Abstract
Successful sexual reproduction depends on normal cell differentiation during early anther development in flowering plants. The anther typically has four lobes, each of which contains highly specialized reproductive (microsporocyte) and somatic cells (epidermis, endothecium, middle layer, and tapetum). To date, six leucine-rich repeat receptor-like protein kinases (LRR-RLK) have been identified to have roles in regulation of anther cell patterning in Arabidopsis thaliana. EXCESS MICROSPOROCYTES1 (EMS1)/EXTRA SPOROGENOUS CELLS (EXS) and SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1/2 (SERK1/2) signal the differentiation of the tapetum. BARELY ANY MERISTEM1/2 (BAM1/2) defines anther somatic cell layers, including the endothecium, middle layer, and tapetum. Moreover, RECEPTOR-LIKE PROTEIN KINASE2 (RPK2) is required for the differentiation of middle layer cells. In addition to process of anther cell differentiation, conserved regulation of anther cell differentiation in different plant species, this review mainly discusses how these receptor-like kinases and other regulators work together to control anther cell fate determination in Arabidopsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The life cycle of flowering plants alternates between the diploid sporophyte and the haploid gametophyte generations. The stamen is the male reproductive organ in a flower. It consists of an anther where the male gametophytes develop, and a filament that anchors the anther to the flower, providing it with water and nutrients. The anther is usually a four-lobed structure (Fig. 1a). Each lobe contains five highly specialized and well-organized cell layers, which are the epidermis, endothecium, middle layer, tapetum, and microsporocytes (pollen mother cells) (Goldberg et al. 1993; Sanders et al. 1999) (Fig. 1a). Microsporocytes are reproductive cells that undergo meiosis and eventually develop into pollen grains. The remaining cell layers are non-reproductive (somatic) cells that are required for the normal development and release of pollen. In particular, the tapetum is critical for pollen development.
In addition to the significance of anthers in the reproductive cycle of flowering plants, studying anther development is important for understanding both fundamental and plant-specific principles. The traits of male sterility are broadly used by plant breeders to produce hybrid varieties for increasing crop yields. Moreover, floral sterility can prevent gene flow from genetically engineered plants through pollen dispersal and increase the overall biomass of plants. Therefore, studying anther development has great impacts on agriculture and the environment. Extensive studies have been conducted to identify genes that control floral organ identity. For example, identity of the six stamens in Arabidopsis flowers is controlled by a combination of the B genes APETALA3 (AP3) and PISTILLATA (PI), the C gene AGAMOUS (AG), and the E genes SEPALLATA1-4 (SEP1-4) (Jack 2004; Ma 2005; Scott et al. 2004; Zhao et al. 2001). However, much less is known about how “organ building” genes specify different cell types of an anther. Following the discovery that the EXCESS MICROSPOROCYTES1 (EMS1) [also known as EXTRA SPOROGENOUS CELLS (EXS)] leucine-rich repeat receptor-like protein kinase (LRR-RLK) regulates tapetal cell fate determination (Canales et al. 2002; Zhao et al. 2002), five more LRR-RLKs have been found to be involved in anther cell differentiation (Albrecht et al. 2005; Colcombet et al. 2005; Hord et al. 2006; Mizuno et al. 2007). Many excellent reviews have summarized advances in understanding of stamen identity, anther cell differentiation, male meiosis, pollen development, and anther dehiscence (Borg et al. 2009; Feng and Dickinson 2007; Goldberg et al. 1993; Jack 2004; Ma 2005; McCormick 2004; Scott et al. 2004; Walbot and Evans 2003; Wilson and Yang 2004; Wilson and Zhang 2009; Yang and Sundaresan 2000; Zhao and Ma 2000; Zhao et al. 2001; Zik and Irish 2003a). In this review, the regulation of signaling during early anther cell differentiation by LRR-RLKs is summarized, mainly using Arabidopsis thaliana as an example.
Anther cell differentiation
Anther development entails cell division, cell differentiation, and cell death, resulting in the specification of both reproductive microsporocytes and somatic cell layers in the same organ. Arabidopsis flower development is divided into 12 stages before flowering using morphological landmarks observed by scanning electron microscopy (Smyth et al. 1990). Following the initiation of stamen primordia at stage 5, the establishment of all anther cell types is finished by stage 9. The development of pollen begins at stage 10, and mature pollen grains are released after anther dehiscence at stage 13.
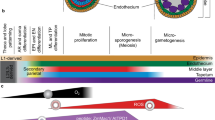
To better understand anther cell differentiation, anther development in Arabidopsis has been divided into two phases and 14 stages based on cellular landmarks visible by light microscopy (Sanders et al. 1999). In phase I, represented by stages from 1 to 8 (approximately corresponding to flower stages 5–10), five anther cell types are established and meiosis occurs. In phase II, represented by stages from 9 to 14 (approximately corresponding to flower stages 10–15), pollen grains are developed and released from the anther after anther tissue degeneration and dehiscence. Cell division and differentiation actively occur from stages 1 to 5, resulting in the specification of all five anther cell types in each lobe. The anther originates from three layers of cells (Fig. 1b), designated L1, L2, and L3. The L1 layer forms epidermis and the L2 layer gives rise to most of the anther cells, including somatic cell layers and reproductive microsporocytes in each lobe. The vascular and connective tissues are derived from the L3 layer at the center of the anther. Archesporial cells are generated from the L2 layer at stage 2, and then divide periclinally to form primary parietal cells (PPC) and primary sporogenous cells (PSC) at stage 3 (Fig. 1b). The primary sporogenous cells will differentiate into microsporocytes. The primary parietal cells further divide into two sets of secondary parietal cells (SPC) at stage 4 (Fig. 1b). The inner set of secondary parietal cells adjacent to the sporogenous cells differentiate into tapetal cells, and the outer secondary parietal cells divide periclinally to form the endothecium and middle layer. Before stage 4, cells of the endothecium, middle layer, and precursors of tapetal cells are not yet organized into layers, because they are not formed synchronously (Sanders et al. 1999; Zhao et al. 2002). By stage 5 (approximately corresponding to the flower stage 9), the anther completes the formation of the four-lobed structure and five types of anther lobe cells. From outside to inside the anther cells in each lobe are the epidermis, endothecium, middle layer, tapetum, and microsporocytes (Fig. 1a, b). Without the availability of molecular markers, there is a disagreement over whether the tapetum alone or both tapetum and middle layers are derived from inner set of secondary parietal cells (Fig. 1b) (Ma 2005; Sanders et al. 1999; Sorensen et al. 2002; Yang et al. 1999; Zhao et al. 2002).
At stage 6, several major events occur. Microsporocytes enter meiosis and become detached from each other. Callose is deposited within the primary cell wall of microsporocytes. In addition, the middle layer becomes thinner and elongated (Owen and Makaroff 1995). Moreover, tapetal cells become vacuolated. Meiosis followed by cytokinesis is completed at stage 7, resulting in the formation of tetrads of microspores. Stage 8 is marked by the release of microspores due to the degeneration of the callose wall surrounding tetrads. At stage 9, surrounded by an exine wall, microspores become vacuolated. In addition, septum is formed. The degeneration of tapetum is initiated at stage 10 and completed at stage 12. At stage 11, pollen undergoes mitotic divisions. Furthermore, cells in the endothecial layer are enlarged in size. To prepare for anther dehiscence, the degeneration of septum cells is initiated and stomium cells start to differentiate. At stage 12, pollen grains become tricellular. The septum is completely degenerated. At stage 13, followed by the breakage along stomium, anther dehiscence occurs, allowing the release of pollen grains. The anther shrinks at stage 14. Although anther morphology was studied by sectioning in Arabidopsis and other species more than a decade ago, only recently have some key genes been identified that directly regulate early anther cell differentiation (Feng and Dickinson 2007; Goldberg et al. 1993; Ma 2005; Sanders et al. 1999; Scott et al. 2004; Walbot and Evans 2003; Wilson and Zhang 2009).
EMS1/EXS and SERK1/2 determine the tapetum
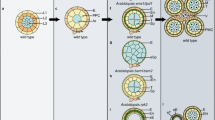
EMS1/EXS is the first LRR-RLK that was identified to play important roles in signaling anther cell differentiation (Canales et al. 2002; Zhao et al. 2002). The ems1/exs mutant anther lacks tapetum, but produces a greater number of microsporocytes than the wild type (Fig. 2). The number of microsporocytes in ems1 mutant anthers is close to the sum of microsporocytes and tapetal cells in wild-type anthers (Zhao et al. 2002). The fact that ems1 anthers produce excess microsporocytes at the expense of tapetal cells indicates that there is a trade-off between somatic and reproductive cells. The EMS1/EXS gene encodes an LRR-RLK, suggesting that EMS1/EXS mediates signals to control tapetal cell fate determination during early anther development.
Diagrams showing the anther structure in wild type and anther phenotypes of ems1/exs, serk1 serk2, tpd1, bam1 bam2, rpk2, and spl/nzz mutants. Anthers in ems1/exs, serk1 serk2, and tpd1 mutants lack tapetum, but produce excess microsporocytes. The bam1 bam2 double mutant anther does not form endothecium, the middle layer, or tapetum, instead it produces extra microsporocytes. The rpk2 mutant anther has no middle layer. The spl/nzz mutant anther fails to produce microsporocytes and anther walls, including the endothecium, middle layer, and tapetum. All the mutants are male sterile
Although neither somatic embryogenesis receptor-like kinase1 (serk1) nor serk2 single mutant exhibits detectable anther phenotypes, anther defects in the serk1 serk2 double mutant resembles that seen in ems1/exs anthers (Fig. 2). (Albrecht et al. 2005; Colcombet et al. 2005). Both SERK1 and SERK2 encode LRR-RLKs with a high level of similarity. Therefore, SERK1 and SERK2 function redundantly in determining tapetal cell fate during anther development. Moreover, SERK1/2 may be involved in the same signaling pathway as EMS1/EXS. Mutation of the TAPETUM DETERMINANT1 (TPD1) gene results in the absence of tapetum and the formation of excess microsporocytes, which is nearly identical to the phenotypes of the ems1/exs single mutant and the serk1 serk2 double mutant (Fig. 2) (Yang et al. 2003). Genetic analysis supports the idea that both TPD1 and EMS1/EXS function in the same genetic pathway (Jia et al. 2008; Yang et al. 2005). Further studies demonstrate that ectopic expression of TPD1 causes abnormal differentiation of tapetal cells and microsporocytes. The abolishment of anther phenotypes caused by the ectopic expression of TPD1 in the ems1 mutant indicates that the TPD1 signaling requires a functional EMS1/EXS. TPD1 encodes a novel small putatively secreted protein and TPD1 interacts with EMS1/EXS in vitro and in vivo (Jia et al. 2008). Furthermore, TPD1 induces the phosphorylation of EMS1/EXS. Therefore, TPD1 could serve as a ligand for the EMS1/EXS receptor kinase.
In animals, signals from somatic cells play critical roles in influencing sex cell fate determination (Santos and Lehmann 2004; Zhao and Garbers 2002). Messenger RNA in situ hybridization experiments show that the EMS1/EXS gene is primarily expressed in the tapetum, while TPD1 is mainly restricted to microsporocytes (Yang et al. 2003; Zhao et al. 2002). Therefore, the TPD1 small protein may be secreted from microsporocytes or their precursors (Fig. 3). TPD1 binds to the EMS1/EXS receptor that is localized on the cell membrane of tapetum precursor cells. Signals relayed by EMS1/EXS might direct tapetum formation by activating the expression of genes that promote tapetal cell fate determination, and oppositely by repressing the expression of genes required for differentiation of microsporocytes. Without the EMS1/EXS receptor in the ems1/exs mutant or the TPD1 ligand in the tpd1 mutant, genes promoting microsporocyte differentiation might be expressed in tapetum precursor cells at a higher level than normal, which consequently results in tapetal precursor cells adopting a microsporocyte fate. Furthermore, the EMS1/EXS-TPD1 signaling complex may contain the SERK1/2 LRR-RLK.
A model for the EMS/EXS-TPD1 signaling pathway in regulating anther cell fate determination. The TPD1 protein is secreted from microsporocytes or their precursors and then binds to the EMS1/EXS receptor that is localized on the cell membrane of tapetum precursor cells. Signals elicited by EMS1/EXS activate a pathway to promote fate determination of tapetal cells and conversely repress a pathway for differentiation of microsporocytes. In either ems1/exs or tpd1 mutant, the EMS/EXS-TPD1 signaling pathway is blocked. Therefore, the pathway for the differentiation of microsporocytes is not restrained in tapetum precursor cells, resulting in tapetal precursor cells adopting a microsporocyte fate
BAM1/2 signals the differentiation of anther somatic cell layers
BAM1 (for BARELY ANY MERISTEM) and BAM2 LRR-RLKs, which share high levels of identity with CLAVATA1, are involved in regulating early anther cell differentiation in Arabidopsis (DeYoung et al. 2006; Hord et al. 2006; Shiu and Bleecker 2001). Neither bam1 nor bam2 single mutants have detectable phenotypes (DeYoung et al. 2006). However, bam1 bam2 double mutants exhibit various developmental abnormalities, including reduced meristem size and aberrant leaves, as well as irregular male and female fertility (DeYoung et al. 2006). Additional detailed morphological analysis of anther development reveals that the bam1 bam2 double mutant anther does not form somatic cell layers, including endothecium, middle layer, and tapetum (Fig. 2) (Hord et al. 2006). Nevertheless, the double mutant anther produces only microsporocytes. Both BAM1 and BAM2 genes are expressed in archesporial cells at as early as stage 2, while in later stages they are preferentially expressed in sporogenous cells and microsporocytes. Therefore, BAM1- and BAM2-mediated signaling is required for the specification of parietal cells that generate cell layers of the anther wall.
It appears that BAM1/2, as well as EMS1/EXS and SERK1/2 play a role that is opposite to that of the SPOROCYTELESS (SPL)/NOZZLE (NZZ) transcription factor in regulating anther cell differentiation. The spl/nzz mutant anther fails to produce microsporocytes and anther walls, suggesting that the SPL/NZZ gene promotes the formation of microsporocytes and anther walls, including the tapetum (Fig. 2) (Schiefthaler et al. 1999; Yang et al. 1999). The anther phenotype of the bam1 bam2 double mutant suggests that BAM1/2 may repress the proliferation of sporogenous cells, but promote the differentiation of adjacent parietal cells. In addition, EMS1/EXS together with SERK1/2 may inhibit the formation of microsporocytes. However, it is not clear whether SPL/NZZ is the target gene of the BAM1/2, EMS1/EXS, or SERK1/2 signal transduction pathway. Without the availability of molecular markers for different anther cell types, the morphological analyses of spl and nzz anthers lead to somewhat different interpretations. It is believed that the spl anther can produce secondary parietal cells and primary sporogenous cells (Yang et al. 1999), while the nzz anther stops cell differentiation after forming a group of archesporial cells (Schiefthaler et al. 1999). SPL/NZZ is expressed in both parietal and sporogenous cells during early stages of anther development and then it is restricted to microsporocytes when anther cells are fully differentiated (Yang et al. 1999). However, the expression of SPL/NZZ in the bam1 bam2 anther is expanded to all, or most of the L2-derived cells (Hord et al. 2006). Therefore, BAM1/2 signaling seems to repress the expression of SPL/NZZ. This might be true for the EMS1/EXS and SERK1/2 signal transduction pathways. According to this assumption, the signal relayed by EMS1/EXS or SERK1/2 represses the expression of genes required for microsporocyte differentiation in tapetal precursors, such as SPL/NZZ. In the ems1/exs single or serk1 serk2 double mutant, SPL/NZZ should be persistently expressed in the tapetal precursor cells, which eventually leads to the formation of excess microsporocytes. The SPL/NZZ gene could also be involved in regulating the expression of EMS1/EXS, SERK1 or SERK2 in parietal cells. In the spl/nzz mutant, the abnormal expression of EMS1/EXS, SERK1 or SERK2 causes the lack of tapetum. Therefore, it is possible that the genetic interactions between the EMS1/EXS or SERK1/2 signal transduction pathway and the SPL/NZZ gene establish a negative feedback loop to control anther cell differentiation. It is also possible that the lack of microsporocytes in the spl/nzz mutant causes the failed differentiation of tapetal cells, since the TPD1 protein is likely produced in microsporocytes.
RPK2 defines the middle layer
The RECEPTOR-LIKE PROTEIN KINASE2 (RPK2) gene, which encodes an LRR-RLK, is required for differentiation of the middle layer during anther development (Fig. 2) (Mizuno et al. 2007). The disruption of RPK2 function results in the formation of anthers without the middle layer. Moreover, the tapetum is affected, displaying hypertrophy. Different from ems1/exs, serk1 serk2, and bam1 bam2 mutants, the rpk2 mutant anther produces tetrads and microspores, although the pollen maturation is abnormal. The expression of RPK2 is strongly detected in the tapetum. Therefore, RPK2 signaling not only controls the differentiation of the middle layer, but also maintains the tapetum.
Integration of multiple LRR-RLK-linked signaling pathways
So far, six LRR-RLKs have been identified that play important roles in signaling early anther cell differentiation. A crucial difference between plant and animal cells is that plant cells have a rigid cell wall, which does not allow cells to move. Therefore, intercellular signaling using diffusible molecules is particularly important for plant cell–cell communication. Receptor-like kinases (RLKs) are key components in plant cell signaling. In Arabidopsis, more than 600 genes encode RLKs, representing 2.5% of the total genes (Shiu et al. 2004; Torii 2004). This number is almost doubled in the rice genome (Shiu et al. 2004). LRR-RLKs, with 223 members in Arabidopsis (www4.ncsu.edu/~sclouse/Clouse2010.htm), form the largest family of RLKs. LRR-RLKs are involved in a wide range of plant growth and developmental processes, including stem cell maintenance, cell fate determination and patterning, steroid hormone signaling, organ size and shape regulation, organ abscission, defense responses, plant transpiration, and nodulation (Becraft 2002; Clouse 2002; Dievart and Clark 2004; Johnson and Ingram 2005; McCarthy and Chory 2000; Morillo and Tax 2006; Morris and Walker 2003; Nakajima and Benfey 2002; Torii 2004; Vert et al. 2005; Waites and Simon 2000). Although mutations of EMS1/EXS, SERK1 and SERK2, BAM1 and BAM2, as well as RPK2 genes cause male sterility, it is not clear how these LRR-RLK-linked signaling pathways integrate to regulate anther cell differentiation.
The bam1 bam2 anther is unable to produce three of the normal layers of the anther wall—the endothecium, middle layer, and tapetum. Instead it produces extra microsporocytes (Hord et al. 2006). The cell layers that are absent from the bam1 bam2 anther wall are derived from parietal cells, suggesting that BAM1/2 signaling is required for the specification of parietal cells from archesporial cells at an early stage of anther development (Fig. 4). The division of secondary parietal cells seems normal in the ems1 mutant (Zhao et al. 2002). This indicates, therefore, that EMS1/EXS signaling acts later than BAM1/2 signaling in determining the fate of tapetal cells, which are generated from the secondary parietal cells. Both BRASSINOSTEROID-INSENSTIVE 1 (BRI1) and EMS1/EXS belong to the LRR-RLK X subfamily, while SERK1, SERK2, and BRI1-ASSOCIATED RECEPTOR KINASE (BAK1, also known as SERK3) are members of LRR-RLK II subfamily (Albrecht et al. 2005; Albrecht et al. 2008; Colcombet et al. 2005). BAK1 and BRI1 form a signaling complex via heterodimerization to mediate plant steroid signaling (Albrecht et al. 2008; Li et al. 2002; Nam and Li 2002; Wang et al. 2005; Wang et al. 2008). The phenotype of the serk1 serk2 double mutant anther resembles that of ems1/exs. Thus, SERK1/2 may function in the same pathway as EMS1/EXS to determine the tapetum (Fig. 4). The rpk2 mutant anther fails to form the middle layer, while the development of tapetum is affected (Mizuno et al. 2007). RPK2 signaling may affect the division of inner or outer secondary parietal cells. RPK2 and EMS1/EXS1 should act in similar developmental stages (Fig. 4). Moreover, it is possible that RPK2 and EMS1/EXS play antagonistic roles in specifying the middle layer and tapetum from their parietal cell precursors. Future molecular genetic analyses hold promise to address how these LRR-RLKs work together to define different cell types during early anther development.
Conserved regulation of early anther cell differentiation
Different plants may use similar mechanisms to control early anther cell differentiation. The rice multiple sporocyte1 (msp1) mutant lacks tapetum, but produces excess microsporocytes in the anther, which is similar to the phenotype of ems1/exs (Canales et al. 2002; Nonomura et al. 2003; Zhao et al. 2002). The MSP1 gene encodes an LRR-RLK with a high level of identity to EMS1/EXS, suggesting that MSP1 is an EMS1/EXS ortholog (Nonomura et al. 2003). Although the mutant gene is not known, the maize mutant multiple archesporial cells (mac1) has a phenotype nearly identical to that of msp1 (Sheridan et al. 1999). Rice has at least two TPD1-like genes, OsTDL1A and OsTDL1B (Zhao et al. 2008). Furthermore, in maize the male sterile converted anther1 (msca1) mutant has a similar phenotype to spl/nzz, which forms archesporial cells, but fails to produce microsporocytes (Chaubal et al. 2003). These results support the idea that eudicots and monocots show conservation in regulating early anther cell differentiation, although they diverged at least 120 million years ago.
Plants have evolved to have different complexities of microsporangium structures. For example, the existence of tapetum is universal in all land plants. Although the tapetum has similar function, the structure varies among species (Pacini et al. 1985). The leptosporangiate ferns have two layers (inner and outer) of tapetum (Hord et al. 2006), while in Psilotum nudum ferns tapetal cells are intermingled with microsporocytes (Pacini et al. 1985). It will be interesting to investigate whether signaling molecules identified in Arabidopsis have conserved functions in controlling microsporangium development in other plant species.
Perspective
The anther is not only vitally important for plant sexual reproduction, but also provides an excellent system to elucidate many fundamental principles, such as the molecular mechanisms of cell fate determination. Normal early anther cell differentiation ensures normal male meiosis, pollen development, and anther dehiscence. Many genes have been found to be linked to anther development based on expression studies using microarray techniques (Alves-Ferreira et al. 2007; Hennig et al. 2004; Lu et al. 2006; Ma et al. 2007; Ma et al. 2008; Wellmer et al. 2004; Wijeratne et al. 2007; Zik and Irish 2003b). However, so far only a few genes have been identified that play direct roles in controlling anther cell differentiation, particularly in cell fate specification of tapetum and microsporocytes. Future studies should focus on identifying genes that regulate cell differentiation in the whole process of anther development. For example, little is known about how the differentiation of archesporial cells is controlled.
Although six LRR-RLKs have been found to signal early anther cell differentiation, significant gaps remain in these LRR-RLK-linked signal transduction pathways. It is not clear what kinds of signaling molecules are involved in relaying signals from the cell surface to the cell interior, and from cell to cell. Furthermore, nothing is known about crosstalk among these signaling networks. Therefore, it will be necessary to identify the signaling components and determine how they are integrated using a combination of molecular genetic, cell biological, genomic, and proteomic approaches. Furthermore, computational and imaging technologies should be employed to study the dynamics of anther cell patterning in the future.
References
Albrecht C, Russinova E, Hecht V, Baaijens E, de Vries S (2005) The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell 17:3337–3349
Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries SC (2008) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol 148:611–619
Alves-Ferreira M, Wellmer F, Banhara A, Kumar V, Riechmann JL, Meyerowitz EM (2007) Global expression profiling applied to the analysis of Arabidopsis stamen development. Plant Physiol 145:747–762
Becraft PW (2002) Receptor kinase signaling in plant development. Annu Rev Cell Dev Biol 18:163–192
Borg M, Brownfield L, Twell D (2009) Male gametophyte development: a molecular perspective. J Exp Bot 60:1465–1478
Canales C, Bhatt AM, Scott R, Dickinson H (2002) EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol 12:1718–1727
Chaubal R, Anderson JR, Trimnell MR, Fox TW, Albertsen MC, Bedinger P (2003) The transformation of anthers in the msca1 mutant of maize. Planta 216:778–788
Clouse SD (2002) Brassinosteroid signal transduction: clarifying the pathway from ligand perception to gene expression. Mol Cell 10:973–982
Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI (2005) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell 17:3350–3361
DeYoung BJ, Bickle KL, Schrage KJ, Muskett P, Patel K, Clark SE (2006) The CLAVATA1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis. Plant J 45:1–16
Dievart A, Clark SE (2004) LRR-containing receptors regulating plant development and defense. Development 131:251–261
Feng X, Dickinson HG (2007) Packaging the male germline in plants. Trends Genet 23:503–510
Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5:1217–1229
Hennig L, Gruissem W, Grossniklaus U, Kohler C (2004) Transcriptional programs of early reproductive stages in Arabidopsis. Plant Physiol 135:1765–1775
Hord CL, Chen C, Deyoung BJ, Clark SE, Ma H (2006) The BAM1/BAM2 receptor-like kinases are important regulators of Arabidopsis early anther development. Plant Cell 18:1667–1680
Jack T (2004) Molecular and genetic mechanisms of floral control. Plant Cell 16(Suppl):S1–S17
Jia G, Liu X, Owen HA, Zhao D (2008) Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase. Proc Natl Acad Sci USA 105:2220–2225
Johnson KL, Ingram GC (2005) Sending the right signals: regulating receptor kinase activity. Curr Opin Plant Biol 8:648–656
Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110:213–222
Lu XC, Gong HQ, Huang ML, Bai SL, He YB, Mao X, Geng Z, Li SG, Wei L, Yuwen JS et al (2006) Molecular analysis of early rice stamen development using organ-specific gene expression profiling. Plant Mol Biol 61:845–861
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56:393–434
Ma J, Duncan D, Morrow DJ, Fernandes J, Walbot V (2007) Transcriptome profiling of maize anthers using genetic ablation to analyze pre-meiotic and tapetal cell types. Plant J 50:637–648
Ma J, Skibbe DS, Fernandes J, Walbot V (2008) Male reproductive development: gene expression profiling of maize anther and pollen ontogeny. Genome Biol 9:R181
McCarthy DR, Chory J (2000) Conservation and innovation in plant signaling pathways. Cell 103:201–209
McCormick S (2004) Control of male gametophyte development. Plant Cell 16(Suppl):S142–S153
Mizuno S, Osakabe Y, Maruyama K, Ito T, Osakabe K, Sato T, Shinozaki K, Yamaguchi-Shinozaki K (2007) Receptor-like protein kinase 2 (RPK2) is a novel factor controlling anther development in Arabidopsis thaliana. Plant J 50:751–766
Morillo SA, Tax FE (2006) Functional analysis of receptor-like kinases in monocots and dicots. Curr Opin Plant Biol 9:460–469
Morris ER, Walker JC (2003) Receptor-like protein kinases: the keys to response. Curr Opin Plant Biol 6:339–342
Nakajima K, Benfey PN (2002) Signaling in and out: control of cell division and differentiation in the shoot and root. Plant Cell 14(Suppl):S265–S276
Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110:203–212
Nonomura K, Miyoshi K, Eiguchi M, Suzuki T, Miyao A, Hirochika H, Kurata N (2003) The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell 15:1728–1739
Owen HA, Makaroff CA (1995) Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. Wassiliewskaja (Brassicaceae). Protoplasma 185:7–21
Pacini E, Franchi GG, Hesse M (1985) The tapetum: its form, function, and possible phylogeny in Embryophyta. Plant Syst Evol 149:155–185
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Santos AC, Lehmann R (2004) Germ cell specification and migration in Drosophila and beyond. Curr Biol 14:R578–R589
Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K (1999) Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc Natl Acad Sci USA 96:11664–11669
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16(Suppl):S46–S60
Sheridan WF, Golubeva EA, Abrhamova LI, Golubovskaya IN (1999) The mac1 mutation alters the developmental fate of the hypodermal cells and their cellular progeny in the maize anther. Genetics 153:933–941
Shiu SH, Bleecker AB (2001) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA 98:10763–10768
Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH (2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16:1220–1234
Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2:755–767
Sorensen A, Guerineau F, Canales-Holzeis C, Dickinson HG, Scott RJ (2002) A novel extinction screen in Arabidopsis thaliana identifies mutant plants defective in early microsporangial development. Plant J 29:581–594
Torii KU (2004) Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways. Int Rev Cytol 234:1–46
Vert G, Nemhauser JL, Geldner N, Hong F, Chory J (2005) Molecular mechanisms of steroid hormone signaling in plants. Annu Rev Cell Dev Biol 21:177–201
Waites R, Simon R (2000) Signaling cell fate in plant meristems. Three clubs on one tousle. Cell 103:835–838
Walbot V, Evans MM (2003) Unique features of the plant life cycle and their consequences. Nat Rev Genet 4:369–379
Wang X, Goshe MB, Soderblom EJ, Phinney BS, Kuchar JA, Li J, Asami T, Yoshida S, Huber SC, Clouse SD (2005) Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell 17:1685–1703
Wang X, Kota U, He K, Blackburn K, Li J, Goshe MB, Huber SC, Clouse SD (2008) Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell 15:220–235
Wellmer F, Riechmann JL, Alves-Ferreira M, Meyerowitz EM (2004) Genome-wide analysis of spatial gene expression in Arabidopsis flowers. Plant Cell 16:1314–1326
Wijeratne AJ, Zhang W, Sun Y, Liu W, Albert R, Zheng Z, Oppenheimer DG, Zhao D, Ma H (2007) Differential gene expression in Arabidopsis wild-type and mutant anthers: insights into anther cell differentiation and regulatory networks. Plant J 52:14–29
Wilson ZA, Yang C (2004) Plant gametogenesis: conservation and contrasts in development. Reproduction 128:483–492
Wilson ZA, Zhang DB (2009) From Arabidopsis to rice: pathways in pollen development. J Exp Bot 60:1479–1492
Yang WC, Sundaresan V (2000) Genetics of gametophyte biogenesis in Arabidopsis. Curr Opin Plant Biol 3:53–57
Yang WC, Ye D, Xu J, Sundaresan V (1999) The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev 13:2108–2117
Yang SL, Xie LF, Mao HZ, Puah CS, Yang WC, Jiang L, Sundaresan V, Ye D (2003) Tapetum determinant1 is required for cell specialization in the Arabidopsis anther. Plant Cell 15:2792–2804
Yang SL, Jiang L, Puah CS, Xie LF, Zhang XQ, Chen LQ, Yang WC, Ye D (2005) Overexpression of TAPETUM DETERMINANT1 alters the cell fates in the Arabidopsis carpel and tapetum via genetic interaction with EXCESS MICROSPOROCYTES1/EXTRA SPOROGENOUS CELLS. Plant Physiol 139:186–191
Zhao GQ, Garbers DL (2002) Male germ cell specification and differentiation. Dev Cell 2:537–547
Zhao D, Ma H (2000) Male fertility: a case of enzyme identity. Curr Biol 10:R904–R907
Zhao DZ, Yu QL, Chen CB, Ma H (2001) Genetic control of reproductive meristems. In: McManus MT, Veit B (eds) Annual plant reviews: meristematic tissues in plant growth and development. Sheffield Academic Press, Sheffield, UK, pp 89–142
Zhao DZ, Wang GF, Speal B, Ma H (2002) The EXCESS MICROSPOROCYTES1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev 16:2021–2031
Zhao X, de Palma J, Oane R, Gamuyao R, Luo M, Chaudhury A, Herve P, Xue Q, Bennett J (2008) OsTDL1A binds to the LRR domain of rice receptor kinase MSP1, and is required to limit sporocyte numbers. Plant J 54:375–387
Zik M, Irish VF (2003a) Flower development: initiation, differentiation, and diversification. Annu Rev Cell Dev Biol 19:119–140
Zik M, Irish VF (2003b) Global identification of target genes regulated by APETALA3 and PISTILLATA floral homeotic gene action. Plant Cell 15:207–222
Acknowledgments
I would like to thank Hong Ma for giving me the opportunity to study anther development when I was in his laboratory. His insight greatly influenced my decision to continue my research in this area. I also thank H. A. Owen and C. Starrett for critical comments on this manuscript, and thank G. Jia for providing the image of the anther section. This work was supported by a grant from the University of Wisconsin-Milwaukee Research Growth Initiative (RGI) Program (to D.Z.), an NSF grant IOS-0721192 (to D.Z.), and the Shaw Scientist Award (to D.Z.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Twell.
Rights and permissions
About this article
Cite this article
Zhao, D. Control of anther cell differentiation: a teamwork of receptor-like kinases. Sex Plant Reprod 22, 221–228 (2009). https://doi.org/10.1007/s00497-009-0106-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-009-0106-3