Abstract
Cocoa pod husk (CPH) waste from chocolate and cocoa powder production is repurposed as raw materials for animal feed using response surface methodology (RSM) with a faced central composite design (FCCD). The investigated variables included fermentation time (ranging from 7 to 21 days), the percentage of molasses (ranging from 0 to 10%), and the liquid-to-solid (L/S) ratio ranging from 0 to 15 (mg/l). Seventeen experimental runs were executed, and the accuracy of the model was verified through ANOVA (R2 = 0.9878, R2adj = 0.9804, p < 0.0001). The optimal conditions for achieving the highest predicted total crude protein content (TCPC) were determined to be as follows: 7.93% TCPC, a fermentation period of 21 days, 10% molasses, and solid-state fermentation (L/S = 0). Aspergillus niger were used in each run to degrade CPH, boosting TCPC compared to unfermented CPH. SEM analysis confirmed CPH degradation by the microorganism. Notably, the sample collected from these optimal conditions exhibited a reduced theobromine content of 0.58 ± 0.02 mg/100 g dry mass, along with heightened antioxidant properties. This result indicates a greater-than-expected increase in protein content, highlighting fermentation's potential to enhance the value of CPH for animal feed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cocoa pod husk (CPH) is an agricultural waste generated during the production of chocolate and cocoa powder. It is derived from the cocoa fruit [1]. The mature cocoa fruit contains CPH approximately 60–70% [2], resulting in approximately 40 thousand metric tons of waste annually in Thailand. Improper disposal of this large CPH volume can cause environmental issues and waste valuable biomass resources [2]. CPH comprises various chemical components: total crude fiber (40–70%), total crude protein (5–10%), lipids (1–5%), and ash (5–10%) on a dry basis [2]. Its substantial quantity makes it suitable for applications such as organic fertilizer production, supplementation in animal feed, and bioenergy generation [3, 4]. Despite containing antioxidant substances like polyphenols and flavonoids that offer potential health benefits, CPH also contains theobromine (C7H8N4O2), a bitter alkaloid [5].
Theobromine has physiological effects, including vasodilation, diuretic properties, potential health benefits, and mood enhancement [6]. However, theobromine can be toxic to certain animals due to slower metabolism, which leads to its accumulation and adverse effects [7, 8]. Therefore, a reduction process is necessary to prevent harm to animals. Fermentation is a process commonly employed to reduce the theobromine content in agricultural products [3, 8,9,10]. Fermentation with an alkaline environment promotes reactions and affects enzymatic activity, leading to the degradation or conversion of theobromine [4]. Molasses, which is abundant in sugars and serves as nutrients for microorganisms, is used in fermentation to break down and metabolize theobromine [11]. This supports enzymatic processes that facilitate theobromine reduction. Furthermore, the fermentation process also contributes to an increase in the total crude protein content (TCPC) of CPH [12]. Microorganisms involved in fermentation can produce proteolytic enzymes that break down proteins into smaller peptides and amino acids [13]. These modified protein structures may exhibit improved nutritional properties and higher bioavailability compared to the original proteins [14].
Additionally, microorganisms such as bacteria or fungi utilized in the fermentation process have the capability to synthesize proteins through their metabolic activities and multiply during fermentation to generate new protein molecules [13, 15]. Response surface methodology (RSM) is a statistical technique used to design experiments and optimize the impact of process variables. It is based on the principles of Design of Experiments (DOE), which is a field of applied statistics focused on planning, conducting, analyzing, and interpreting controlled tests to assess factors influencing parameter values [14]. The faced central composite design (FCCD) extends the widely used central composite design (CCD) for research in engineering and science. It adds “face” points on the design space boundaries, improving coverage for exploring response surface curvature [16]. The FCCD models the relationship between input variables and the response variable. By strategically collecting data, regression models describe this relationship. It combines factorial, axial, and face points to evaluate main effects, interaction effects, and curvature effects. The FCCD optimizes process exploration, aids in prediction under different conditions, and efficiently studies the impact of input variables on the response of interest [17].
The aims of this study were to enhance the TCPC in cocoa pod husk (CPH). Fermentation was employed to increase TCPC content in CPH, considering independent parameters such as fermentation time, % molasses, and liquid-to-solid ratio (L/S). DOE and RSM were utilized to optimize the single response variable, employing a FCCD. Analysis of variance (ANOVA) was carried out to validate the experimental results and ensure the acceptability of the developed model. Following this, the optimal conditions were applied to investigate the theobromine content and antioxidant substances that supported these findings, as compared to unfermented CPH.
2 Material and methods
2.1 Materials and chemical reagents
Cocoa mature fruits [Pa7+Na32] were obtained from Tah-Sala in Nakhon Si Thammarat Province, Southern Thailand. The fruits were washed with tap water, and the seeds were removed to obtain cocoa pod husk (CPH). The CPH was ground to a small size using a commercial grinder machine and then kept in a vacuum bag. It was stored in a freezer at 0°C until use. The chemical reagents used in this research were of analytical grade. Standard theobromine (purity 98.5%), absolute ethanol, acetonitrile, phosphoric acid, quercetin, the 2,2-diphenylpicrylhydrazyl (DPPH), gallic acid, sodium carbonate, Kjeldahl catalyst, sodium hydroxide (98%), and Folin-Ciocalteu reagent were purchased from Sigma-Aldrich Pte Ltd. (Nucleos, Singapore).
2.2 Growth of A. Niger in a controlled environment
The A. niger TISTR3013 used in this study was purchased from Thailand Institute of Scientific and Technological Research, Thailand. To culture A. niger, the laboratory utilized potato dextrose agar for cultivation, followed by an incubation at 30°C for a period of 5 days. The spores of A. niger were collected through gentle tapping of the inverted plate. Utilizing the Fuch-Rosenthal technique [18, 19], spore quantification was conducted using a hematocytometer, resulting in an estimated count of 108 spores/ml.
2.3 Utilizing scanning electron microscopic for microanalysis
Fermented and unfermented CPH′ samples were analyzed for their structure by scanning electron microscopic (SEM) that was performed in a SEM SU3900 device (Hitachi Instruments), operating at 15 keV. Before analysis, samples were vacuum dried and coated/metallized with a layer of gold in a sputter coater and then dried.
2.4 The increase in total crude protein in CPH
RSM DOE was employed to examine the effects of fermentation conditions on the increase in TCPC. Fermentation time (X1), percentage of additional molasses (X2), and the ratio of additional water to CPH (X3) were the parameters of interest in this study. The alpha coefficient value (⍺) was set to face the center, resulting in 17 experimental runs obtained from the DOE. The effects of these three independent variables were represented as “−1” (low level), “0” (normal level), and “1” (high level), as shown in Table 1. The axial or star points in the FCCD variables were statistically examined. Multiple regressions were used to fit a quadratic polynomial model to the experimental design data, as shown in Eq. (1).
where Y is the predicted crude protein content and b0, bi, bii, and bij are the regression coefficients for the model intercept, linear quadratic, and interaction terms, respectively. The independent variables in the model were fermentation time (X1), percentage of molasses (X2), and liquid-to-solid ratio (X3).
The FCCD design was carried out using Design-Expert® software for Windows, version 13. The acceptability of the model was assessed based on the correlation coefficient (R2), adjusted correlation coefficient (R2adj), and the lack-of-fit test. Regression analysis and three-dimensional (3D) response surface plots were utilized to determine the optimal condition for protein content.
2.5 Preparation of fermented CPH sample
CPH was blended into a paste, then weighed (50g) and placed into 1000-ml Duran bottles to serve as the fermentation reactor. The fermentation process involved mixing the CPH paste with 1 ml of the A. niger solution (108 spores/ml) in each reactor. This fermentation procedure was conducted under aerobic conditions in a room maintained at temperatures between 30 and 40°C, with fermentation times determined based on the experimental design.
2.6 Determination of TCPC
The TCPC of the fermented samples was determined using the modified Kjeldahl method [20]. Two grams of dried CPH samples was placed in a Kjeldahl flask, and 20 ml of sulfuric acid and 2 g of Kjeldahl catalyst were added. The flasks were then placed in a block heater at 420°C for 3 h. The resulting solutions were diluted by adding 30 ml of distilled water, followed by the addition of 30 ml of 40% sodium hydroxide (w/v). The flasks were placed on the heater blocks and connected to a condenser for distillation. The distillates were collected using 30 ml of 4% boric acid (w/v) solution and were analyzed by titration with 0.1 N HCl as the titrant. A few drops of methyl red were used as an indicator. The total nitrogen content of CPH from the Kjeldahl method was calculated according to Eq. (2) below:
where V1 is the volume of HCL used in the titration (ml), n1 is the normality of HCL, F1 is the acid factor, and Mw is the molecular weight of nitrogen. To calculate the TCPC, Eq. (3) below was used.
where factor value of 6.25 was used for food and feed, and F2 is dilute factor in the dilution.
CPH extract preparation
The fermented CPH sample with the highest TCPC was extracted. The chosen sample was dried in a convection oven, and precise amounts of 1.5 g were then placed into 250-ml Erlenmeyer flasks. The flask was subsequently filled with 45 ml of 55% ethanol and placed in an ultrasonic bath at 50°C for 60 min. Afterwards, the sample was centrifuged for 10 min at the centrifugation force of 4430g at room temperature. The resulting supernatants, containing the extract solutions, were collected in individual vials and stored at 0°C until they were used to determine the theobromine content and antioxidant properties.
2.7 Determination of theobromine content
The theobromine content in the CPH extracts was analyzed by an Agilent 1200 HPLC system based on the modified method of Nguyen et al. [21]. In summary, theobromine samples (100 μg/mL in methanol) and extracts were filtered through 0.45-μm nylon membranes. Then, 50 μL of each sample was injected using an auto-injection system. A calibration curve for theobromine ranged from 0 to 400 mg/L, utilizing a C18 column (Hypersil ODS, 5 μm, 4.0 × 250 mm cartridge). The column was maintained at 35°C. Isocratic elution was achieved with a mobile phase of 80% distilled water (A) and 20% pure acetonitrile (B) at a flow rate of 1 mL/min. Detection of theobromine content in the extracts was performed at 274 nm using an Agilent Multi λ fluorescence detector (EX:330, EM:440 nm). The theobromine content in the extracts was quantified as mg/100 g dry mass using Eq. (4).
where A is the peak area of sample/slope of calibration curve, B is the weight of extract obtained per 100g of dry mass, and C is the weight of extract diluted in 55% ethanol before HPLC analysis.
2.8 Determination of total phenolic content
The total phenolic content (TPC) of the sample was determined using the Folin-Ciocalteu method [22]. The Folin-Ciocalteu reagent was diluted at a ratio of 1:10 with pure distilled water. Sodium carbonate with a purity of 99.5%, weighing 57.5 g, was diluted with distilled water to a final volume of 500 ml. In a test tube, 0.1 ml of CPH extract sample was added to 5 ml of Folin-Ciocalteu reagent, followed by the addition of 3.5 ml of the sodium carbonate solution. The mixture was incubated in a water bath at 40°C for 1 h. Subsequently, the samples were measured using a UV-vis spectrophotometer at a wavelength of 738 nm. Gallic acid, at concentrations ranging from 0.05 to 0.5 mg/ml, was used as the standard to generate a standard curve. The results were expressed as milligrams of gallic acid equivalents (GAE) per 100 g of sample dry mass (d.m.).
2.9 Determination total flavonoid content
The quantification of the total flavonoid content in the selected sample was accomplished by employing a modified methodology, which was derived from the approach initially proposed by Hu et al. [23]. In a screw-capped tube, 1 mL of CPH extract solutions was diluted to 10 mL with 55% ethanol. Then, 0.5 mL of each sample extract was added, followed by the addition of 0.1 mL of 5% NaNO2 solution. Next, 1 mL of 10% AlCl3 solution was added to the tube. Finally, 1.0 mL of 1M NaOH solution and 3 mL of distilled water were added to the tube. The flavonoid content in each extract sample was determined using a UV-Vis spectrophotometer at a wavelength of 510 nm. The experiment was conducted in triplicate, and no sample was utilized as a blank. The results were reported as milligrams of quercetin equivalent (QE) per 100 g of dry mass (d.m.), with quercetin used as the standard to generate a calibration curve.
2.10 Determination of %DPPH scavenging activity
The DPPH radical scavenging activity of the extract samples was examined and adapted following the method of Thabti et al. [24]. Firstly, 3.0 mL of DPPH solution (200 mM DPPH in 95% ethanol) was thoroughly mixed with 1.0 mL of extract solution at concentrations of 333, 666, and 1332 μg/mL in a screw-capped tube. The absorbance was measured at 517 nm using a UV-Vis spectrophotometer. The DPPH scavenging activity (%) was calculated according to Eq. (5) below:
where Acontrol is the light absorption value of the DPPH solution, and Asample is the light absorbance value of the extract mixed with DPPH solution.
2.11 Statistical analysis
All experiments were performed in triplicate. The data were presented as mean ± standard deviation, with statistical analysis conducted using Design-Expert® software (version-13). One-way ANOVA was used to analyze results, with a significance level set at less than 5% (p ≤ 0.05).
3 Results and discussion
3.1 Statistical analysis of the model by ANOVA
The experimental results obtained in this study demonstrated that the desired dependent variable was influenced by the main parameters of fermentation time (days), percentage of molasses, and liquid-to-solid ratio (L/S). The mutual and simultaneous effects of these parameters were found to be significant in relation to the TCPC (%). The p-value of the model for the desired parameter was <0.0001, indicating the significance of the model and the obtained results. After excluding the quadratic polynomial models, Eq. (6) represents the most significant empirical relationship between the predictors (independent process variables) and their response. This relationship was determined based on various criteria, such as lack of fit, p-value, highest R2, predicted R2, and adjusted R2 values. The statistical parameters are provided in Table 4.
Table 2 presents the values of various terms related to Eq. (6), encompassing fermentation time, the percentage of added molasses, and the liquid-to-solid ratio for TCPC determination. The model showcased in this table holds significance, supported by a low p-value (<0.0001) and a high F-value (134.47). A commendable model accuracy is indicated by the R2 value of 0.9878, aligning with the theoretical approach that favors lower p-values, higher F-values, and elevated R2 values to establish a substantial and well-fitting model. Moreover, the lack of fit test for TCPC (p = 0.4852) revealed that the quadratic models retained statistical significance and could be applied for further analysis. Additionally, a robust correlation between the experimental and predicted values was observed, substantiated by R2, adjusted R2, and predicted R2 values all surpassing 0.95. This underscores the adequacy and goodness of fit of the models [25].
3.2 Accuracy check of the model using diagnostic charts
The assessment of data normality involves examining the alignment of data points around a straight line. In the normal distribution plot, shown in Fig. 1a, the correlation between variables is depicted. The results closely adhere to the straight-line pattern, confirming the normal distribution of the data. Additionally, upon comparing the predicted versus residuals data in Fig. 1b and predicted versus run in Fig. 1c, it becomes apparent that the residuals versus actual plot closely aligns with a straight line. This suggests that the results obtained from the experiments closely resemble the data generated from the runs, as illustrated in Fig. 1d. These findings underscore the consistency between predicted values and the values acquired from experiments, thereby affirming the accuracy of the designed model.
3.3 Contour plots of variants parameters
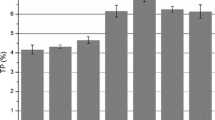
The outcomes derived from the experiments and model predictions are displayed in Table 3. The influence of chosen input variables and their interactions on TCPC percentage was simulated. This simulation is illustrated in Fig. 2, showcasing how TCPC varies between two variables while keeping the third one constant at its mean value. In Fig. 2a–c, distinct conditions are depicted for TCPC. Notably, the fermentation time, %molasses, and L/S ratio were found to exert a significant impact on TCPC. Increasing %molasses from 0 to 10% led to a rise in TCPC from approximately 5 to 8%. Conversely, an initial increase in the L/S ratio resulted in a decrease in TCPC from about 8 to 5%. Furthermore, following 21 days of fermentation, the TCPC escalated from roughly 5 to 8%. The initial TCPC in fresh CPH was approximately 4.75%. These findings conclusively demonstrate that the chosen input variables particularly fermentation time, %molasses, and L/S ratio play a pivotal role in determining TCPC.
Response surface plots and effects of variants variables
The model equation indicates a positive correlation between TCPC and fermentation time as well as % molasses, while the L/S ratio has a negative effect. Graphs depicting the actual results against the predicted values for TCPC were generated using RSM in Design-Expert® software. The 3D surface plot illustrates the impact of fermentation time and % molasses on TCPC in Fig 3. The TCPC increases significantly with longer fermentation time (from 7 to 21 days) and the addition of molasses (from 0 to 10%). Natural microorganisms play a crucial role in biodegradation, breaking down organic materials into simpler compounds, and the addition of molasses provides a sufficient food source to activate their growth [12]. Microorganisms synthesize proteins using the nutrients derived from the degradation of organic matter, and they also produce enzymes for biodegradation, many of which are proteins themselves [12]. The variety of microorganisms participating in the fermentation process can also impact the protein content. Various microorganisms might exhibit differing rates of protein synthesis and specific requirements, collectively contributing to an overall rise in protein levels. Conversely, as the fermentation progresses, microorganisms could selectively target constituents of CPH, and molasses based on their metabolic capabilities. Consequently, this selective utilization of carbohydrates and other nutrients can lead to an enhanced synthesis of proteins [12]. A high L/S ratio, referred to as submerged fermentation, significantly affects protein synthesis. Insufficient or imbalanced nutrients in the medium can limit the microorganisms’ ability to efficiently produce protein, resulting in reduced TCPC. In this study, TCPC ranged from 4.12 to 7.85%. Through modeling calculations, the maximum predicted TCPC was 7.93% at 21 days of fermentation time with the addition of 10% molasses, and without additional liquid media.
3.4 Optimal state validation
The optimal conditions were determined to be a fermentation time of 21 days, with the addition of 10% molasses, and employing solid-state fermentation (L/S=0). To validate the model, the optimal predicted values generated by Design-Expert® software were compared with the actual values. The optimal predicted values are accompanied by a 95% confidence interval (CI). The actual value of TCPC was 7.85 ± 0.02%, whereas the predicted value stood at 7.93%. For a 95% confidence interval, the lower bound value was calculated at 7.67%, while the upper bound value was determined to be 8.19%. The optimal conditions, namely 10% molasses addition and a fermentation period of 21 days, conducted under solid-state (L/S = 0) fermentation, were successfully scaled up to a 20-l fermentation system. TCPC of the optimal condition was measured at 7.99% ± 0.08%. The observed value exceeded the predicted value obtained through RSM, indicating that the experimental conditions yielded a more favorable outcome than initially anticipated.
3.5 Examination of the structural configurations of CPH using SEM
Figure 4a–c present SEM images of unfermented cocoa pod husk (CPH) samples, while Fig 4d–f display SEM images of fermented CPH samples at a 1000× magnification. These images reveal significant alterations in surface texture, porosity, and the exposed structural characteristics.
Scanning electronic microscopic photo of cocoa pod husk (CPH) sample: a outer layer (epicarp) of fresh CPH; b middle layer (mesocarp) of fresh CPH; c inner layer (endocarp) of fresh CPH; d outer layer (epicarp) of fermented CPH; e middle layer (mesocarp) of fermented CPH; f inner layer (endocarp) of fermented CPH, all magnified at 1000×
In Fig 4a, we observe the epicarp, which is the outer layer of the CPH where the deposition of epicuticular waxes on the fruit takes place. Turning our attention to Fig 4d, it becomes evident that the epicarp has undergone substantial changes following the fermentation process, showcasing the effective decomposition of epicuticular waxes by microorganisms.
Fig 4b and e provide cross-sectional views of fresh CPH and fermented CPH, respectively. A marked contrast is observable when comparing fresh CPH, characterized by gaps between porous cell walls due to the rapid evaporation of moisture during the drying process while preserving the overall cell structure, with fermented CPH. The latter exhibits clear signs of digestion by microorganisms, resulting in the fragmentation of the mesocarp layer. Notably, fungal growth was also detected during the fermentation process.
Lastly, when analyzing the endocarp layer of the CPH, a striking disparity emerges between picture (c), representing the state before fermentation, and picture (f), illustrating the post-fermentation condition. In picture (f), decomposition is evident, manifesting as a rough and cracked surface [26]. Consequently, it is reasonable to conclude that the fermentation process has indeed led to the decomposition of CPH, a phenomenon in line with the observed increase in the TCPC value.
A. niger employs diverse mechanisms to effectively break down and utilize CPH, encompassing both plant biomass and industrial byproducts. The fungus secretes specific extracellular enzymes, including cellulases, hemicellulases, pectinases, amylases, and proteases, to target components within the CPH. This process results in the decomposition of cellulose and hemicellulose into simpler sugars like glucose, xylose, and mannose [27]. Pectinases work on complex polysaccharides such as pectin, transforming them into sugars and oligomers. Amylases play a crucial role in the hydrolysis of starch into maltose and glucose, while proteases break down proteins into amino acids present in the CPH [28]. A. niger strategically utilizes the breakdown products from CPH as sources of carbon and energy for its metabolic processes. The fungus demonstrates versatility by engaging in fermentation processes under suitable conditions, leading to the production of organic acids, notably citric acid, contributing to its energy supply [13]. This capacity to generate organic acids enhances the acidic environment, supporting the efficiency of enzymatic activities specific to the CPH. The fungal hyphae of A. niger exhibit their ability to penetrate CPH, facilitating efficient exploration and utilization of available nutrients. Crucially, A. niger exemplifies a sophisticated regulatory mechanism in enzyme production, adjusting its enzymatic toolkit based on the unique composition of CPH. This dynamic interplay of enzymatic degradation, metabolic utilization, and regulatory adjustments underscores the fungus’ effectiveness in breaking down and assimilating CPH.
3.6 Theobromine reduction
The HPLC analysis of the theobromine standard and fermented CPH extracts revealed a peak at 2.509 min. As depicted in Fig 5, the standard theobromine peaks and theobromine contents were observed under the optimal conditions of fermented CPH samples. In Fig 5a, the initial theobromine content in unfermented CPH was quantified at 59.50 ± 1.22 mg/100 g dry mass. Following fermentation with 10% molasses, without additional water, and a fermentation period of 21 days, as shown in Fig 5b, the theobromine content in CPH decreased to 0.58 ± 0.02 mg/100 g dry mass. This reduction can be attributed to the proliferation of indigenous microorganisms during fermentation, which facilitates the breakdown of theobromine into smaller molecules [29]. Xanthine, theophylline, and methylxanthines are some of the compounds formed through the destruction of theobromine [6]. Although these compounds share structural similarities, they possess distinct chemical properties. Under the fermentation conditions of excess molasses and a solid-state setup (L/S=0) and conditions, the microorganisms, predominantly A. niger, exhibited growth under these optimal conditions. A. niger is recognized for its capacity to generate diverse enzymes, including cellulases and hemicellulases, which play a crucial role in breaking down complex carbohydrates such as fibers. Furthermore, A. niger exhibits the capability to produce organic acids, with citric acid being a prominent example. According to findings reported by other study, it has been observed that when the pH of the fermentation process is decreased, there is a significant reduction in the theobromine content during the fermentation of CPH [30,31,32]. The reduction in theobromine content of cocoa pod husk (CPH) after the fermentation process enhances the efficiency of CPH as an alternative animal feed material, thereby mitigating potential harmful effects on animals [33].
3.7 Total phenolic content
The value of total phenolic content from optimum conditions was determined equal to 2127.76 ± 19.29 mgGAE/100g dry mass. The presence of molasses in the fermentation process can facilitate the breakdown of complex organic compounds into simpler compounds, leading to an increase in the total phenolic content [34, 35]. This breakdown process allows larger phenolic compounds to be transformed into smaller, and more bioavailable forms. The increase in total phenolic content observed after A. niger fermentation is a result of various factors working together. These include enzymatic activity, bioconversion, substrate metabolism, release from the matrix, and microbial synthesis. A. niger utilizes enzymatic processes to break down complex compounds in the substrate, releasing phenolic compounds that were previously bound in the cellular structure of the raw material. Additionally, A. niger can convert precursor compounds into phenolic compounds with enhanced antioxidant activity, leading to an accumulation of total phenolic content in the final product. As A. niger metabolizes substrates for growth, the breakdown of these substrates generates intermediate products, some of which may be phenolic compounds, contributing to an overall increase. Furthermore, phenolic compounds that were bound or conjugated in the raw material matrix can be released by microbial activity, making them more available and adding to the observed rise in total phenolic content. The microbial synthesis of phenolic compounds during metabolic processes further contributes to the overall accumulation of phenolic content in the fermented product. These complex mechanisms highlight the dynamic nature of changes in phenolic composition facilitated by A. niger during fermentation. A study conducted by Ouattara et al. investigated the effect of fermentation with Lactobacillus plantarum on the phenolic content, antioxidant capacity, and bioactive compounds of cocoa pod husk from Ghana. Their results showed that the total phenolic content increased from 626.66 ± 22.01 mg GAE/100g dry mass to 2127.76 ± 19.29 mg GAE/100g dry mass after fermentation [36].
3.8 Total flavonoid content
The total flavonoid content value obtained under the optimal conditions was determined to be 4198.30 ± 24.71 mg/100g dry mass. The highest total flavonoid content was observed in the optimal conditions. This discovery suggests that the fermentation process has the potential to enhance the overall flavonoid content, with the addition of 10% molasses (w/w) contributing to the highest value. Flavonoids, encompassing flavanols, flavones, and condensed tannins, are recognized to experience an increase during the fermentation process due to the proliferation of microorganisms. A. niger play a role in catalyzing the conversion of flavonoids into flavonoid derivatives [22, 37, 38] Additionally, fermentation can influence the extraction and solubility of flavonoids within the fermented matrix. It has been observed that some flavonoids become more soluble in solvents compared to their non-fermented counterparts, which contributes to an increase in the measured total flavonoid content [39].
3.9 %DPPH scavenging
In this investigation, the extracts for free radical scavenging potential was studied using the DPPH assay. The %DPPH values from the optimal conditions was 88.33 ± 4.32%. The addition of 10% molasses strongly influenced the change in %DPPH, as the molasses acted as a nutrient source for natural microorganisms, thereby activating their growth. The increased presence of microorganisms involved in fermentation can produce metabolites with antioxidant properties [40]. The increase in %DPPH can also be attributed to the formation of new bioactive compounds during fermentation or the breakdown of larger antioxidant molecules into smaller, more bioavailable forms, which enhances the %DPPH scavenging activity [41].
Essentially, antioxidants play a crucial role in maintaining optimal animal health by neutralizing free radicals and preventing oxidative stress. Their anti-inflammatory effects help preserve tissues, support the immune system, and provide essential cellular protection. With links to cardiovascular health and disease prevention, antioxidants are vital for promoting overall well-being. Incorporating these compounds into animal nutrition is essential to reduce the risks associated with oxidative stress and ensure a healthier life [42,43,44]. The antioxidant properties of the optimal conditions determined through RSM were compared with those of unfermented CPH, as depicted in Table 4.
4 Conclusion
The optimal conditions for fermentation to enhance TCPC were achieved through 21 days of fermentation period, with 10% molasses, an L/S ratio of 0, and 1 ml of A. niger solution (108 spores/ml). The successful application of response surface methodology (RSM) enabled the identification of key variables influencing TCPC increase. This was further supported by statistical parameters confirmed through ANOVA. The quadratic model, boasting an R2 value of 0.9878, R2adj value of 0.9804, and a p-value of <0.0001, effectively demonstrated its predictive capacity. SEM analysis indicated that the CPH was decomposed by A. niger, leading to an enhancement in the production of simpler substances, especially TCPC. Fermentation time and the percentage of molasses exhibited positive impacts on TCPC, while the L/S ratio exerted a notably strong negative influence. As a result of these conditions, the theobromine content saw a significant reduction from 59.50 ± 1.22 to 0.58 ± 0.02 mg/100 g dry matter compared to the unfermented CPH sample. Remarkably, these optimized conditions not only minimized the theobromine content but also maximized antioxidant activities, including TPC (total phenolic content), TFC (total flavonoid content), and %DPPH (percentage of DPPH radical scavenging), which measured 2127.76 ± 19.29 mg GAE/100g dry mass, 4198.30 ± 24.71 mg QE/100g dry mass, and 88.33 ± 4.32%, respectively. The fermentation of CPH using A. niger can increase the TCPC while simultaneously reducing the amount of theobromine, which may cause harm to animals. Furthermore, it provides a comprehensive understanding of the variables that positively influence the experiment. Moreover, it was discovered that the fermented CPH exhibited a higher level of antioxidant substances compared to the samples that did not undergo the fermentation process. These findings carry significant implications for potential CPH utilization across various industries, particularly in the realms of food and agriculture. The developed model serves as a valuable tool for future research and holds the potential for application on a larger scale within the industrial domain.
Data availability
All obtained data during this work are included in this manuscript.
References
Meza-Sepúlveda DC, Castro AM, Zamora A, Arboleda JW, Gallego AM, Camargo-Rodríguez AV (2021) Bio-based value chains potential in the management of cacao pod waste in Colombia: a case study. Agronomy 11(4):693. https://doi.org/10.3390/agronomy11040693
Oddoye EOK, Agyente-Badu CK, Gyedu-Akoto E (2013) Cocoa and its by-products: identification and utilization. In: Chocolate in Health and Nutrition. Humana Press Inc, pp 23–37. https://doi.org/10.1007/978-1-61779-803-0_3
Bhatia SK, Jagtap SS, Bedekar AA, Bhatia RK, Patel AK, Pant D, Yang YH (2020) Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges. Bioresour Technol 300. https://doi.org/10.1016/j.biortech.2019.122724
Lorenci Woiciechowski A, Dalmas Neto CJ, Porto de Souza Vandenberghe L, de Carvalho Neto DP, Novak Sydney AC, Letti LAJ, Soccol CR (2020) Lignocellulosic biomass: acid and alkaline pretreatments and their effects on biomass recalcitrance – Conventional processing and recent advances. Bioresour Technol 304. https://doi.org/10.1016/j.biortech.2020.122848
Oduro-Mensah D, Ocloo A, Nortey T, Antwi S, Okine LK, Adamafio NA (2020) Nutritional value and safety of animal feed supplemented with Talaromyces verruculosus-treated cocoa pod husks. Sci Rep 10(1). https://doi.org/10.1038/s41598-020-69763-9
del Brunetto MR, Gutiérrez L, Delgado Y, Gallignani M, Zambrano A, Gómez Á, Ramos G, Romero C (2007) Determination of theobromine, theophylline and caffeine in cocoa samples by a high-performance liquid chromatographic method with on-line sample cleanup in a switching-column system. Food Chem 100:459–467. https://doi.org/10.1016/j.foodchem.2005.10.007
Smit HJ (2011) Theobromine and the pharmacology of cocoa. In: Methylxanthines, Handbook of Experimental Pharmacology, vol 200. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-13443-2_7
Reddy BS, Reddy LV (2013) Chocolate poisoning in a dog. Int J Vet Health Sci Res 01(03):16–17. https://doi.org/10.19070/2332-2748-130004
Dahunsi SO, Adesulu-Dahunsi AT, Izebere JO (2019) Cleaner energy through liquefaction of Cocoa (Theobroma cacao) pod husk: pretreatment and process optimization. J Clean Prod 226:578–588. https://doi.org/10.1016/j.jclepro.2019.04.112
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. Elsevier Ltd. https://doi.org/10.1016/j.biortech.2015.10.009
Dahunsi SO, Osueke CO, Olayanju TMA, Lawal AI (2019) Co-digestion of Theobroma cacao (Cocoa) pod husk and poultry manure for energy generation: effects of pretreatment methods. Bioresour Technol 283:229–241. https://doi.org/10.1016/j.biortech.2019.03.093
Yaya OL, Kouadio Appiah KE, Doudjo S, Mahamane Nassirou AK, Didier FG, Benjamin YK, Drogui AP, Tyagi DR (2023) Multi-response and multi-criteria optimization of acid hydrolyzate detoxification of cocoa pod husks: effect on the content of phenolic compounds and fermentable sugars. Heliyon 9(4):e15409. https://doi.org/10.1016/j.heliyon.2023.e15409
Chebaibi S, Leriche Grandchamp M, Burgé G, Clément T, Allais F, Laziri F (2019a) Improvement of protein content and decrease of anti-nutritional factors in olive cake by solid-state fermentation: a way to valorize this industrial by-product in animal feed. J Biosci Bioeng 128(3):384–390. https://doi.org/10.1016/j.jbiosc.2019.03.010
Gupta R, Hemansi Gautam S, Shukla R, Kuhad RC (2017) Study of charcoal detoxification of acid hydrolysate from corncob and its fermentation to xylitol. J Environ Chem Eng 5(5):4573–4582 https://doi.org/10.1016/j.jece.2017.07.073
Kim H, And Keeney PG (1984) (-)-Epicatechin content in fermented and unfermented cocoa beans. J Food Sci 49(4):1090–1092. https://doi.org/10.1111/j.1365-2621.1984.tb10400.x
Roelofsen PA (1958) Fermentation, drying, and storage of cacao beans. Adv Food Res 8(C):225–296. https://doi.org/10.1016/S0065-2628(08)60021-X
Vásquez ZS, de Carvalho NDP, Pereira GVM, Vandenberghe LPS, de Oliveira PZ, Tiburcio PB, Soccol CR (2019) Biotechnological approaches for cocoa waste management: a review. Waste Manag 90:72–83. https://doi.org/10.1016/j.wasman.2019.04.030
Roy S, Kr Saha A, Panda S, Dey G (2023) Optimization of turmeric oil extraction in an annular supercritical fluid extractor by comparing BBD-RSM and FCCD-RSM approaches. Mater Today: Proc 76:47–55. https://doi.org/10.1016/j.matpr.2022.09.039
Aspevall O, Hallander H, Gant V, Kouri T (2001) European guidelines for urinalysis: A collaborative document produced by European clinical microbiologists and clinical chemists under ECLM in collaboration with ESCMID. Clin Microbiol Infect 7(4):173–178. https://doi.org/10.1046/j.1198-743X.2001.00237.x
Kouri T, Gyory AZ, Rm R, Force IUT (2003) ISLH recommended reference procedure for the enumeration of particles in urine. Lab Hematol Official Public Int Soc Lab Hematol 92:58–63 https://api.semanticscholar.org/CorpusID:20591325
Jacobs S (1960) The determination of nitrogen in organic compounds by the indanetrione hydrate method. Analyst 85(1009):257–264. https://doi.org/10.1039/AN9608500257
Nguyen TH, Sunwoo IY, Jeong GT, Kim SK (2019) Detoxification of hydrolysates of the red seaweed Gelidium amansii for improved bioethanol production. Appl Biochem Biotechnol 188(4):977–990. https://doi.org/10.1007/s12010-019-02970-x
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Hu Z, Tang X, Liu J, Zhu Z, Shao Y (2017) Effect of parboiling on phytochemical content, antioxidant activity and physicochemical properties of germinated red rice. Food Chem 214:285–292. https://doi.org/10.1016/j.foodchem.2016.07.097
Thabti I, Marzougui N, Elfalleh W, Ferchichi A (2011a) Antioxidant composition and antioxidant activity of white (Morus alba L.), black (Morus nigra L.) and red (Morus rubra L.) mulberry leaves. Acta Botanica Gallica 158(2):205–214. https://doi.org/10.1080/12538078.2011.10516267
Aghel B, Rahimi M, Sepahvand A, Alitabar M, Ghasempour HR (2014) Using a wire coil insert for biodiesel production enhancement in a microreactor. Energy Convers Manag 84:541–549. https://doi.org/10.1016/j.enconman.2014.05.009
Sarmiento-Vásquez Z, Vandenberghe L, Rodrigues C, Tanobe VOA, Marín O, de Melo Pereira GV, Soccol CR (2021) Cocoa pod husk valorization: alkaline-enzymatic pre-treatment for propionic acid production. Cellulose 28(7):4009–4024. https://doi.org/10.1007/s10570-021-03770-5
Muharja M, Darmayanti RF, Fachri BA, Palupi B, Rahmawati I, Rizkiana MF, Halim A (2023) Biobutanol production from cocoa pod husk through a sequential green method: depectination, delignification, enzymatic hydrolysis, and extractive fermentation. Bioresour Technol Rep 21:101298. https://doi.org/10.1016/j.biteb.2022.101298
de Oliveira PZ, de Souza VLP, Rodrigues C, de Melo PGV, Soccol CR (2022) Exploring cocoa pod husks as a potential substrate for citric acid production by solid-state fermentation using Aspergillus niger mutant strain. Process Biochem 113:107–112. https://doi.org/10.1016/j.procbio.2021.12.020
Nieves LM, Panyon LA, Wang X (2015) Engineering sugar utilization and microbial tolerance toward lignocellulose conversion. Front Bioeng Biotechnol 3. https://doi.org/10.3389/fbioe.2015.00017
Rakhmani S.I , and Puastuti W (2022) Utilization of Fermented Cocoa Pod Husk (CPH) as Feed Ingredient for Sheep.
Sánchez M, Laca A, Laca A, Díaz M (2023) Cocoa bean shell: a by-product with high potential for nutritional and biotechnological applications. Antioxidants 12(5):1028. https://doi.org/10.3390/antiox12051028
Iyayi EA (2004) Changes in the cellulose, sugar and crude protein contents of agro-industrial by-products fermented with Aspergillus niger, Aspergillus flavus and Penicillium sp. Afr J Biotechnol 3(3):186–188. https://doi.org/10.5897/ajb2004.000-2033
Camps-Bossacoma M, Pérez-Cano FJ, Angels F, Castell M (2018) Theobromine is responsible for the effects of cocoa on the antibody immune status of rats. J Nutr 148(3):464–471. https://doi.org/10.1093/jn/nxx056
Brito E, García N, Gallao M, Cortelazzo A, Fevereiro P, Braga M (2000) Structural and chemical changes in cocoa (Theobroma cacao L) during fermentation, drying and roasting. J Sci Food Agric 81:281–288
Camu N, Winter T, Addo S, Takrama J, Bernaert H, De Vuyst L (2008) Fermentation of cocoa beans: influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J Sci Food Agric 88:2288–2297. https://doi.org/10.1002/jsfa.3349
Ouattara HG, Honoré O, Droux M, Reverchon S, Nasser W, Niamke S (2017) Lactic acid bacteria involved in cocoa beans fermentation from Ivory Coast: species diversity and citrate lyase production. Int J Food Microbiol 256:11–19. https://doi.org/10.1016/j.ijfoodmicro.2017.05.008
Juan MY, Chou CC (2010) Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol 27(5):586–591. https://doi.org/10.1016/j.fm.2009.11.002
Hussain OA, Abdel REA, Badr AN, Hathout AS, Rashed MM, Fouzy ASM (2022) Total phenolics, flavonoids, and antioxidant activity of agricultural wastes, and their ability to remove some pesticide residues. Toxicol Rep 9:628–635. https://doi.org/10.1016/j.toxrep.2022.03.038
Misnawi Selamat J, Bakar J, Saari N (2002) Oxidation of polyphenols in unfermented and partly fermented cocoa beans by cocoa polyphenol oxidase and tyrosinase. J Sci Food Agric 82(5):559–566. https://doi.org/10.1002/jsfa.1075
Gan C-Y, Latiff A (2011) Extraction of antioxidant pectic-polysaccharide from mangosteen (Garcinia mangostana) rind: optimization using response surface methodology. Carbohydr Polym 83:600–607. https://doi.org/10.1016/j.carbpol.2010.08.025
Utami RR, Armunanto R, Rahardjo S, Supriyanto (2016) Effects of cocoa bean (Theobroma cacao L.) fermentation on phenolic content, antioxidant activity and functional group of cocoa bean shell. Pak J Nutr 15(10):948–953. https://doi.org/10.3923/pjn.2016.948.953
Yapo BM, Besson V, Koubala BB, Koffi KL (2013) Adding value to cacao pod husks as a potential antioxidant-dietary fiber source. Am J Food Nutrit 1(3):38–46. https://doi.org/10.12691/ajfn-1-3-4
Anees Ali Jafri S, Mehmood Khalid Z, Rizwan Khan M, Ashraf S, Ahmad N, Mahmoud Karami A, Aslam S (2023) Evaluation of some essential traditional medicinal plants for their potential free scavenging and antioxidant properties. J King Saud Univ Sci 35(3). https://doi.org/10.1016/j.jksus.2023.102562
Funding
This work was supported by Songklanakarin Graduate Student Scholarship from the Prince of Songkla University (PSU), Graduate School Research Support Funding, and facilities and equipment support from the Discipline of Excellence in Department of Chemical Engineering, Department of Chemical Engineering, Faculty of Engineering, Prince of Songkla University.
Author information
Authors and Affiliations
Contributions
NS was responsible for writing the original draft and conducting formal analysis, while PP and JC were responsible for conceptualization, reviewing, and editing.
Corresponding author
Ethics declarations
Ethical approval
The manuscript is prepared in compliance with the Publishing Ethics Policy.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sianoun, N., Pongyeela, P. & Chungsiriporn, J. Aspergillus niger fermentation of cocoa pod husk for increased total crude protein utilizing response surface methodology. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05303-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05303-z