Abstract
Copra meal is a good source of galactomannan and its mannooligosaccharides have prebiotic properties. However, limited data are available concerning the ideal requirements for mannan hydrolysis. Thus, optimum hydrolysis conditions for the production of oligosaccharides from copra meal hydrolysate were investigated using response surface methodology. Model validation provided good agreement between experimental results and predicted responses. Maximum oligosaccharide of 14.41 ± 0.09 mg/ml (20 ml) was obtained at an enzyme concentration of 16.52 U/ml, substrate concentration 15% and reaction time 12 h. On a larger scale, this increased to 15.76 ± 0.04 mg/ml (200 ml) and 16.89 mg/ml (2000 ml). Defatted copra meal hydrolysate promoted the growth of beneficial bacteria as lactobacilli and bifidobacteria, while inhibiting pathogens Salmonella serovar Enteritidis S003, Escherichia coli E010, Staphylococcus aureus TISTR 029 and Shigella dysenteriae DMST 1511. Higher yield of oligosaccharides under optimum conditions indicated the potential of this method for production of mannooligosaccharides from copra meal hydrolysate on an industrial scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copra meal, as well-dried coconut kernel, is a natural source of high amounts of galactomannan (Balasubramaniam 1976). In Thailand, 25 million metric tons per year of copra meal waste are produced from the coconut oil industry (Index Mundi 2014). Mannans can be obtained from legume seeds (Marcos et al. 1995), coconut kernel (Balasubramaniam 1976) and tubers of konjac (Wootton et al. 1993). The mannan of legume and coconut is galactomannan (Marcos et al. 1995; Balasubramaniam 1976) and that of konjac is glucomannan (Wootton et al. 1993). Mannooligosaccharides are generated when mannan is hydrolyzed by endo-1,4-β-d-mannanase (EC 3.2.1.78), which catalyzes the random cleavage of β-d-1,4-mannopyranosyl linkages within the main chain of galactomannan, glucomannan, galactoglucomannan and mannan (McCleary and Matheson 1974).
Beta-mannanases are useful in many fields including biobleaching of pulp in the paper industry (Gübitz et al. 1996), bioconversion of biomass wastes to fermentable sugars (Chandrakant and Bisaria 1998), upgrading of animal feedstuff (Ray et al. 1982), and reducing the viscosity of coffee extracts (Hashimoto and Fukumoto 1969). They also have potential application in the production of mannooligosaccharides that are selectively utilized by intestinal Bifidobacterium spp. and used as valuable food sweeteners or additives (Tomotari 1990).
Bacillus circulans NT 6.7 was isolated from soil taken from a coconut factory in Nakon Pathom Province, Thailand. Beta-mannanases from B. circulans NT 6.7 hydrolyze defatted copra meal to mannooligosaccharides which have prebiotic properties suitable for industrial applications. Mannooligosaccharide displayed broad inhibition to pathogens such as Salmonella serovar Enteritidis S003 and Escherichia coli E010 and promoted growth of probiotics such as Lactobacillus reuteri AC-5 (Phothichitto et al. 2006).
Oligosaccharides were produced from copra meal hydrolysate by identifying optimum hydrolysis conditions using response surface methodology (RSM). Defatted copra meal derived oligosaccharides were detected by high performance liquid chromatography (HPLC) and their prebiotic properties were assessed.
Materials and methods
Materials
Copra meal was dried at 60 °C for 4 h, and then blended and milled using a hammer mill to obtain particle size of 0.5 mm. Defatted copra meal was prepared by oil extraction in Soxhlet apparatus for 4–6 h.
Methods
Strain and enzyme
Bacillus circulans NT 6.7 (Phothichitto et al. 2006) was used for mannanase production using a 5-l fermenter containing 2 l of mineral salts medium (MSM). Cultivation was conducted at an agitation speed of 600 rpm, aeration rate of 0.75 vvm (vol. of air/vol. of medium/min) and inoculum of 1% (v/v) at 45 °C for 15 h. The cells were removed by centrifugation at 9500 rpm for 15 min at 4 °C, and the supernatant was assayed for mannanase activity following the method of Pangsri et al. (2015). Mannanase was assayed by measuring the reducing sugars released using dinitrosalicylic acid (DNS) method (Miller 1959). The mannanase assay mixture contained 0.1 ml of 1% (w/v) locust bean gum prepared in 50 mM potassium phosphate buffer, pH 6 and 0.1 ml of appropriately diluted culture broth. The reaction mixture was maintained at 50 °C for 60 min. After incubation, 0.2 ml of DNS reagent was added and the mixture was boiled for 5 min. The developed red brown color was measured at 540 nm. One unit of enzyme activity was defined as the amount of enzyme liberating 1 µmol of mannose per minute under assay conditions. Controls were routinely included with enzyme preparation or substrate omitted.
Experimental design
The parameters of copra meal hydrolysis were optimized using the central composite design (CCD) of response surface methodology (RSM) with the creation of a series of experiments utilizing Minitab 16 Software (Minitab Inc., USA) to investigate three independent variables of enzyme concentration, substrate concentration, and reaction time for five different levels (− 1.682, 1, 0, + 1, + 1.682) (Table 1).
Optimization of the process variables was combined with factorial CCD following RSM principles to study their impacts through simultaneous modification of the responses by conducting a limited number of experiments. Optimum hydrolysis conditions were determined for mannooligosaccharide production from copra meal hydrolysate, using CCD as an effective tool for sequential experimentation, while providing acceptable data to quantify lack of fit by ignoring the unusually large number of design points (Özer et al. 2009).
Minitab 16 Software (Minitab Inc., USA) achieved regression and graphical analyses of the data, with evaluation of model variance using ANOVA. CCD matrices of 20 experiments were employed for full factorial design of enzyme concentration, substrate concentration, and reaction time and quadratic models were developed.
The response was expressed as a second-order polynomial equation as:
where Y is the predicted response, xi and xj are variables, k is the number of independent variables (factors), β0 is the constant coefficient, β i , β ii and β ij are interaction coefficients of linear, quadratic and cross product coefficients, respectively, and ε represents residual error (Moghaddam et al. 2010).
Connections of variables as actual values, together with predicted and experimental responses are presented in Table 2 as a complete 23 factorial design with six axial and center points.
Twenty experiments were performed on a complete quadratic model of 3 factors at five levels from 23 + 2(3) + 6 total points, where 23 represents cube points, and 2(3) represents axial points and 6 replicates at center points. Multivariate regression analysis was performed on Eq. (1) to produce Eq. (2) which was then used to optimize product responses.
Each model was examined for significance and lack of fit, with response surface plots designed using the same software. Model terms were evaluated by P values at 95% confidence level. Quality of the polynomial model was expressed by coefficients of determination R2 and R2 (adj).
Production of mannooligosaccharides from defatted copra meal hydrolysate
Defatted copra meal (0.5–15%) in 10 ml 0.05 M potassium phosphate buffer, pH 6.0 was mixed with 10 ml β-mannanase (1–25 U/ml) in a Duran bottle and incubated in a water bath shaker at 50 °C 150 rpm for reaction time from 0.5 to 12 h as shown in Table 2. The reaction was stopped by boiling the samples for 10 min and then centrifuging at 4 °C with 9500 rpm for 15 min. Supernatant was tested for reducing sugar using the DNS method (Miller 1959) and total carbohydrate by the phenol–sulfuric method (Dubois et al. 1956). Designated experiments were fulfilled and the related results analyzed by Minitab 16 Software. The optimum condition was used for upscale production of oligosaccharide from copra meal hydrolysate. The large-scale experiment used 2000 ml reaction volume in a reactor at 50 °C, 200 rpm.
Analysis of hydrolyzed products from defatted copra meal
Hydrolysis products were detected by high pressure liquid chromatography (HPLC) using a Waters HPLC system analyzed by Breeze 2 program equipped with an Aminex-HPX-42C column (Bio-Rad, USA) and a refractive index detector (2414 RID; Waters, Milford, MA, USA). Operation conditions were column temperature at 75 °C, detector temperature at 40 °C, mobile phase of deionized water with a flow rate of 0.4 ml/min, and an injection volume of 20 µl. Calibration was performed using a series of standard sugar solutions including mannose, mannobiose, mannotriose, mannotetraose, mannopentaose and mannohexaose (Megazyme, Ireland).
Effect of defatted copra meal hydrolysate on growth of beneficial and pathogenic bacteria
Defatted copra meal hydrolysate from three scales consisting of 20 ml (wet sample), dried samples from 200, and 2000 ml were prepared under optimum conditions. Each scale was used to evaluate percentage (%) enhancement and inhibition of bacteria comprising four strains of beneficial bacteria and four strains of pathogenic bacteria. Beneficial bacteria were cultivated in 5 ml of De Man Rogosa and Sharpe broth (MRS) (Difco), MRS broth (Difco) + 0.05% l-cysteine (Sigma). Pathogenic bacteria were cultivated in 5 ml of nutrient broth (NB) (Merck). The test medium contained 1% (v/v) inoculum with or without (control) and 1% (w/v) defatted copra meal hydrolysate.
Cell growth was determined by spread plating appropriate dilution factors on their respective media (Table 3) and grown at 37 °C. Pathogenic bacteria incubated at around 12–15 h, Lactobacillus incubated at around 18–24 h and Bifidobacteria incubated at around 24–27 h. Percentage enhancement and inhibition activities were calculated as described by Phothichitto et al. (2006) using the equations below:
where SB is the number of cells in the medium with copra meal hydrolysate and CB is without copra meal hydrolysate.
Results and discussion
Production of mannooligosaccharides from copra meal hydrolysate
Central composite design for response surface methodology was applied to investigate the optimum combination of the three variables. The corresponding uncoded variables at their levels and set of 20 experiments are presented as Table 2. Results showed that experimental oligosaccharide values varied from 4.07 to 12.06 mg/ml. Analyses of independent and dependent variables were performed to obtain a regression equation that predicted the response under a specified range. The regression obtained for oligosaccharide value (Y) was as follows:
where X1 = enzyme concentration, X2 = substrate concentration and X3 = reaction time.
A regression model was used to calculate predicted values of oligosaccharide compared with experimental values. Statistical analysis showed that regression was significant (P < 0.05), but linear quadratic terms and interaction coefficients were not significant. The ANOVA also revealed a significant lack of fit which did not validate the model (data not shown). The lack of fit may not be good theoretically, because P value showed significance for the model. However, this study suggested the model as best, because the condition provided maximum yield at surface constraints. Substrate concentration was not increased more than 15% which was the concentration limitation due to problems of water-substrate absorption. When extending reaction time above 12 h (14.41 ± 0.09 mg/ml), product quantity only slightly increased (24 h; 15.23 ± 0.48 mg/ml). Hence, 12 h was selected to save costs. Only enzyme concentration showed the highest point at 16.52 U/ml in range of optimization plot.
To determine optimum levels of each variable for maximum oligosaccharide production, RSM plots were constructed against any two independent variables, fixed at their respective zero level. Oligosaccharide production was affected by enzyme concentration, substrate concentration, and reaction time as shown in Fig. 1.
Figure 1a shows the relationship of oligosaccharide with substrate concentration and enzyme concentration, with reaction time fixed at coded zero level or 6.25 h. Changes in substrate concentration and enzyme concentration revealed maximum oligosaccharide increase when substrate concentration was at the highest limit and enzyme concentration was at 16.52 U/ml. Figure 1b shows the relationship of oligosaccharide with reaction time and enzyme concentration with substrate concentration fixed at coded zero level or 7.75%. Changes in reaction time and enzyme concentration revealed that maximum oligosaccharide increase occurred when reaction time was at the higher limit and enzyme concentration was at 16.52 U/ml. Figure 1c shows the relationship of oligosaccharide with reaction time and substrate concentration, with enzyme concentration fixed at coded zero level or 13.00 U/ml. Changes in reaction time and substrate concentration revealed maximum oligosaccharide increase when reaction time was at the higher limit and substrate concentration was at the highest limit.
Optimum conditions for hydrolysis are presented in Table 4. The major goal of RSM optimization was to maximize the yield of mannooligosaccharides by enzymatic hydrolysis of defatted copra meal. Predicted optimum conditions for oligosaccharide production were enzyme concentration of 16.52 U/ml, substrate concentration of 15%, and reaction time at 12 h with the corresponding predicted maximum response as 12.53 mg/ml. A confirmative test was carried out using optimum levels of the independent variables. The predicted value was found to be lower than the experimental value. From the regression equation, 12.53 mg/ml oligosaccharide was predicted at optimum values of the independent variables, whereas the experimental value of oligosaccharide was 14.41 mg/ml. Therefore, the confirmative test validated the experimental results as well as the regression model.
Analysis of hydrolyzed product from defatted copra meal
HPLC analysis results of hydrolyzed products as composition of mannooligosaccharides from defatted copra meal are shown in Table 5. Yield of oligosaccharides was used to determine the optimum condition. Various oligosaccharides were detected in copra meal hydrolysate as mannobiose (M2), mannotriose (M3), mannotetraose (M4), mannopentaose (M5) and mannohexaose (M6).
For the small scale (20 ml), Run 1 recorded mannooligosaccharides of M6, M4 and M3 but M5, M2 and M1 were not detected. This could result from the lower reaction time compared to Run 5.
Meanwhile, other runs gave mannooligosaccharides consisting of two to six mannose units as well as mannose. However, in some runs such as Runs 11–20, none or low M5 was detected, possibly because of lower substrate concentration in Run 11, while low M5 in Run 13 could be caused by low reaction time compared to other runs. In other runs, absence of M5 was attributed to enzyme activity. This is likely, because at higher volumes (200 and 2000 ml) where freeze dried samples were used, M5 was detected. Moreover, results suggested that total product concentration was more affected by substrate and enzyme concentration than reaction time. Pangsri (2014) also used defatted copra meal, and M5 was detected at varying concentrations of enzyme (1, 5, 10, 15 and 20 U/ml) with fixed substrate concentration (1%) and reaction time (30 min). Hence, this might be the differences in experimental conditions.
The pattern of hydrolysis products at optimum conditions (Table 6) showed that mannooligosaccharides resulting from mannanase hydrolysis of defatted copra meal contained a mixture of mannose-containing oligosaccharides (6.81 ± 0.24 mg/ml) with mannohexaose (M6) as the predominant product, followed by (2.86 ± 0.10 mg/ml) mannobiose (M2), (2.69 ± 0.14 mg/ml) mannotriose (M3), and (2.05 ± 0.09 mg/ml) mannotetraose (M4). This result concurred with Piwpankaew et al. (2014) who reported that beta-mannanase from B. circulans NT 6.7 hydrolyzed the mannan backbone in a random fashion to release oligosaccharides of different sizes, and mannooligosaccharides of two to six mannose units. They concluded that the wild-type enzyme from B. circulans NT 6.7 could be used for the production of prebiotic mannooligosaccharides from defatted copra meal.
Oligosaccharide copra meal hydrolysate at optimum condition was used for upscale production. Results showed highest products at 15.76 ± 0.04 mg/ml (200 ml) and 16.89 mg/ml (2000 ml) (Tables 7, 8). All scales of hydrolysis products showed similar HPLC profiles (Fig. 2). Hydrolysis product profiles before and after freeze drying showed the same pattern. However, M5 was detected only after freeze drying when the product was concentrated up to 14.59-fold (Fig. 3).
Previous authors, using similar enzymes and pH but lower substrate concentration at 50 °C and short time (50 min) reported very low product concentration (Pangsri 2014). Therefore, higher yields can be achieved by increasing the concentration of enzymes and substrates to the optimum level, thus, prolonging reaction time under optimum enzyme temperature and pH.
Enhancement/inhibition properties of defatted copra meal hydrolysate
Results showed that defatted copra meal hydrolysate (3 scales) at optimum conditions supported the growth of L. reuteri KUB-AC5, Lactobacillus crispatus JCM 5810, and Bifidobacterium bifidum TISTR 2129, and Bifidobacterium animalis subsp. animalis TISTR 2194 (Fig. 4). Conversely, defatted copra meal hydrolysate inhibited the growth of pathogenic bacteria (E. coli E010, Salmonella serovar Enteritidis S003, Staphylococcus aureus TISTR 029 and Shigella dysenteriae DMST 1511) as shown in Fig. 5.
Results were consistent with Phothichitto et al. (2006) who showed that culture filtrate of B. circulans NT 6.7 growth on copra meal promoted growth of L. reuteri KUB-AC5, but inhibited Salmonella serovar Enteritidis S003 and E. coli E010. A report by Pangsri et al. (2015) suggested that copra meal hydrolysate produced by mannanase from B. circulans NT 6.7 showed enhanced activity for growth of L. reuteri KUB-AC5 and inhibition of Salmonella serovar Enteritidis S003, E. coli E010, S. aureus TISTR 029 and S. dysenteriae DMST 1511. Furthermore, Titapoka et al. (2008) reported that copra meal hydrolysate by mannanase from Klebsiella oxytoca KUB-CW2-3 showed enhanced activity for growth of L. reuteri KUB-AC5. Therefore, commercial mannooligosaccharides from yeast cell walls stimulated growth of beneficial bacteria and inhibited growth of three strains of pathogenic bacteria (S. dysenteriae DMST 1511, S. aureus TISTR 029, and Salmonella enterica serovar Enteritidis DMST 17368). Similarly, Line et al. (1997) reported that yeast cell wall carbohydrates, especially mannose residue, were effective in preventing Salmonella sp. colonization. Not all oligosaccharides were prebiotics, but copra meal hydrolysate mannooligosaccharides obtained here showed prebiotic properties, since it promoted the growth of desirable bacteria and inhibited pathogenic bacteria as previously reported.
Conclusions
Optimum conditions were investigated for the production of oligosaccharides from copra meal hydrolysate. Concentration of oligosaccharides at 14.34 ± 0.11 mg/ml was obtained under experimental conditions of enzyme concentration (16.52 U/ml), substrate concentration (15%) and reaction time (12 h). Model validation provided good agreement between experimental and predicted response.
The optimum condition was suitable for upscale mannooligosaccharides production, since 2000 ml showed the highest product amount (16.89 mg/ml) with the same pattern of hydrolysis. High yield of oligosaccharide under the optimum conditions indicated the potential of this method for production of mannooligosaccharides from copra meal hydrolysate on an industrial scale.
Defatted copra meal hydrolysate promoted probiotic bacteria (Lactobacillus group and Bifidobacterium group) and inhibited pathogenic bacteria; E. coli E010, Salmonella serovar Enteritidis S003, S. aureus TISTR 029 and S. dysenteriae DMST 1511. Hence, B. circulans NT 6.7 defatted copra meal hydrolysate can be used as a prebiotic.
References
Balasubramaniam K (1976) Polysaccharides of the kernel of maturing and mature coconuts. J Food Sci 41:1370–1373
Chandrakant P, Bisaria VS (1998) Simultaneous bioconversion of cellulose and hemicellulose to ethanol. Crit Rev Biotechnol 18:295–331
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Gübitz GM, Hayn M, Ürbanz G, Steiner W (1996) Purification and properties of an acidic β-mannanase from Sclerotium rolfsii. J Biotechnol 45(2):165–172
Hashimoto Y, Fukumoto J (1969) Studies on the enzyme treatment of coffee beans. Nippon Nogeikagaku Kaishi 43:317–322
Index Mundi (2014) IndexMundi country reports, Massachusetts. http://www.indexmundi.com. Accessed 13 Jul 2014
Line JESB, Cox NA, Stern NJ (1997) Yeast treatment to reduce Salmonella and Campylobacter, populations associated with broiler chickens subjected to transport stress. Poult Sci 76:1227–1231
Marcos SB, Valeria RP, Dalva CR, Sonia MCD (1995) Seed galactomannan in the classification and evolution of the Leguminosae. Phytochem 38:871–875
McCleary VB, Matheson NK (1974) Galactomannan structure and β-mannanase and β-mannosidase activity in germinating legume seeds. Phytochemistry 14:1187–1194
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moghaddam SS, Moghaddam MRA, Arami M (2010) Coagulation/flocculation process for dye removal using sludge from water treatment plant: optimization through response surface methodology. J Hazard Mater 175:651–657
Özer A, Gürbüz G, Çalımlı A, Körbahti BK (2009) Biosorption of copper(II) ions on Enteromorpha prolifera: application of response surface methodology (RSM). Chem Eng J 146:377–387
Pangsri P (2014) Characterization of mannanase from Bacillus circulans NT 6.7 and its application in mannooligosaccharides preparation. Ph.D. thesis, Kasetsart University
Pangsri P, Piwpankaew Y, Ingkakul A, Nitisinprasert S, Keawsompong S (2015) Characterization of mannanase from Bacillus circulans NT 6.7 and its application in mannooligosaccharides preparation as prebiotic. http://www.springerplus.com/content/4/1/77
Phothichitto K, Nitisinprasert S, Keawsompong S (2006) Isolation, screening and identification of mannanase producing microorganisms. Kasetsart J (Nat Sci) 40(Suppl):26–38
Piwpankaew Y, Sakulsirirat S, Nitisinprasert S, Ngu-yend TH, Haltrich D, Keawsompong S (2014) Cloning, secretory expression and characterization of recombinant β-mannanase from Bacillus circulans NT 6.7. http://www.springerplus.com/content/3/1/430
Ray S, Pubols MH, Mgginnis J (1982) The effect of a purified guar degrading enzyme on chicken growth. Poult Sci 61:488–494
Titapoka S, Keawsompong S, Haltrich D, Nitisinprasert S (2008) Selection and characterization of mannanase-producing bacteria useful for the formation of prebiotic manno-oligosaccharides from copra meal. World J Microbiol Biotechnol 24:1425–1433
Tomotari M (1990) Bifidobacteria and their role in human health. J Ind Microbiol 6:263–268
Wootton AN, Luker-Brown RJ, Cheetham PSJ (1993) The extraction of a glucomannan polysaccharide from konjac corms (elephant yam, Amorphophallus rivieri). J Sci Food Agric 61:429–433
Acknowledgements
This research was financially supported by the Graduate School of Kasetsart University and the National Research Council of Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Rungruangsaphakun, J., Keawsompong, S. Optimization of hydrolysis conditions for the mannooligosaccharides copra meal hydrolysate production. 3 Biotech 8, 169 (2018). https://doi.org/10.1007/s13205-018-1178-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1178-2


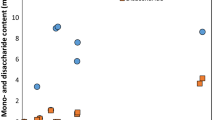






 20 ml,
20 ml,
 200 ml and
200 ml and
 2000 ml) on beneficial bacteria
2000 ml) on beneficial bacteria
 20 ml,
20 ml,
 200 ml and
200 ml and
 2000 ml) on pathogenic bacteria
2000 ml) on pathogenic bacteria