Abstract
Copra meal is a by-product of coconut milk extraction, contained 4.60 ± 0.01 g/100 g DM and 62.19 ± 0.53% of protein and fiber, respectively. The optimal condition for quality improvement of copra meal was investigated using Box–Behnken design combined with response surface methodology (RSM). The simultaneous saccharification and fermentation (SSF) of Copra meal was performed by mannanase enzyme and yeast, Saccharomyces cerevisiae. The concentration of mannanase was determined as the most important factor to increase protein content in copra meal. The protein content was increased by 64% when 0.7% of enzyme per copra meal dry weight, the ratio of copra meal to water at 1:4.56 and fermentation time of 90.25 h at 30 °C were used. The program predicted an increase of 3.06 g of protein/100 g dry matter; however, the experimental result showed an increase of 3.35 g/100 g DM of protein in copra meal. The 10 kg of copra meal SSF in Koji reactor, the protein content increased to 4.18 g/100 g DM, while fiber content decreased 49%. Moreover, amino acids were increased by 64.05% and oligosaccharides, especially mannohexaose, were increased to 0.708 g/g DM. Results showed that fermentation of copra meal with mannanase and yeast offers a potential method to improve the nutrition of copra meal as animal feed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodegradation of macromolecules and release of microbial metabolites during fermentation is a commonly used method to improve nutritional value through utilization of many agricultural by-products. Copra meal is an important feed ingredient as the by-product of coconut oil and coconut milk extraction from coconut kernels. In Thailand, the annual production of copra meal varies from 17,000–25,000 metric tons (Index Mundi 2018). The by-product is mainly used in the animal feed industry due to its affordable price and nutritional composition. Copra meal from the coconut milk industry contains 4.07%, 36.22% and 72.61% crude protein, fat and crude fiber, respectively (Limmanee and Rumpagaporn 2017). Copra meal has been reported to be free of any harmful anti-nutritional factors as obtained in grain legumes, however, their use in the feeding of monogastric animals is limited on them by their branched polysaccharides constituents (Knudsen 1997). However, the high content of insoluble fiber, mainly in the form of galactomannan (Balasubramaniam 1976) reduces copra meal potential as both a protein and energy source by increasing the viscosity of intestinal contents and suppressing nutrition intake. Moreover, lysine and methionine have been reported to be deficient in copra meal which also limits their use in poultry feed (Dairo and Ogunmodede 2004).
Reducing insoluble fiber in copra meal by means of enzymes and fermentation has been used as an efficient method to improve nutritional composition. Mannanase is important in increasing the quality of animal feed (Marini et al. 2006) and bioconversion of biomass to fermentable sugars (Chandrakant and Bisaria 1998). Mannanase releases oligosaccharides such as mannobiose, mannotriose, mannotetraose, mannopentaose and mannohexaose as major products, and also has a reducing sugar production capability such as mannose and glucose by randomly cleaving mannosidic linkages in backbone structures of lignocellulosic substrates (McCleary 1988; Naughton et al. 2001). After digestion, these micro-molecules of oligosaccharide depend on the source of the enzyme (Titapoka et al. 2008). Filamentous fungi are commonly used for enzyme production. Enzyme growth and release by specific fungi such as Trichoderma and Aspergillus species hydrolyze complex carbohydrates, lessen the anti-nutritional effect and improve the protein content of copra meal (Pham et al. 2010; Pangsri 2014, Pangsri and Pangsri 2017). However, the use of fungi fermented with animal feed is also associated with mycotoxin release. Despite the potential of mannanase in reducing insoluble fiber and degrading antinutritional factors, existing bioactive metabolites in copra meal increase through the incorporation of yeast. By growing on reducing sugar released from copra meal by the action of mannanase, yeast can increase nutrients, i.e. vitamins and especially protein content and amino acids. Therefore, optimizing fermentation conditions to improve nutrient content is necessary (Oboh and Akindahunsi 2003; Oboh 2006a, 2006b).
Therefore, mannanase and yeast activities were investigated during simultaneous saccharification and fermentation (SSF) to improve the nutritional value of copra meal as feed for animals. This study focused on optimizing conditions for nutritional addition, especially protein to copra meal by mannanase and yeast using a Box–Behnken experimental design combined with response surface methodology (RSM). The effects of three independent variables as enzyme concentration, water ratio and fermentation time, as well as the interaction of yeast and mannanase on the change of protein content in copra meal were also examined.
Materials and methods
Materials
Dried copra meal after coconut milk extraction was obtained from Ampol Food Processing Limited, Nakhon Pathom Province, Thailand and used as a substrate for fermentation. Mannanase “BGM” 10 from Aspergillus niger EC no. 3. 2. 1. 78 was kindly supplied by Amano Enzyme Inc., (Japan). Instant dry baker’s yeast “Saf-instant” (Saccharomyces cerevisiae) was used.
Box–Behnken experimental design
Optimization conditions aimed to maximize protein increase of copra meal (ΔProtein = final copra meal protein content—initial coprameal protein content) were performed using a Box–Behnken experimental design combined with response surface methodology (RSM). Three independent variables were investigated as enzyme concentration, water ratio and fermentation time for three different levels coded as − 1, 0, + 1 (Table 1). Design-Expert software (Demo version) (Stat-Ease, Inc., Minneapolis, USA) was used to design the experiment. Factors were studied in a single block of 12 sets of test conditions and 5 center points (Table 2). The response was expressed as a quadratic polynomial model equation as:
where Y is the process response or output, k is a number of the independent variables (factors), and β0 is a constant coefficient of the model. The regression coefficients (β1, β2 and β3), (β1, β22 and β33) and (β12, β12 and β23) represent linear, quadratic, and interaction effects of the model, respectively. Multiple regression analysis by X1 (enzyme concentration), X2 (water ratio) and X3 (fermentation time) were coded, ranging from − 1 to + 1. Each model was examined for statistical significance of coefficients (P < 0.05). Quality of the polynomial model was expressed by coefficients of determination R2 and R2 (adj).
Copra meal fermentation
Following the experimental conditions in Table 2, the copra meal (200 grams) was mixed with water and enzyme in a 5-L fermenter tank. Then the mixture was inoculated with 0.1% DM of rehydrated baker’s yeast and incubated at 30 °C for 30 min. The increase rate of protein content was analyzed by Design-Expert 7.0.0 software program (Demo version). In addition, the optimal condition of protein production was tested to confirm the validity of the model.
The optimal condition was used for large-scale production (10 kg) in a 491-L Koji preparation reactor, equipped with a mixing system, stirring occasionally.
Determination of viable cell yeast
2 grams of sample was placed in 8 mL of 0.85% NaCl and was serially diluted. An aliquot of 0.1 mL of diluted sample was plated on yeast extract peptone dextrose (YPD) agar mixed with 100 μg/mL of chloramphenicol (Pupchem; U.S.A.). The plate was incubated at 30 °C for 48 h.
Proximate composition of fermented copra meal
Copra meal and fermented copra meal sampling at different time were dried at 80 °C for 48 h and keep in a plastic bag in a desiccator before proximate analyses.
Proximate analyses of the copra meal and fermented copra meal (ash, fat, carbohydrate, and crude fiber) were performed using the AOAC method (AOAC 2000). Moisture content was determined using an oven drying method at 105 ± C, and total protein content was determined by the Kjeldahl method with a conversion factor of 6.25. Nitrogen content was multiplied with a factor 6.25, to express the results as total protein or crude protein.
For reducing sugar and total sugar measurement, solid phase and liquid phase were separated by a hydraulic press. The solid was dried at 80 °C for 48 h and kept in plastic bags in a desiccator before analysis. Liquid part was stored at − 20 °C.
The liquid phase was tested for reducing sugar using the DNS method (Miller 1959) and total carbohydrate by the phenol–sulfuric method (Dubois et al. 1956).
Determination of amino acid content of fermented copra meal
Nineteen primary amino acids in fermented copra meal were analyzed by the Institute of Food Research and Product Development, Kasetsart University following the method of Herbert et al. (2000). Samples were hydrolyzed with 6 M HCl for 24 h at 110 °C and analyzed by high-pressure liquid chromatography (HPLC) with a narrow bore C18 HP Amino Acid Analysis and Fluorescence Detector for excitation/emission wavelengths at 340/450 nm.
Determination of mannose and mannooligosaccharide contents of fermented copra meal
Following Rungruangsaphakun and Keawsompong (2018), mannooligosaccharide content of fermented copra meal with yeast and mannanase was analyzed by a Waters HPLC system with a Breeze 2 program equipped with an Aminex-HPX-42C column (Bio-Rad, USA) and refractive index detector (2414 RID; Waters, Milford, MA, USA). Conditions were column temperature 75 °C, detector temperature 40 °C, mobile phase of the deionized water flow rate of 0.4 mL/min, and injection volume 20 μL. Calibration was performed using a series of standard sugar solutions including mannose, mannobiose, mannotriose, mannotetraose, mannopentaose and mannohexaose (Megazyme, Ireland).
Determination of mannanase activity
Mannanase activity was measured following the method of Pangsri et al. (2015) by reducing sugars released using the dinitrosalicylic acid (DNS) method (Miller 1959). Mannanase assays followed the incubation mixture of 0.1 mL of 1% (w/v) locust bean gum (substrate) in 50 mM citrate buffer at pH 5.5 and 0.1 mL of appropriately diluted enzyme. The mixture was incubated at 50 °C for 60 min. After incubation, 0.2 mL of DNS reagent was added and boiled for 5 min. The developed red-brown color was measured at 540 nm. One unit of enzyme activity was defined as the amount of enzyme liberating 1 μmol of mannose per minute under the assay condition. Controls were routinely included with enzyme preparation or substrate omitted.
Results and discussion
Optimization of copra meal fermentation
To determine the protein increase with respect to the optimal combination of enzyme concentration, water ratio and fermentation time, a total of 17 experiments were conducted in triplicate (Table 2). A second-order polynomial model proposed by Box–Behnken design was used to calculate the predicted values by a mathematical model (Eq. 2). The regression equation for change of protein in copra meal (ΔProtein; g/100 g DM) in terms of actual factors was expressed as follows:
where X1 is the enzyme (g/100 g DM), X2 is the water ratio and X3 is the fermentation time.
Here, Y is the predicted ΔProtein (g/100 g DM) and a regression model was used to calculate optimal values of protein change compared with observed values. The analysis of variance (ANOVA) (Table 3) demonstrated the significance of the model with a P value of less than 0.0001, and high F value at 102.20. The lack of fit was also tested with a P value at 0.4051 indicated that the model fits well. The multiple correlation coefficient of R2 at 0.9924 indicated the good quality of the model. Variables X1, X2 and X3 indicated significant effects of enzyme concentration, water ratio and fermentation time on increase in protein content in fermented copra meal. Considering the interaction between parameters with P value higher than 0.05, no interaction was shown by X23, while X12 and X13 showed interaction effects on protein increase (P < 0.05).
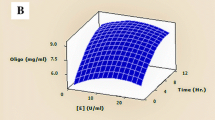
Three-dimensional response surface graphs (Fig. 1) were plotted to show the interaction of factors and optimal conditions of change in protein during copra meal fermentation. Concerning the response for interaction factors between enzyme content and water ratio (Fig. 1a) and between enzyme content and fermentation time (Fig. 1b), the appearance of the graph was not linear. The red area depicted protein content that changed to high value. Maximum amounts of enzyme gave the highest protein increase. When using a sufficiently high amount of enzyme, optimal fermentation time was 48–120 h. Fermentation times were influenced by the amount of enzyme. If using high amounts of enzyme, fermentation was faster in a shorter time. Both factors had coherent influences. Increasing the water ratio higher than 6.25 reduced protein increase. However, if protein increase was set to the maximum, enzyme concentration and water ratio were 0.95 g/100 g DM and 1:4.92, respectively, with fermentation time at 99.23 h, giving protein increase of 3.62 g/100 g DM.
After completing all 17 experiments, various conditions were designed that required a set of criteria and boundaries for optimal results. Criteria for finding the best condition were (1) use the optimal amount of water ratio, (2) the optimal fermentation time, and (3) the least amount of enzyme so that the fermentation process did not have spoilage and yeast grew well. From the experimental results, copra meal treated with enzyme concentration lower than 0.7% DM gave low amounts of reducing sugar that led to slow growth of yeast and contamination by other microorganisms. Copra meal was spoiled within 48 h of fermentation. Based on the possible minimum enzyme concentration, the optimal conditions to increase the protein content in copra meal were proposed as 0.7% DM enzyme, 1:4.56 water ratio, and 90.25 h. Protein increase of 3.06 g/100 g DM was predicted by the model and also validated by other independent fermentations.
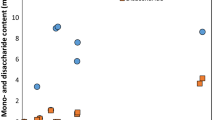
From the validation fermentations (Fig. 2), protein content increased rapidly during the first 24 h, consistent with rapidly growing yeast cell volume. In the early stages, amounts of live yeast cells were relatively stable. In this period, the yeast was in the stationary phase with the same birth rate and death rate. The subsequent autolysis of cells resulted in increased amounts of proteins and amino acids. After 90.25 h, the yeast cells reduced considerably. During fermentation, nutrition elements were utilized, and yeast cells entered the death phase. From the validation fermentations, protein content of copra meal after the end of the fermentation process at 90.25 h was predicted to be 7.96 g/100 g DM. However, the protein content after the end of the fermentation process was 8.32 ± 0.34 g/100 g DM, indicating that the amount of protein increased by 3.35 g/100 g DM. The protein value obtained from the validation fermentation was 8.66% higher than predicted. However, the difference from the predicted value might come from the deviation of analysis.
Change of protein content (closed circle), viable cell (open circle), reducing sugar (open square) and total sugar (open triangle) during copra meal fermentation with mannanase and Saccharomyces cerevisiae. (validated experimentation, 200 g experimentation). (Data shown are means of triplicate experiments)
Production of copra meal fermentation 10 kg
An experiment was carried out by expanding the capacity using the original fermentation conditions to confirm the results. This experiment was performed using a Koji preparation reactor equipped with a mixing system. Ten kilograms of copra meal with 4.60 g/100 g DM initial protein content were mixed with 45.6 L of water, 70.8 g mannanase enzyme and 10 g of rehydrated dried yeast.
With the expansion of production capacity in the Koji tanks with stirred leaves (Fig. 3), protein content and yeast cells increased rapidly during the first 24 h and then stabilized. This was consistent with results in the validation experiments. Carbohydrate content in copra meal was reported as high as 45–70% and comprised mannose (79.8%), glucose (12.8%), galactose (6.1%) and arabinose (1.3%) (Khuwijitjaru et al. 2012, 2014; Yalegama et al. 2013). In this study, amounts of reducing sugar and total sugar content increase by enzymatic hydrolysis using β-mannanase were also tracked. Reducing sugar and total sugar increased to the highest value at the beginning of fermentation and then gradually decreased and became stable after 18 h, implying that the sugar produced was used in the growth of yeast as the population is significantly increased.
Despite the predicted optimal time at 90.25 h, the predicted protein increase in copra meal was calculated every 6 h along the fermentation time length. After 6 h, increased protein content in copra meal was higher than the predicted value throughout the fermentation process, ending up at 8.78 g/100 g DM, indicating 4.18 g/100 g DM of increasing protein. The actual protein content was more than the predicted value of the program by 26.79%. The difference of the predicted value and the validated result might be from the effect of mixing. In the 10-kg experiment, the koji tank was equipped with the mixing system and needed 15-min mixing before sampling. It could provide well mixing and resulted in higher oxygen transfer rate than the small tank which was stirred by hand only 2–3 min before taking samples. The significant of mixing might also affect the yield of the fermented copra meal too. As during the fermentation, solid phase of copra meal was decreased from the action of enzyme hydrolysis and the result shows that the solid loss in 10 kg experimentation was 38.80% while in validated experimentation (200 g scale), the solid loss was only 26.86%.
Proximate composition of fermented copra meal
The proximate composition of copra meal is given in Table 4. Copra meal contained 4.60% crude protein which was very low compared to other vegetable protein sources but high mannooligosaccharide (MOS) which acts as a prebiotic for rumen microbes (Moorthy and Viswanathan 2009).
After the end of the fermentation process of expansion level experimentation, various chemical elements were compared between dried copra meal and copra meal treated with mannanase enzyme and yeast (validation experiment and expansion scale) as shown in Tables 4 and 5. The protein content increased by approximately 90.87%, which is made up of amino acids as well while mannanase enzymes digested copra meal to get reducing sugar and various types of oligosaccharides which is consistent with the objective of this research.
Copra meal is used as a protein source in the diets of fish (Kim et al. 2001); however, the protein quality of copra is poor both in terms of its amino acid balance and digestibility (Swick 1999). Copra meal is deficient in important essential amino acids such as lysine and methionine but high in arginine, which is known to have antagonizing effects on lysine utilization (Swick 1999). Adding yeast to copra meal to increase some essential amino acids such as lysine and methionine could solve the problem of arginine antagonism and also improve its protein quality. In this study, the dried copra meal consisted of 2825.53 mg/100 g DM total amino acids. The amino acids in copra meal were comparable to other vegetable oil meals but methionine was low compared to groundnut oil cake, and lysine was low compared to soybean meal (Moorthy and Viswanathan 2009; Creswell and Brooks 1971). Protein properties were improved by fermentation with mannanase enzyme and yeast, yielding a total amount of amino acid at 4635.43 mg/100 g DM. This increased amino acid content to 64.05%, in agreement with a previous study on soybean meal fermentation by Hassaan et al. (2015). This result was due to the high production of yeast cell mass and, consequently, protein production within the yeast population. Almost all amino acids are essential for the growth of animals. In particular, lysine increased by 151.5% from its original value, while methionine analysis was limited, and it was not possible to determine any increase. Methionine content was low compared to other amino acids. These amino acids are added directly from yeast cells when yeast grows for a specified period. When the nutrients required by the yeast for growth begin to decrease, the yeast cells develop a higher mortality rate. Yeast cells undergo autolysis and digest themselves, resulting in various amino acids dissolving as found in this experiment that 1297.64 mg/100 g DM of amino acid was detected in the liquid part which account for 38.88% of total amino acid. Importantly, amino acids are subunits of proteins. With increasing protein content, amino acid content also increases.
Action of mannanase in polysaccharide fiber degradation also showed an improvement, and fiber content decreased by 50% after enzyme treatment and yeast fermentation. The enzyme hydrolyzed and converted the mannan to either mannooligosaccharides or more simple sugar molecules as soluble carbohydrates (Khanongnuch et al. 2006). Here, copra meal treated by mannanase enzyme and simultaneous fermentation by S. cerevisiae reduced anti-nutritional factors.
Oligosaccharides resulting from copra meal digestion by enzymatic hydrolysis may contribute to maintaining the gut flora, leading to an improvement in host health (Graham and Balnave 1995). These oligosaccharides cannot be degraded by digestive enzymes. They pass through the GI tract and are believed to benefit the host animal by improving intestinal microflora balance. Thus, further analyses on mannooligosaccharides in fermented copra meal were conducted as shown in Fig. 4. Oligosaccharide products were determined as mannobiose (M2), mannotriose (M3), mannotetraose (M4), mannohexaose (M5) and mannohexaose (M6), while mannose (M1) was also examined.
The amount of mannose was highest at an early stage (6 h) and gradually decreased during fermentation, while M2–M5 occurred in small amounts during the fermentation process. Mannohexaose recorded the highest volume at the beginning of the fermentation process and then remained constant. This might be the result of yeast that can use M1 and also small oligosaccharides as a carbon source for growth. Final oligosaccharide products of fermented copra meal at optimal conditions contained a mixture of mannose (M1) and mannohexaose (M6) as the highest amount. Asano et al. (2001) reported that mannooligosaccharide was used by Bifidobacterium adolescentis, Lactobacillus acidophilus and Lactobacillus gasseri but could not be used by bad bacteria such as Clostridium perfringens and Escherichia coli. This suggested that mannooligosaccharides had the potential to promote the improvement of healthy human intestinal microflora as prebiotics. The present of mannooligosaccharide in fermented copra meal indicated the advantage of this product over than other commonly used vegetable protein source as it potential as prebiotics source in animal feed.
Conclusion
Improving copra meal nutritional properties by increasing the protein content using mannanase enzyme, and fermentation with Saccharomyces cerevisiae with the aid of the RSM technique, gave an optimal yield of fermented copra meal production. Protein in fermented copra meal increased by 64% when 0.7% of enzyme per 100 g dry matter and water ratio at 1:4.56 were used at 90.25 h of fermentation. Amino acid content, especially lysine, increased by 2.5 fold. Anti-nutritional values decreased as fiber content reduced by 50%. Results showed that the improved copra meal could be used as a source of protein supplement in the feed industry.
Our study results showed an increase in oligosaccharides and total amino acids, especially lysine in copra meal. Consequently, on an industrial scale, fermentation of copra meal with mannanase and yeast could be a potential method to improve its quality as animal feed.
References
AOAC (2000) Official methods of analysis of AOAC International, 17th edn. AOAC International, Gaithersburg
Asano I, Nakamura Y, Hoshino H, Aoki K, Fujii S, Imura N, Iino H (2001) Use of mannooligosaccharides from coffee mannan by intestinal bacteria. Nippon Nogeikagaku Kaishi 75:1077–1083 (in Japanese)
Balasubramaniam K (1976) Polysaccharides of the kernel of maturing and matured coconuts. J Food Sci 41:1371–1373
Chandrakant P, Bisaria VS (1998) Simultaneous bioconversion of cellulose and hemicellulose to ethanol. Crit Rev Biotechnol 18(4):295–331
Creswell DC, Brooks CC (1971) Effect of coconut meal on Coturnix quail and of coconut meal and coconut oil on performance, carcass measurements and fat composition in swine. J Anim Sci 33(2):370–375
Dairo FAS, Ogunmodede BK (2004) Performance of laying hens fed coconut meal as replacement for groundnut cake. Trop J Anim Sci 2:87–90
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Graham H, Balnave D (1995) Dietary enzyme for increasing energy availability. In: Wallace RJ, Chesson A (eds) Biotechnology in animal feeds and animal feeding. VCH Verlagsgesellschaft, Weinheim, p 302
Hassaan MS, Soltan MA, Abdel-Moez AM (2015) Nutritive value of soybean meal after solid state fermentation with Saccharomyces cerevisiae for Nile tilapia, Oreochromis niloticus. Anim Fd Sci Technol 201:89–98
Herbert P, Barros P, Ratola N, Alves A (2000) HPLC determination of amino acids in musts and port wine using OPA/FMOC derivatives. J Food Sci 65(7):1130–1133
Index Mundi (2018) IndexMundi country reports, Massachusetts. http://www.indexmundi.com. Accessed 15 Aug 2019
Khanongnuch C, Sa-nguansook C, Lumyong S (2006) Nutritive quality of β-mannanase treated copra meal in broiler diets and effectiveness on some fecalbacteria. Int J Poult Sci 5:1087–1091
Khuwijitjaru P, Watsanit K, Adachi S (2012) Carbohydrate content and composition of product from subcritical water treatment of coconut meal. J Ind Eng Chem 18:225–229
Khuwijitjaru P, Pokpong A, Klinchongkon K, Adachi S (2014) Production of oligosaccharides from coconut meal by subcritical water treatment. Int J Food Sci Technol 49(8):1946–1952
Kim BG, Lee JH, Jung HJ, Han YK, Park KM, Han IK (2001) Effect of partial replacement of soybean meal with palm kernel meal and copra meal on growth performance, nutrient digestibility and carcass characteristics of finishing pigs. Asian Aust J Anim Sci 14(6):821–830
Knudsen KEB (1997) Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Fd Sci. Technol 67:319–338
Limmanee M, Rumpagaporn P (2017) Preparation of coconut residue for chemical modification and addition in food products. In: The 55th Kasetsart University Annual Conference, pp 809–816
Marini AM, Yatim AM, Babji AS, Annuar BO, Noraini S (2006) Evaluation of nutrient contents and amino acid profiling of various types of PKC. Anal Chem 2:135–141
McCleary BV (1988) b-D-Mannanase. Methods Enzymol 160:596–610
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moorthy M, Viswanathan K (2009) Nutritive value of extracted coconut (Cocos nucifera) meal. Res J Agric Biol Sci 5:515–517
Naughton PJ, Mikkelsen LL, Jensen BB (2001) Effects of nondigestible oligosaccharides on Salmonella enterica serovar Typhimurium and nonpathogenic Escherichia coli in the pig small intestine in vitro. Appl Environ Microbiol 67(8):3391–3395
Oboh G (2006a) Nutrient enrichment of Cassava peels using a mixed culture of Saccharomyces cerevisiae and Lactobacillus spp. solid media fermentation techniques. Electron. J Biotechnol 9:4649
Oboh G (2006b) Nutrient enrichment of Cassava peels using a mixed culture of Saccharomyces cerevisiae and Lactobacillus spp solid media fermentation techniques. Electron J Biotechnol 9:4649
Oboh G, Akindahunsi AA (2003) Biochemical changes in Cassava products (flour & gari) subjected to Saccharomyces cerevisiae solid media fermentation. Food Chem 82(4):599–602
Pangsri P (2014) Characterization of mannanase from Bacillus circulans NT 6.7 and its application in mannooligosaccharides preparation. Ph.D. thesis, Kasetsart University
Pangsri P, Pangsri P (2017) Mannanase enzyme from Bacillus subtilis P2-5 with waste management. Energy Proc 138:343–347
Pangsri P, Piwpankaew Y, Ingkakul A, Nitisinprasert S, Keawsompong S (2015) Characterization of mannanase from Bacillus circulans NT 6.7 and its application in mannooligosaccharides preparation as prebiotic. http://www.springerplus.com/content/4/1/77
Pham TA, Berrin JG, Record E, To KA, Sigoillot JC (2010) Hydrolysis of softwood by Aspergillus mannanase: role of a carbohydrate-binding module. J Biotechnol 148:163–170
Rungruangsaphakun J, Keawsompong S (2018) Optimization of hydrolysis conditions for the mannooligosaccharides copra meal hydrolysate production. 3 Biotech 8:169
Swick RA (1999) Considerations in using protein meals for poultry and swine. American Soybean Association, Technical Bulletin, Washington, p 19
Titapoka S, Keawsompong S, Haltrich D, Nitisinprasert S (2008) Selection and characterization of mannanase-producing bacteria useful for the formation of prebiotic manno-oligosaccharides from copra meal. World J Microbiol Biotechnol 24:1425–1433
Yalegama LLWC, Nedra Karunaratne D, Sivakanesan R, Jayasekara C (2013) Chemical and functional properties of fibre concentrates obtained from by-products of coconut kernel. Food Chem 141:124–130
Acknowledgements
This research was supported by Research and Researchers for Industries (RRI), the Thailand Research Fund under the Office of the Prime Minister, Royal Thai Government (code: MSD5810009) and Ampol Food Processing Limited, Nakhon Pathom Province, Thailand.
Author information
Authors and Affiliations
Contributions
Conceptualization: [SM, SK]; Methodology: [JK, SM and SK]; Formal analysis and investigation: [JK]; Writing—original draft preparation: [JK, SM and SK]; Writing—review and editing: [SM and SK]; Funding acquisition: [SM and SK]; Resources: [SM and SK]; Supervision: [SM and SK].
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kraikaew, J., Morakul, S. & Keawsompong, S. Nutritional improvement of copra meal using mannanase and Saccharomyces cerevisiae. 3 Biotech 10, 274 (2020). https://doi.org/10.1007/s13205-020-02271-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02271-9