Owen HA, Zhao D (2016) Control of anther cell differentiation by the small protein ligand TPD1 and its receptor EMS1 in Arabidopsis. PLoS Genet 12:e1006147
Ito T, Wellmer F, Yu H, Das P, Ito N, Alves-Ferreira M, Riechmann JL, Meyerowitz EM (2004) The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 430:356–360
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T et al (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36:D480–D484
Li X, Wang A, Zu F, Hu Z, Lin J, Zhou G, Tu J (2016) Identification of a nuclear-recessive gene locus for male sterility on A2 chromosome using the Brassica 60 K SNP array in non-heading Chinese cabbage. Genes & Genom 38:1151–1157
Li DD, Xue JS, Zhu J, Yang ZN (2017) Gene regulatory network for tapetum development in Arabidopsis thaliana. Front Plant Sci 8:1559
Libeau P, Durandet M, Granier F, Marquis C, Berthome R, Renou JP, Taconnat-Soubirou L, Horlow C (2011) Gene expression profiling of Arabidopsis meiocytes. Plant Biol (Stuttg) 13:784–793
Lu P, Chai M, Yang J, Ning G, Wang G, Ma H (2014) The Arabidopsis CALLOSE DEFECTIVE MICROSPORE1 gene is required for male fertility through regulating callose metabolism during microsporogenesis. Plant Physiol 164:1893–1904
Ma H (2006) A molecular portrait of Arabidopsis meiosis. Arabidopsis Book 4:e0095
Ma H (2013) A battle between genomes in plant male fertility. Nat Genet 45:472–473
Parish RW, Li SF (2010) Death of a tapetum: a programme of developmental altruism. Plant Sci 178:73–89
Reddy TV, Kaur J, Agashe B, Sundaresan V, Siddiqi I (2003) The DUET gene is necessary for chromosome organization and progression during male meiosis in Arabidopsis and encodes a PHD finger protein. Development 130:5975–5987
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sexual Plant Reprod 11:297–322
Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K (1999) Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc Natl Acad Sci USA 96:11664–11669
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16(Suppl):S46–S60
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511-U174
Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578
Wang D, Guo Y, Wu C, Yang G, Li Y, Zheng C (2008) Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genomics 9:44
Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26:136–138
Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun JH, Bancroft I, Cheng F et al (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039
Wang A, Hu J, Huang X, Li X, Zhou G, Yan Z (2016a) Comparative transcriptome analysis reveals heat-responsive genes in Chinese cabbage (Brassica rapa ssp. chinensis). Front Plant Sci 7:939
Wang J, Niu B, Huang J, Wang H, Yang X, Dong A, Makaroff C, Ma H, Wang Y (2016b) The PHD finger protein MMD1/DUET ensures the progression of male meiotic chromosome condensation and directly regulates the expression of the condensin gene CAP-D3. Plant Cell 28:1894–1909
Wei F, Zhang GS (2010) Meiotically asynapsis-induced aneuploidy in autopolyploid Arabidopsis thaliana. J Plant Res 123:87–95
Xie C, Mao XZ, Huang JJ, Ding Y, Wu JM, Dong S, Kong L, Gao G, Li CY, Wei LP (2011) KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39:W316–W322
Xin Q, Shen Y, Li X, Lu W, Wang X, Han X, Dong FM, Wan LL, Yang GS, Hong DF et al (2016) MS5 mediates early meiotic progression and its natural variants may have applications for hybrid production in Brassica napus. Plant Cell 28:1263–1278
Yang SL, Xiea LF, Mao HZ, Puah CS, Yang WC, Jiang LX, Sundaresan V, Ye D (2003a) TAPETUM DETERMINANT1 is required for cell specialization in the Arabidopsis anther. Plant Cell 15:2792–2804
Yang X, Makaroff CA, Ma H (2003b) The Arabidopsis MALE MEIOCYTE DEATH1 gene encodes a PHD-finger protein that is required for male meiosis. Plant Cell 15:1281–1295
Ye J, Fang L, Zheng HK, Zhang Y, Chen J, Zhang ZJ, Wang J, Li ST, Li RQ, Bolund L et al (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34:W293–W297
Yi B, Zeng F, Lei S, Chen Y, Yao X, Zhu Y, Wen J, Shen J, Ma C, Tu J et al (2010) Two duplicate CYP704B1-homologous genes BnMs1 and BnMs2 are required for pollen exine formation and tapetal development in Brassica napus. Plant J 63:925–938
Zhang D, Yang L (2014) Specification of tapetum and microsporocyte cells within the anther. Curr Opin Plant Biol 17:49–55
Zhang W, Sun YJ, Timofejeva L, Chen CB, Grossniklaus U, Ma H (2006) Regulation of Arabidopsis tapetum development and function by dysfunctional tapetum1 (dyt1) encoding a putative bHLH transcription factor. Development 133:3085–3095
Zhao D (2009) Control of anther cell differentiation: a teamwork of receptor-like kinases. Sex Plant Reprod 22:221–228
Zhou Z, Dun X, Xia S, Shi D, Qin M, Yi B, Wen J, Shen J, Ma C, Tu J et al (2012) BnMs3 is required for tapetal differentiation and degradation, microspore separation, and pollen-wall biosynthesis in Brassica napus. J Exp Bot 63:2041–2058
Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN (2008) Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J 55:266–277
Zhu J, Lou Y, Xu X, Yang ZN (2011) A genetic pathway for tapetum development and function in Arabidopsis. J Integr Plant Biol 53:892–900
Zhu EG, You CJ, Wang SS, Cui J, Niu BX, Wang YX, Qi J, Ma H, Chang F (2015) The DYT1-interacting proteins bHLH010, bHLH089 and bHLH091 are redundantly required for Arabidopsis anther development and transcriptome. Plant J 83:976–990
Acknowledgements
This study was supported by the Innovation Fund of Wuhan Academy of Agricultural Science and Technology (CX201719-03), the National Key Research and Development Program of China (2017YFD0101803), Bulk Vegetable Industry Technology Systems of China (CARS-23-G-26) and the Natural Science Foundation of Hubei Province Youth program (ZRMS2019001622). We thank anonymous reviewers very much for providing constructive comments.
Author information
Authors and Affiliations
Contributions
LS and XL contributed equally to this study. Conceptualization, LS; Formal analysis, XL and FZ; Investigation, LS and XL; Methodology, LS and FZ; Resources, AW; Supervision, AW, CG, BW, CL, JT and GZ; Visualization, LS; writing—original draft, LS and XL; writing—review and editing, JT and GZ.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Human and animal rights
No animals/humans were used for studies that are base of this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, L., Li, X., Zu, F. et al. Comparative transcript profiling and cytological observation of the newly bred recessive genic male sterility non-heading Chinese cabbage (Brassica rapa ssp. chinensis) line WS24-3A. Genes Genom 41, 1475–1492 (2019). https://doi.org/10.1007/s13258-019-00867-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-019-00867-x