Abstract
Brown spot (BS) disease causes significant losses to rice productivity. In this study, a roving survey in the Karnataka state of India revealed a wider distribution of BS with a percent disease index range of 20.56–50.74. From the symptomatic geo-distinct samples, pure cultures of 63 isolates were obtained. Based on the conidial morphology, 63 isolates were identified as Bipolaris oryzae (Bo) (n = 40), Curvularia lunata (Cl) (n = 15), and Exserohilum rostratum (Er) (n = 08). The taxonomic identity was further confirmed via ITS-sequencing. A pathogenicity assay on a BS-susceptible rice cultivar GNV-05–01 confirmed the pathogenicity of all three pathogens, which induces typical BS disease on test plants. Further, on PDA media, all isolates of three pathogens showed significant cultural diversity for mycelial color, colony type, and sporulation. We further studied the in-planta distribution of three pathogens on a randomly collected 600 BS spots from 10 different rice fields, which indicated that 77.83%, 17.33%, and 4.83% of the typical BS were produced by Bo, Cl, and Er, respectively. The ITS region was sequenced for selected 9, 7, and 3 isolates of Bo, Cl, and Er, respectively, and analyzed for their nucleotide and haplotype diversity, and phylogenetic relationships. A phylogenetic study identified the unique clustering patterns, and haplotyping indicated 3, 4, and 6 haplotypes. Tajima’s D (D) test showed several rare alleles in the ITS regions. This is the first comprehensive study reporting the three fungal pathogens causing BS of rice and it is useful for re-designing the screening protocol for the host plant resistance breeding program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is a staple food crop in Asia, Africa, South America, and, to some extent, in the United States (Asma et al. 2023). Rice crops are affected by several bacterial and fungal diseases; one of them is rice brown spot (BS), a most prevalent disease that causes significant damage to rice yield and quality. BS of rice caused by Bipolaris oryzae (teleomorph: Cochliobolus miyabeanus) has been known to occur in Japan since 1900. In India, the first report of this disease was from Madras in 1919 by Sundraraman (Sunder et al., 2014). The BS has been associated with two major epidemics in India, the first in 1918–19 (in the Krishna–Godavari delta) and the second in 1942 (in India and Bangladesh) (Chakrabarti 2001). The 1942 epidemic led to the Great Bengal Famine in the Indian sub-continent (India and Bangladesh) (Padmanabhan 1973; Chakrabarti 2001). The BS of rice is a chronic disease that affects millions of hectares of rice every growing season and causes yield losses from 4 to 52% (Barnwal et al. 2013). This disease is particularly severe in rice fields where the water supply is improper, combined with imbalances in chemical fertilizers, especially nitrogenous fertilizers (Ou 1985; Barnwal et al. 2013). Such predisposing factors are usually associated with the farmers of resource-poor locations and the direct seeded crop establishment method (Ou 1985).
Historically, BS was known to be caused by B. oryzae in different parts of the world, including India (Barnwal et al. 2013; Sunder et al., 2014). But, in recent years, this disease has also been reported to be caused by several other fungal pathogens, such as Curvularia spp. and Exserohilum rostratum, along with B. oryzae in some of the rice-growing countries of the world (Kusai et al. 2015; Khemmuk et al. 2016; Majeed et al. 2016a, b). The association of C. lunata with BS of rice has been reported in India (Kamaluddeen et al., 2013), Malaysia (Kusai et al. 2015), Pakistan (Majeed et al. 2016a, b), and in Northern Queensland (Khemmuk et al. 2016). Curvularia spp. are the important phytopathogens reported worldwide and are more destructive on grasses and cereal plants, including rice (Kusai et al. 2015). In addition to causing disease in plants, Curvularia spp. are known to have a broad host range and have been reported as opportunistic human and animal pathogens (Al-Odaini et al. 2022). The first report of C. lunata infecting rice in India was documented in the Allahabad district of Uttar Pradesh on the Pant-12 variety in 2012 (Kamaluddeen et al., 2013). After that, no systematic investigations have been conducted to study the distribution of C. lunata in the Indian rice ecosystem.
E. rostratum (Syn: Setosphaeria rostrata) is a member of the class Dothideomycetes, which can cause disease in many plant species and human beings. The pathogen was first described as a plant pathogen in 1923 (Drechsler 1923). E. rostratum has been reported to cause brown spot disease in rice (Kusai et al. 2015; Majeed et al. 2016a, b), sugarcane (Ahmadpour et al. 2013), bottle gourd (Choudhary et al. 2018), cucumber (Dhara et al. 2020), mulberry (Arunakumar et al. 2019), banana (Lin et al. 2011) and many grasses (Gauthier and Keller 2013; Wu and Turgeon 2013). This fungus is also an opportunistic human pathogen that causes fungal meningitis (Gauthier and Keller 2013; Sharma et al. 2014). The association of E. rostratum with rice seeds was reported in 1987 (Sivanesan 1987); however, its rice pathogenic nature has not been explored systematically.
In addition to B. oryzae, a study in India reports the association of C. lunata in causing BS disease in rice. Still, there is no literature regarding the association of E. rostratum in causing BS disease of rice. Most previous studies on BS of rice, such as host plant resistance, pathogen characterization, and disease management, have focused only on B. oryzae-BS-pathosystem, and no studies have been conducted to study the multiple pathogens of BS in India. Considering the broad host range of E. rostratum and C. lunata and their opportunistic pathogenic ability on humans, systematic investigations are required. In this study, we report the association of E. rostratum and C. lunata in causing BS disease of rice along with B. oryzae, their morpho-molecular characterization, and in-planta distribution of three species in the diseased plant samples. The information reported in this study is essential to redesign the studies related to BS of rice, which was otherwise primarily focused only on the B. oryzae-BS pathosystem.
Materials and methods
Collection of diseased samples and pathogen isolation
A roving survey was conducted during Kharif 2017 in different rice-growing regions of Karnataka state of India, namely Belagavi, Ballari, Chikkamagaluru, Davanagere, Dharwad, Koppal, Mandya, Raichur, Shivamogga, Vijayanagara, Uttar Kannada, and Yadgir (Table 1). During the survey, ten plots of 1 m2 each were randomly selected in each field, and the observations on disease severity were recorded. Initially, the disease was first measured following a 0–9 scale (IRRI 2013) and the recorded grades were converted into Percent Disease Index (PDI) as described previously (Wheeler 1969). The rice leaves showing typical BS disease symptoms were collected from all the surveyed locations. The associated fungal pathogen/s was isolated on potato dextrose agar media (PDA) following the standard fungal isolation technique (Tuite 1969). The disease lesions of the leaf tissue were cut into 5 × 5 mm pieces, surface sterilized with 1% sodium hypochlorite for 1 min, rinsed in sterile distilled water five times, and placed on PDA and incubated at room temperature (25 ± 2 °C). After 7 days of incubation, single-spore isolations were performed to obtain pure culture, where conidia were picked under a binocular microscope using a sterile inoculation loop and transferred to a fresh PDA medium. Pure cultures for 63 isolates were recovered and further used for taxonomic identification.
Initially, the colony appearance for all 63 isolates was recorded after 7 days post-inoculation on PDA. Later, conidial morphology was recorded under a bright LED field and phase contrast microscope with a digital color camera (BX-53, Olympus, USA). Conidial morphology, size and number of septations and other special features like hilum were recorded for each isolate. Based on the colony shape and conidial morphology, 63 isolates were tentatively identified for their taxonomy. Further, about 19 isolates representing each district, colony type, and conidial morphology were selected for taxonomic identification through ITS sequence analysis.
Cultural and morphological variability
All isolates, irrespective of their taxonomic identity, were grown on PDA plates to study cultural and morphological characteristics. The Petri plates containing PDA medium were inoculated in the center with actively growing 5 mm mycelial discs and incubated at 25 ± 2 °C. The experiment was repeated thrice using the same isolate and media. The cultural characteristics, viz., colony diameter, colony color, and growth pattern, were recorded after ten days of inoculation. The morphological characters, viz., sporulation, color, size (length and width) of the conidia, and the number of septa were also recorded under a bright LED field and phase contrast microscope attached with a digital color camera (BX-53, Olympus, USA).
DNA isolation, Primer synthesis, and PCR amplification
Total DNA from selected 19 isolates was extracted from mycelium using the cetyl trimethylammonium bromide (CTAB) method described by Murray and Thompson (1980). About 0.5 g of 5-day-old mycelium grown on PDA was ground to a fine powder using liquid nitrogen and was used for total DNA extraction. The quality of DNA was assessed on 1% agarose gel electrophoresis and quantified using a Qubit 4.0 Fluorometer (Qubit 4.0, Invitrogen, USA). A previously reported primer pair (ITS 1 and 4) was designed to amplify the conserved ITS 1 and 4 regions (White et al. 1990; Gardes and Bruns 1993). Primer sequences were synthesized at a commercial facility (Shrimpex, Tamil Nadu, India). The PCR reaction mixture consisted of 0.2 mM of dNTPs, 5 units of Taq DNA polymerase, 10Χ Taq buffer, 1.5 mM of MgCl2, 0.2 µM each forward and reverse primer, 50 ng of template DNA and MilliQ water was used to make the final volume to 50 μl. The PCR Amplifications were conducted in a thermal cycler (Eppendorf, Hamberg, Germany) with an initial denaturation of 5 min at 94 °C, 35 cycles (each) 1 min at 94 °C, 1 min at 60 °C, and 1 min at 72 °C; and a final extension of 10 min at 72 °C and hold at 4 °C. The amplified product was analyzed using 1% agarose gel electrophoresis.
Sequencing and sequence analysis
The PCR amplified products were purified using the HiPura® PCR Product Purification Kit (HiMedia™ Laboratories Pvt Ltd, Mumbai, India) following the manufacturer’s instructions. The PCR-amplified products of 19 isolates were sequenced using a commercial facility (Eurofins, Bangalore, India). The raw sequences obtained were aligned using BioEdit software (Version 7.2.5), and the purified sequences obtained were subjected to the BLAST analysis in the NCBI GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for taxonomic matching. Finally, the consensus sequences obtained for each isolate/pathogen were deposited in the NCBI GenBank with accession numbers (Table 1).
Pathogenicity test for different pathogens
Pure cultures of all three identified pathogens (B. oryzae, C. lunata, and E. rostratum) were used to establish Koch’s postulates on a BS-susceptible cultivar, Gangavathi Sona (cv. GNV-05-01). Disinfected viable seeds (treated with carbendazim 50 WP at the rate of 2 g/kg of seeds) of the rice variety GNV-05-01 were sown in plastic pots filled with sterilized soil. The plants were maintained under an environmentally controlled growth chamber with an optimum temperature of 30 ± 2 °C. Three isolates of each pathogen, viz., B. oryzae (BO-BGV, BO-UK, and BO-VJN), C. lunata (CL-DWD, CL-CKM, and CL-RCR1), E. rostratum (SR-KPL-1, SR-BLR-1, and SR-SMG-1) were mass multiplied on PDA for seven days at 25 ± 2 °C. The mycelial mat was scrapped from the PDA plates using a sterile blade and ground with sterile distilled water to make the suspension comprised of spores and mycelial bits. The spore suspension was sieved through a double-layered muslin cloth to remove mycelial clumps and traces of media. Inoculum suspension of each isolate/pathogen was spray inoculated (until run-off) to the leaves of 30 days seedlings, whereas water-inoculated plants served as control. The experiment was replicated thrice and inoculated plants were covered with polythene until symptom development.
Phylogenetic analysis
The ITS sequences of 19 isolates of three pathogens were used to deduce the evolutionary relationship between the intra- and interspecies. The ITS sequences for other reference strains (from different countries and hosts) of each species available in the NCBI GenBank were also retrieved and used in the analysis. Phylogenetic analysis was performed in MEGA 11 (Tamura et al. 2021). The evolutionary history was inferred using the neighbor-joining method. Sequences were assembled to generate the consensus sequence identity matrix using BioEdit (Version 7.2.5) (Hall 1999). A combined phylogenetic tree was constructed for all three pathogens studied in this work.
Nucleotide diversity and haplotype analysis
The DNA sequences of B. oryzae, C. lunata, and E. rostratum samples were separately used for multiple sequence alignment using the Clustal W multiple alignment method of MEGA 11 (Tamura et al. 2021), and the aligned sequences were exported for DNA polymorphism analysis using DnaSP v6.12.03 software (Rozas et al. 2017). The number of polymorphic/segregating sites (S), nucleotide diversity (Pi), Theta (per site and sequence) from S (Theta-W), Average number of nucleotide differences (k), Tajima’s D (D), the number of haplotypes and haplotype diversity were analyzed. Further, the haplotype data exported from DnaSP v6.12.03 software was utilized to construct the haplotype tree based on the median-joining algorithm calculation method using NETWORK 10.2.0.0. (https://fluxus-engineering.com/).
In-planta pathogen distribution and their frequency
After isolation and confirming B. oryzae, C. lunata, and E. rostratum as pathogens associated with the BS of rice, we conducted a field experiment to test their distribution on the diseased leaves. The BS-infected leaf samples were collected randomly from the BS-infected fields (n = 10) at the Agricultural Research Station, Gangavathi, India. Each field sample comprised three infected plants; from each infected plant, about 20 typical brown spots were excised, amounting to 60 samples from each field (20 × 3 = 60). These excised leaf bits (brown spots) were cultured on the PDA medium and incubated at 25 ± 2 °C for 7 days. After the incubation, the associated pathogen was identified based on the colony appearance, growth characteristics, and conidial characters. Based on the pathogen identity, the per cent distribution of each pathogen was calculated.
Results
Survey and sample collection
Among the surveyed districts, the presence of BS disease was recorded in all the locations with varied levels of disease severity (PDI, 20.56–50.74). Disease severity was recorded in all the surveyed locations, right from the nursery to the maturity stage, on all the rice cultivars. The spots resembled sesame seeds with cylindrical to oval brown spots surrounded by a yellow halo on the leaves. The symptoms were similar irrespective of cultivars, region, and stage of the crop. The overall mean disease severity varied between 20.56% and 50.74% in the surveyed districts. The disease was found to be more severe in Ballari (50.74%), followed by Koppal (39.05%) and Dharwad (38.77%); in comparison, the severity was found to be lowest in Shivamogga (20.56%) (Table 1). With respect to a different type of cultivation, the disease was found to be more severe in direct seeded rice (DSR) under a rainfed system (94.44 PDI) as compared to the transplanted-irrigated system (35.55 PDI) irrespective of the cultivars and geographical region. Among the cultivars, cv. Intan and cv. GNV-05-01 (Gangavathi Sona) were found to be severely infected with BS disease (Table 1).
Isolation and morphological identification of the pathogens
Associated fungal pathogens were isolated from all the diseased leaf samples collected from different geographical locations of Karnataka. Based on the cultural and microscopic characteristics, the pathogens were tentatively identified as B. oryzae, C. lunata, and E. rostratum.
Initially, B. oryzae produced greyish to black color colonies all along the margins of inoculated leaf bits and formed a greyish radial mycelial colony. Later, 10 days after incubation, radial growth of the colony increased and showed a whitish-grey-light brown fluffy growth. Microscopic observation revealed the presence of septate mycelium, which was light brown, on which conidiophores were produced. The conidiophores were thick and dark at the base and lighter towards the tip, and they produced the conidia with 5–10 septa (n = 30). The fully matured conidia were brownish in color (26.47 × 8.56 µm) (Fig. 1). After 10 days of incubation, C. lunata produced flattened blackish to greyish mycelial colonies with black pigmentation on the reverse side of the Petri Plate. The conidia were fusiform in shape, curved slightly at the second cell with three septations (21.15 × 9.53 µm) (Fig. 1). Whereas E. rostratum produced white mycelium, which later turned to grey and finally converted to black after 8–10 days. Cylindrical to ellipsoid-shaped conidia were formed at the apex of the conidiophores, were straight to slightly curved with 5–8 dissertate, and were darker in color towards the basal region. The conidial wall is darker in color except near the apex region and measures about 46.2–49.12 × 12–14.5 µm with a characteristic protruding truncated hilum (Fig. 1).
Cultural and morphological characteristics of brown spot associated pathogens; A whitish-grey colony (Bipolaris oryzae), Blackish colony (Curvularia lunata) and grey color (Exserohilum rostratum); B Conidial and mycelial characters (100X); C Conidial morphology of B. oryzae, C. lunata, and E. rostratum observed under bright LED field and phase contrast microscope (400X (BX-53, Olympus, USA)
In total, following the single spore isolation technique, we obtained the pure culture for 40 isolates of B. oryzae (63.5%), 15 isolates of C. lunata (23.8%), and eight isolates of E. rostratum (12.6%).
Cultural and morphological variability
Based on the colony color and type, all 40 isolates of B. oryzae were categorized into four (greyish, greyish-white, greyish-brown, and greyish-black) and three (flat, cottony, and fluffy) groups, respectively (Table 2). Among the 40 isolates, the majority of the isolates produced greyish (n = 21) and greyish-white mycelia (n = 14) with cottony growth (n = 20) (Online Resource 1). Fifteen isolates of C. lunata were categorized into two groups with respect to their colony color (blackish and greyish) and type (flat and raised) (Online Resource 1, Table 2). The E. rostratum isolates were considered as one group with greyish flat colony growth and a good spore former (Online Resource 1, Table 2). But surprisingly, the majority of B. oryzae isolates did not sporulate on the PDA medium and were categorized as non-sporulating (n = 17); the rest of the isolates were categorized into poor (n = 16), moderate (n = 4), and good (n = 3) sporulating isolates. In comparison, the majority of C. lunata isolates were good at sporulating (n = 10), even though some isolates were poor (n = 4) to moderate (n = 1) spore formers.
Molecular identification
Further, nine isolates of B. oryzae, seven isolates of C. lunata, and three isolates of E. rostratum were selected for ITS-based taxonomic identification. The ITS sequences of all three pathogen strains were deposited in the NCBI GenBank with accession numbers (Table 1). The BLAST analysis of the ITS sequences of nine isolates of B. oryzae, six isolates of C. lunata, and three isolates of E. rostratum revealed the 99–100% nucleotide sequence identity with the B. oryzae, C. lunata, and E. rostratum strains, respectively in the NCBI database which confirmed the taxonomic identity of three pathogens as initially indicated by the cultural and microscopic characters.
Pathogenicity test
A pathogenicity test was conducted for three isolates of each of the three pathogens on a BS-susceptible rice cultivar in an environmentally controlled growth chamber. After 5–8 days post-inoculation, a minute pin head-shaped brownish flecks appeared on the upper surface of the leaves inoculated with B. oryzae, C. lunata, and E. rostratum after 5 days post-inoculation. Later (10 dpi), such pin-head-shaped flecks matured into brown to dark brown lesions, which are the characteristics of the brown spot. In contrast, no symptoms were produced on the plants sprayed with distilled water. All 30 seedlings inoculated with B. oryzae, C. lunata, and E. rostratum showed the characteristic symptoms of BS disease (Table 3). The symptoms produced on the artificially diseased plants were similar to the BS symptoms recorded in the field. Further, all three pathogens were re-isolated from the artificially inoculated plants and confirmed based on the culture-microscopic method. It was revealed that B. oryzae can produce symptoms 2–3 days earlier than C. lunata and E. rostratum. Symptomatically, three pathogens are indistinguishable even after 30 dpi.
Phylogenetic analysis
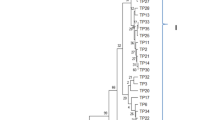
For carrying out phylogenetic analysis, consensus sequences of nine isolates of B. oryzae, seven isolates of C. lunata, and three isolates of E. rostratum, along with ITS sequences of reference isolates available in NCBI GenBank, were used. A neighbor-joining tree constructed using 36 strains of Bipolaris spp., 34 strains of Curvularia spp., and 37 strains of Exserohilum spp., diverged into three major genus-specific clusters (Fig. 2). A separate cluster was formed among the strains of Bipolaris spp. in which all nine isolates of the study were grouped into one cluster, along with the other strains of B. oryzae from Thailand, China, Sri Lanka, Mexico, Kenya, and the USA. This result indicated that the B. oryzae strains causing BS in Karnataka share common ancestorial evolution as that of other strains used in the study (Fig. 2). Phylogenetic analysis of ITS sequences of seven strains of C. lunata from this study, along with 27 strains of Curvularia spp., formed a major cluster separated from Bipolaris spp., and Exserohilum spp. (Fig. 2). Interestingly, one strain, CL-VJN-IND-Rice, formed a sub-cluster separated from other strains of the study, indicating an independent evolutionary history (Fig. 2). Phylogenetic analysis of three strains of E. rostratum of this study along with 34 geo-distinct strains of Exserohilum spp., indicated the separate genus-specific-clustering patterns (Fig. 2) in a neighbor-joining tree. Within this Exserohilum-cluster, all three strains of this study were grouped together as separate sub-cluster along with mulberry (Accession No. MH244432), Jatropha (Accession No. OP861483), coconut (Accession No. OK271378), lesser joy weed (Accession No. ON331993), and cucumber (Accession No. MN337265) strains of E. rostratum from Indian origin. Interestingly, a human strain of E. holmii from India (Accession No. MH319028) was also clustered with E. rostratum strains infecting plants in India (Fig. 2).
Phylogenetic tree showing the evolutionary relationships among the geo-distinct isolates of Bipolaris spp., Curvularia spp. and Exserohilum spp. The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the p-distance method and are in the units of the number of base differences per site. Evolutionary analyses were conducted in MEGA 11. The isolates of this study are highlighted in green circles (Bipolaris oryzae), orange rhomboid (Exserohilum rostratum) and blue triangles (Curvularia lunata)
Nucleotide diversity and haplotyping analysis
A total of 167 sites (excluding sites with gaps/missing data) were observed in 36 sequences of B. oryzae. The polymorphic (segregating) sites among the 36 samples of B. oryzae studied was 3. The average number of nucleotide difference (k), Theta (per site) from S (Theta-W), Theta (per sequence) from S, (Theta-W), and nucleotide diversity (Pi) were found to be 0.171, 0.00436, 0.728 and 0.00103, respectively. Tajima’s D (D) test was used to test the neutrality of nucleotide mutation, and it was found to be negative (D = 1.72516) and non-significant at P value > 0.10. The number of haplotypes (h) was found to be 3, distributing all 35 isolates with a haplotype diversity (hd) of 0.113, a variance of Haplotype diversity of 0.00516, and a standard deviation of Haplotype diversity of 0.072. Among the three haplotypes, Hap_1 has the highest number of isolates, with 33 isolates, followed by one each in Hap_2 (MH481660) and Hap_3 (MH481658). The major haplotype Hap_1 consisted of 33 isolates (Fig. 3A). The three haplotype groups have been represented as haplotype tree drawn based on nucleotide sequences representing the differentiation of isolates (Fig. 3A).
Haplotype tree showing different haplotype groups among the different pathogens of brown spot disease based on nucleotide polymorphisms. A Presence of 3 haplotype groups among the 35 isolates of Bipolaris spp. B The presence of 4 haplotype groups among the 34 isolates of Curvularia spp. C The existence of 6 haplotype groups among the 38 isolates of Exserohilum spp
Similarly, a total of 102 sites (excluding sites with gaps/missing data) were observed in 34 sequences of C. lunata. The polymorphic (segregating) sites among the 34 sequences of Curvularia were 6. The average number of nucleotide difference (k), Theta (per site) from S (Theta-W), Theta (per sequence) from S, (Theta-W), and nucleotide diversity (Pi) were found to be 0.520, 0.01439, 1.467 and 0.00510, respectively. Tajima’s D (D) test is used to test the neutrality of nucleotide mutation, and it was found to be negative (D = − 2.16734) and significant at P value < 0.05. The number of haplotypes (h) was found to be 4, distributing all 34 isolates with a haplotype diversity (hd) of 0.171, a variance of Haplotype diversity of 0.00739, and a standard deviation of Haplotype diversity of 0.086. Among the four haplotypes, Hap_2 has the highest number of isolates, with 31 isolates of Curvularia followed by one each in Hap_1 (MH568685), Hap_3 (MH478165) and Hap_4 (MH478169) (Fig. 3B). The four haplotype groups of Curvularia have been represented as haplotype tree drawn based on nucleotide sequences representing differentiation of isolates.
In the case of E. rostratum, a total of 156 sites (excluding sites with gaps/missing data) were observed in 37 sequences. The polymorphic (segregating) sites among the 37 sequences of Exserohilum spp. isolates was 85. The average number of nucleotide difference (k), Theta (per site) from S (Theta-W), Theta (per sequence) from S, (Theta-W), and nucleotide diversity (Pi) were found to be 6.057, 0.12968, 20.230, and 0.03883, respectively. Tajima’s D (D) test is used to test the neutrality of nucleotide mutation, and it was found to be negative (D = − 2.67285) and significant at P value < 0.001. The number of haplotypes (h) was found to be 6, distributing all the 37 isolates with a haplotype diversity (hd) of 0.249, a variance of Haplotype diversity of 0.00856, a standard deviation of Haplotype diversity of 0.093. Among the six haplotypes, Hap_2 has the highest number of Exserohilum isolates with 32 isolates, followed by one each in Hap_1 (MH478168), Hap_3 (MH201152), Hap_4 ([MH319028), Hap_5 (KR263036) and Hap_6 (NR_138225). The six haplotype groups of Exserohilum isolates have been represented as haplotype tree drawn based on nucleotide sequences representing differentiation of isolates (Fig. 3C).
In-planta pathogen distribution
After confirming the association of three pathogens in causing the BS of rice, we conducted a field experiment on the in-planta distribution of three pathogens on the same diseased leaf/plant. The spots were subjected to pathogen isolation on PDA, followed by microscopic identification for taxonomy. Out of the 600 disease spots collected from different locations of the same field, about 77.83% (467/600) spots were caused by B. oryzae. Meanwhile, 17.33% (104/600) and 4.83% (29/600) of the brown spots were produced by C. lunata and E. rostratum, respectively (Fig. 4, Online Resource 2). The results showed that all three pathogens can able to cause BS disease in the field. However, B. oryzae was found to be the predominant pathogen.
Discussion
BS of rice is an important disease affecting millions of hectares worldwide every year. Now, the disease is prevalent in almost all the rice-growing states of the country in sporadic to epidemic form, especially in the regions where rice is being grown as direct seeded rice (DSR) under rainfed conditions (Gangopadhyay 1983; Anonymous 2022). Moreover, in India, in recent times, the rice crop establishment method has been shifting from transplantation to the DSR system due to a shortfall in irrigation water, which is leading to changes in the pathogen profile and their severity in the new system of rice cultivation (Anonymous 2022). Therefore, in the present study, we aim to identify the status of BS disease in different ecosystems of Karnataka state, a predominantly rice-growing southern state in India. A roving survey during Kharif 2017 at different stages of the crop suggested the wider occurrence of the disease in all the surveyed ecosystems irrespective of the genotype and crop establishment methods. Our study reported up to 50% PDI, which was significantly more compared to the previous reports (up to 17%) reported by the earlier studies (Channakeshava 2016; Chethana et al. 2016; Satishkumar 2017). Our study also reported the difference in the severity of BS disease in different crop establishment methods, wherein the transplanted paddy had a lesser disease severity (up to 35.55 PDI) compared to DSR (up to 94.44 PDI) irrespective of cultivar and geographical region. This increased disease severity in DSR could be due to high in-field nutrient leaching (Surendhar et al. 2021), improper water distribution due to poor land leveling, high weed infestation, and improper plant-to-plant spacing under DSR cultivation method (Zadoks 1974; Ou, 1985; Sunder et al. 2005; Barnwal et al. 2013; Priyadashani et al. 2022).
Historically, BS has been reported to be caused by B. oryzae; however, its etiology has become more complex as several reports suggest the association of more than one fungal pathogen, such as C. lunata, from India, Malaysia, Pakistan, Australia, and Cambodia (Kamaluddeen et al., 2013; Kusai et al. 2015; Khemmuk et al. 2016; Majeed et al. 2016a, b; Tann and Soytong 2017), E. rostratum from Pakistan (Majeed et al. 2016a, b). In India, a report on the association of E. rostratum with rice crops was reported in 1987 by Sivanesan (1987). However, its pathogenic abilities on rice have not been explored systematically. Similarly, a report on the association of C. lunata as a causal agent of BS of rice has been published (Kamaluddeen et al., 2013), but no further studies were made to unravel its on-field distribution, genetic diversity, and in-planta distribution along with other BS-causing pathogens.
From the typical leaf lesions of BS disease, associated fungi were isolated following the standard fungal isolation protocol and were purified using single-spore isolation techniques. All the pure cultures were initially observed for their conidial morphology and were tentatively identified as three pathogens (Fig. 1). Of 63 pure cultures, 40 were identified as B. oryzae, 15 as C. lunata, and eight cultures as E. rostratum. Further, the taxonomic identity was confirmed through ITS sequence analysis of the selected pure cultures. Here, we employed both conventional microscopic-spore morphology and sequencing of ITS for pathogen species identification to rule out any possible error in species identification. All three identified pathogens (three isolates of each) were tested for their pathogenicity on a BS-susceptible rice cultivar (cv. GNV-05-01), which revealed that all three suspected fungal pathogens (B. oryzae, C. lunata, and E. rostratum) could induce the typical BS disease symptoms on rice. We found that both E. rostratum and C. lunata could induce all the characteristic symptoms of BS disease, as that of B. oryzae, thus proving its pathogenic nature in causing the BS disease of rice. However, B. oryzae can induce the disease 2–3 days early compared to E. rostratum and C. lunata. During the in-planta study, B. oryzae was predominant compared to E. rostratum and C. lunata. A previous study in Australia highlighted the predominance of B. oryzae in the BS-infected samples compared to C. lunata, although both pathogens were recovered from the infected samples (Khemmuk et al. 2016).
Based on the symptoms, it was not possible to detect the mixed infection in the field or in-planta experiments. Previous reports also concluded similarly that, based on the symptomatology, BS-causing pathogens could not be identified (Khemmuk et al. 2016). A detailed study to identify the cultivar differentials to distinguish the symptomatology of these three pathogens, if any, is required. A new study from India investigated the host range and symptoms produced by E. rostratum on different hosts; they provided evidence of E. rostratum infecting wheat, rice, and finger millet (Korra et al. 2023). Even though the pathogen has a wide host range, the symptoms produced on wheat and rice were similar, often mimicking the symptoms of BS (Korra et al. 2023). A recent report suggests that B. oryzae can be a cross-infective pathogen with other crops; very recently, a new study from India has highlighted the cross-infection of B. oryzae with wheat and the co-existence of B. oryzae and B. sorokiniana in regions where the rice–wheat cropping system was predominant (Singh et al. 2021). This information further supports our findings that B. oryzae can co-exist with other fungal pathogens, which can cause disease symptoms similar to those of B. oryzae.
In addition to causing disease in several crop plants, E. rostratum and C. lunata are known to be opportunistic human pathogens and represent typical cross-kingdom pathogens that infect hosts of different taxonomic groups (Rinaldi et al. 1987; Yau et al. 1994; Gauthier and Keller 2013; Sharma et al. 2014). There are several well-known cases of cross-kingdom host jump, such as Burkholderia cepacia from maize to humans (Di Cello et al. 1997); a plant endophyte Cryptococcus gattii from forest trees to humans (Datta et al. 2009), plant pathogenic Alternaria alternata, Aspergillus flavus, and Fusarium oxysporum to humans (Gauthier and Keller 2013). A previous study has reported the cross-kingdom pathogenicity of a plant strain of E. rostratum on humans and visa-versa (Sharma et al. 2014). Therefore, our study has expanded the scientific literature that documented the host range of these two cross-kingdom pathogens.
Further, the studies on diversity among the geo-distinct isolates of each pathogen revealed the significant morpho-cultural-genetic diversity within the pathogen population. Previous studies have reported the morphological diversity among the isolates of B. oryzae (Kumar et al. 2011; Valarmathi and Ladhalakshmi 2018). Interestingly, six isolates of B. oryzae collected from the severely infected fields (on field PDI 32.22–94.44%) produced a greyish mycelia with fluffy growth (n = 6). Therefore, it could be a morphological marker that can be used for quick identification of virulent isolates of B. oryzae; however, this observation requires further research to confirm this marker-trait association. In a previous study, morphological characters have been used to co-relate the potential virulence of the B. oryzae isolates (Kumar et al. 2016).
In our study, we adopted the ITS sequence-based strategy, as reported previously for other rice fungal pathogens (Sharanabasav et al. 2021; Amoghavarsha et al. 2022), to study the genetic diversity among different isolates of three pathogens. The ITS sequencing was employed for analyzing the genetic diversity among C. lunata isolates of maize from China (Liu et al. 2015) and from rice (Kusai et al. 2015; Khemmuk et al. 2016; Majeed et al. 2016a, b; Tann and Soytong 2017). The phylogenetic analysis among the 36 isolates of Bipolaris spp. indicated that all Indian strains of B. oryzae grouped in the same cluster and were genetically related to all other strains used in the study, indicating their common ancestral origin. Similarly, Indian C. lunata isolates have also clustered together in a single cluster except CL-VJN-IND-Rice, which diverged from other isolates and formed separate sub-cluster. Three isolates of E. rostratum grouped together with other geo-distinct strains of E. rostratum isolated from diverse hosts. The analysis indicated that the rice strain E. rostratum grouped with strains (of Indian origin) isolated from mulberry, jatropha, coconut, lesser joy weed, and cucumber. Interestingly, a human strain of E. holmii from India (Accession No. MH319028) was also clustered with E. rostratum strains infecting plants in India. These results provided preliminary evidence that the different strains of E. rostratum, infecting different crops, share a common ancestral origin, and this needs to be confirmed further using complete genome sequences of all available strains.
The phylogeny-based genetic diversity among the isolates of three pathogens has also been supported by the nucleotide diversity in the ITS sequences and haplotyping. The haplotype analysis has identified three haplotypes among the geo-distinct isolates Bipolaris spp. However, there was an incongruence with respect to isolates grouped in different phylogenetic clusters and haplotype groups. Isolates BO-VJN and BO-MND formed separate haplotypes, although these two formed the same phylogenetic cluster (Fig. 2). Similar results have also been reported for Magnaporthe oryzae isolates from India (Amoghavarsha et al. 2022). Whereas isolates of Curvularia spp. formed four haplotypes compared to two sub-clusters in the phylogenetic analysis. Unlike in the phylogeny, isolates such as CL-DWD and CL-CKM formed a separate haplogroup, indicating their genetic divergence from other isolates. Haplotype analysis indicated a greater number of haplotypes (n = 06) among the distinct strains of Exserohilum spp. indicating the high degree of genetic diversity among them.
The present investigation provides conclusive evidence of the wide prevalence of BS disease in all rice ecosystems and also an association of three fungal pathogens such as B. oryzae, C. lunata, and E. rostratum, in causing BS disease in rice in India. To the best of our knowledge, this is the first study to report the genetic diversity among cross-kingdom infecting E. rostratum and C. lunata isolates sampled from rice. Although a preliminary report was available on the association of C. lunata causing the BS disease of rice in India, we are providing conclusive evidence for the multiple pathogens associated with it. This study is very important for re-designing the BS disease research, which was otherwise focused only on B. oryzae.
Data availability
The NCBI GenBank accession numbers of genomic sequences used in this study are given in the text.
References
Ahmadpour A, Karami S, Heidarian Z, Javan-Nikkhah M (2013) Exserohilum rostratum causing sugarcane leaf spot in Iran. Australasian Pl Dis Notes 8:97–99
Al-Odaini N, Pan KS, Liao LW, Mo NF, Jiang ZW, Li TT, Li XY, He XJ, Zheng DY, Cao CW (2022) Experimental Phaeohyphomycosis of Curvularia lunata. J Clin Med 11(18):5393
Amoghavarsha C, Pramesh D, Naik GR, Naik MK, Yadav MK, Ngangkham U, Chidanandappa E, Raghunandana A, Sharanabasav H, Manjunatha SE (2022) Morpho-molecular diversity and avirulence genes distribution among the diverse isolates of Magnaporthe oryzae from Southern India. J Appl Microbiol 132(2):1275–1290
Anonymous, 2022, Production Oriented Survey, All India Coordinated Research Project on Rice ICAR-Indian Institute of Rice Research, Rajendranagar, Hyderabad-500 030, TS, India
Arunakumar GS, Gnanesh BN, Pooja D, Sivaprasad V (2019) First report of Setosphaeria rostrata causing leaf spot on mulberry in India. Plan Dis 103(4):1–4
Asma J, Subrahmanyam D, Krishnaveni D (2023) The Global Lifeline: A Staple Crop Sustaining TwoThirds of the World's Population. Agri Arch 2(3):15–18
Barnwal MK, Kotasthane A, Magculia N, Mukherjee PK, Savary S, Sharma AK, Singh HB, Singh US, Sparks AH, Variar M, Zaidi N (2013) A review on crop losses, epidemiology and disease management of rice brown spot to identify research priorities and knowledge gaps. Eur J Pl Pathol 136:443–457
Chakrabarti NK (2001) Epidemiology and disease management of brown spot of rice in India. Major fungal disease of rice: recent advances. Kluwer Academic Publishers, Dordrecht, Netherlands, pp 293–306
Channakeshava (2016) Studies on brown spot disease of paddy. Dissertation, University of Agricultural Sciences, Bangalore
Chethana BS, Deepak CA, Rajanna MP, Ramachandra C, Shivakumar N (2016) Current scenario of rice diseases in Karnataka. Intl J Sci and Nat 7(2):405–412
Choudhary M, Sardana HR, Bhat MN, Gurjar MS (2018) First report of leaf spot diseases caused by Exserohilum rostratum on bottle gourd in India. Pl Dis 102:2042–2042
Datta K, Bartlett KH, Marr KA (2009) Cryptococcus gattii: emergence in Western North America: exploitation of a novel ecological niche. Interdisciplinary Persp Inf Dis. https://doi.org/10.1155/2009/176532
Dhara B, Maity A, Mondal P (2020) First report of Excerohillum leaf spot: a unique halophilic pathogen in Cucumis sativus in the South Bengal area of India. Aust Pl Pathol 49:257–266
Di Cello F, Bevivino A, Chiarini L, Fani R, Paffetti D, Tabacchioni S, Dalmastri C (1997) Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different plant growth stages. Appl Environ Microbiol 63(11):4485–4493
Drechsler C (1923) Some graminicolous species of Helminthosporium. J Agric Res 24:641–740
Gangopadhyay S (1983) Current concepts on fungal diseases of rice. Today and tomorrow’s printers and publishers, New Delhi., p 349
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118
Gauthier GM, Keller NP (2013) Crossover fungal pathogens: the biology and pathogenesis of fungi capable of crossing kingdoms to infect plants and humans. Fungal Genet Biol 61:146–157
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic acids symposium series 41: 95-98
IRRI (2013) Standard evaluation system for rice, 5th edn. International Rice Research Institute, Manila, Philippines
Kamaluddeen SS, Lal AA (2013) A new blight disease of rice caused by Curvularia lunata from Uttar Pradesh. Int J Agri Sci Res 3(5):13–16
Khemmuk W, Shivas RG, Henry RJ, Geering ADW (2016) Fungi associated with foliar diseases of wild and cultivated rice (Oryza sativa) in northern Queensland. Aust Pl Pathol 45:297–308
Korra T, Navathe S, Biradar S, Chand R (2023) Pathogenicity and infection behaviour of Exserohilum rostratum on wheat and associated collateral hosts. J Pl Pathol 105(3):695–709
Kumar P, Anshu V, Kumar S (2011) Morpho-pathological and molecular characterization of Bipolaris oryzae in rice (Oryzae sativa). J Phytopathol 159:51–56
Kumar A, Solanki IS, Akhtar J, Gupta V (2016) Morpho-molecular diversity of Bipolaris oryzae causing brown spot of paddy. Indian J Agric Sci 86(5):615–620
Kusai NA, Azmi MMZ, Zulkifly S, Yusof MT, Zainudin NAIM (2015) Morphological and molecular characterization of Curvularia and related species associated with leaf spot disease of rice in Peninsular Malaysia. Rend Fis Acc Lincei. https://doi.org/10.1007/s12210-015-0458-6
Lin SH, Huang SL, Li QQ, Hu CJ, Fu G, Qin LP, Ma YF, Xie L, Cen ZL, Yan WH (2011) Characterization of Exserohilum rostratum, a new causal agent of banana leaf spot disease in China. Aust Pl Pathol 40:246–259
Liu T, Zhao FZ, Wang YY, Hou JM, Liu LZ, Shen YQ, Liu Z, Zhang HT, Zuo YH (2015) Comparative analysis of phylogenetic relationships, morphologies, and pathogenicities among Curvularia lunata isolates from maize in China. Gene Mol Res 14(4):12537–12546
Majeed RA, Shahid AA, Ashfaq M (2016a) First report of Curvularia lunata causing brown leaf spot of rice in Punjab. Pak Pl Dis 100:219
Majeed RA, Shahid AA, Liaqat GA, Saleem K, Asif M, Shafiq M (2016b) First report of Setosphaeria rostrata causing brown leaf spot of rice in Pakistan. Pl Dis 100(11):2162–2163
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acid Res 8:4321
Ou SH (1985) Rice diseases, 2nd edn. England, CMI, Kew, p 370
Padmanabhan SY (1973) The Great Bengal famine. Ann Rev Phytopathol 11:11–26
Priyadashani C, Wickramasinghe ADM, Priyanka C, Egodawatta ADB, Weerasinghe APA, Devasinghe AU (2022) Effect of rates and sources of N fertilizer application on dynamics of rice brown leaf spot disease (Bipolaris oryzae) incidences in the dry zone of Sri Lanka. J Tropical Crop Sci 9(3):165–173
Rinaldi MG, Phillips P, Schwartz JG, Winn RE, Holt GR, Shagets FW, Elrod J, Nishioka G, Aufdemorte TB (1987) Human Curvularia infections: report of five cases and review of the literature. Diagnostic Microbiol and Infect Dis 6(1):27–39
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol 34(12):3299–3302
Satishkumar (2017) Studies on brown leaf spot of rice and its management. Dissertation. University of Agricultural Sciences, Dharwad.
Sharanabasav H, Pramesh D, Prasannakumar MK, Chidanandappa E, Yadav MK, Ngangkham U, Parivallal B, Raghavendra BT, Manjunatha C, Sharma SK, Karthik N (2021) Morpho-molecular and mating-type locus diversity of Ustilaginoidea virens: an incitant of false smut of rice from Southern parts of India. J Appl Microbiol 131(5):2372–2386
Sharma K, Goss EM, Dickstein ER, Smith ME, Johnson JA, Southwick FS, Van Bruggen AH (2014) Exserohilum rostratum: characterization of a cross-kingdom pathogen of plants and humans. PLoS ONE 9(10):e108691
Singh K, Aggarwal R, Sharma S, Gurjar MS, Manjunatha C, Choudhary M (2021) Molecular and phenotypic analysis reveals cross infection of Bipolaris species in wheat and rice. Indian Phytopathol 74:929–938
Sivanesan A (1987) Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycol Pap 158:1–261
Sunder S, Ramsingh RA (2014) Brown spot of rice: an overview. Indian Phytopathol 67(3):201–215
Sunder S, Singh R, Dodan DS, Mehla DS (2005) Effect of different nitrogen levels on brown spot (Drechslera oryzae) of rice and its management through host resistance and fungicides. Plant Dis Res 20:111–114
Surendhar M, Anbuselvam Y, Ivin J (2021) Status of rice brown spot (Helminthosporium oryzae) management in India: a review. Agril Rev 43(2):217–222
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027
Tann H, Soytong S (2017) Biological control of brown leaf spot disease caused by Curvularia lunata and field application method on rice variety IR66 in Cambodia. AGRIVITA J Agric Sci 39:111–117
Tuite J (1969) Plant pathological methods: fungi and bacteria. Burgess Publishing Company, Minneosta
Valarmathi P, Ladhalakshmi D (2018) Morphological characterization of Bipolaris oryzae causing brown spot disease of rice. Int J Curr Microbiol App Sci 7(2):161–170
Wheeler BEJ (1969) An introduction to plant diseases. John Wiley and Sons Ltd, London, p 301
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18(1):315–322
Wu D, Turgeon BG (2013) Setosphaeria rostrata: insights from the sequenced genome of Setosphaeria turcica. Fungal Gen Biol 61:158–163
Yau YC, de Nanassy J, Summerbell RC, Matlow AG, Richardson SE (1994) Fungal sternal wound infection due to Curvularia lunata in a neonate with congenital heart disease: case report and review. Clin Infect Dis 19(4):735–740
Zadoks JC (1974) The role of epidemiology in modern Phytopathology. Phytopathol 64:918–929
Acknowledgements
The authors thank the Director of Research, UAS Raichur, for the research facilities. This project was funded by the fungicide testing projects (Ab. Ac. 8096) to DP.
Author information
Authors and Affiliations
Contributions
DP and MKP conceived the project, arranged the funds, designed the experiments, and reviewed the manuscript. MKK, PPB, and BK conducted the survey, collected isolates, cultured and performed a morpho study. AR, UN, HDP, and CM analyzed the data, wrote the first draft, reviewed the draft, and prepared the fig and tables. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Supplementary Information
Below is the link to the electronic supplementary material.
13205_2024_4033_MOESM1_ESM.jpg
Supplementary file1 Online resource 1. Morpho-cultural variability of pathogens associated with brown spot disease of rice A. Bipolaris oryzae: Greyish, Greyish-white, Greyish-brown, Greyish-black; B. Curvularia lunata: Blackish, Greyish; C. Exserohilum rostratum (JPG 223 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pramesh, D., Prasannakumar, M.K., Raghunandana, A. et al. Identification and characterization of multiple fungal pathogens associated with brown spot disease of rice in India. 3 Biotech 14, 187 (2024). https://doi.org/10.1007/s13205-024-04033-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-024-04033-3