Abstract
The ubiquitous presence of MPs in water bodies presents an escalating concern, as these minuscule plastic particles could ultimately reach humans via the drinking water supply. This study explores the efficacy and underlying mechanisms of removing PE and PVC MPs using Abelmoschus esculentus seeds (commonly known as okra), a natural and environmentally benign coagulant. Through experiments conducted under varying conditions—such as pH level, coagulant dosage, MP concentration, and EC—using the standard method and a Jar test apparatus, the sedimentation rate was assessed. ZP analysis revealed that charge neutralization and bridging cause pivotal in enhancing the removal efficiency of MPs. FESEM and FTIR analyses corroborated the formation of new bonds during the interaction between the MPs and the okra seed-based coagulant. The findings indicate that the optimal parameters for PVC removal were a coagulant dosage of 70 mg/L, a pH of 10, and an MP concentration of 20 mg/L, achieving a removal efficiency of 80.11%. Conversely, for PE, the maximum removal efficiency of 64.76% was realized at a coagulant dosage of 70 mg/L, a pH of 3, and an MP concentration of 20 mg/L. Abelmoschus esculentus seeds offer a practical and eco-friendly option, potentially substituting chemical coagulants, to efficiently eliminate MPs from aquatic environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic has become an integral part of modern society, with its affordability and durability making it a material of choice across various industries (Frias and Nash 2019). This ubiquity has led to the current era being dubbed the “Age of Plastics” (Zahmatkesh Anbarani et al. 2024). However, the excessive and indiscriminate use of plastic compounds has led to substantial environmental challenges, causing widespread pollution and ecosystem degradation globally. Plastic production and disposal processes emit hazardous chemicals, contaminating soil, water, and air. These contaminants threaten wildlife, destabilize ecosystems, and endanger human health by polluting food chains (Donuma et al. 2024; Yari et al. 2024).
Through mechanical, chemical, and biological decomposition processes, plastics break down into micron-scale and nano-scale particles, collectively known as MPs (Bai et al. 2024; Bajt 2021; Klein et al. 2018). According to NOAA, MPs are defined as plastic particles with a diameter of less than 5 mm (Jahanpeyma and Baranya 2024). These MPs can originate from primary sources, such as industrial production, or secondary sources, where larger plastic items degrade over time (Chaudhry and Sachdeva 2021). Once liberated or detached from their initial plastic products, MPs can navigate through watercourses and disperse across a wide array of environments. These include oceans, wastewater treatment facilities, coastal regions, marine sediments, surface waters, freshwater ecosystems, and even glacial landscapes in the Arctic and Antarctic (Barari and Bonyadi 2023; Zahmatkesh Anbarani et al. 2023b; Zhou et al. 2024).
Their small size and large surface area (Enfrin et al. 2020; Yang et al. 2019) allow them to adsorb a range of hazardous compounds, including PAHs, cyanide (Bonyadi et al. 2012), pesticides (Pirsaheb et al. 2013), antibiotics (Li et al. 2018; Lotfi Golsefidi et al. 2023), polychlorinated biphenyls (Pathak et al. 2020), and heavy metals (Bai et al. 2022; Zafarzadeh et al. 2021). This capability underscores the potential risk MPs pose to both terrestrial and aquatic ecosystems, as well as human health, due to their capacity to accumulate and concentrate these harmful substances. The ingestion or inhalation of MPs by marine organisms (Botterell et al. 2019; Egbeocha et al. 2018) and even humans has raised significant ecological and health concerns. MPs have the unique capability to permeate cell membranes across various organs, leading to disruptions in the normal functioning of critical biological systems. These pollutants affect the digestive, respiratory, nervous, and endocrine systems, potentially leading to systemic disruptions (Esmaeili Nasrabadi et al. 2023). In the digestive system, MPs can cause physical damage and alter the intestinal microbiome, impacting digestion and potentially leading to secondary poisoning from adsorbed toxins. Respiratory issues arise from inhalation of MPs, inducing inflammation and oxidative stress in the lungs, which can progress to chronic obstructive pulmonary disease COPD and other respiratory conditions (Barari et al. 2024). The nervous system may also be affected indirectly through systemic inflammation and oxidative stress, though direct links to neurological effects are still emerging. Endocrine disruption occurs due to MPs’ interference with hormone production and metabolism, leading to a range of health issues, including thyroid dysfunction and reproductive disorders (Bajt 2021; Kim et al. 2021; Shi et al. 2023).
Research indicates the presence of MPs in both surface water and sediment samples from various rivers across Iran, notably in the Zayandeh-rud River, where 588 items per kilogram of dry weight were detected (Behmanesh et al. 2023; Rami et al. 2023). Moreover, MPs have been identified in the effluent of wastewater treatment plants, specifically in District 22 of Tehran, averaging 2.15 MPs particles per (Feizi et al. 2022). Among the various types of MPs found in water bodies, PS and PVC are of particular interest, as their densities are similar to that of natural water (Almujally et al. 2024; Fernández-González et al. 2022; Lee et al. 2021). Freshwater ecosystems have been increasingly recognized as an important source of MPs in the oceans, warranting greater attention (Lee et al. 2021).
Removal of pollutants from aquatic environments employs a variety of methods, including membrane reactors (Mishra et al. 2022), rapid sand filtration (Bayo et al. 2020), photocatalysis, absorption (Padervand et al. 2020), and coagulation processes such as flocculation (Gao and Liu 2022; Ma et al. 2019b). Biological treatments involving organisms like algae (Esmaili et al. 2023; Nasoudari et al. 2023) and yeasts (Anbarani et al. 2023; Mazloomi et al. 2021) are also utilized. A notable observation from reviews indicates the successful removal of PS particles from water using polyaluminum chloride as a coagulant (Li et al. 2021; Liu et al. 2022).
The imperative to maintain environmental equilibrium, protect public health, and preserve the integrity of the food chain has propelled the investigation and adoption of natural coagulants as a compelling alternative to chemical options (Nasrabadi et al. 2023b). The use of natural, sustainable coagulants, such as starch, okra (Badawi et al. 2023) cellulose (Yu et al. 2016; Zhu et al. 2015), pectin (Ibarra-Rodríguez et al. 2017), chitosan (Badawi et al. 2023), lignin (Couch et al. 2016), plant gum (Shahadat et al. 2017) and microbial flocculants (Zahmatkesh Anbarani et al. 2023a), has shown promising results in addressing this issue. In the present study, the highest removal efficiency of PE MPs was achieved at 84% using C. vulgaris (Eydi and Bonyadi 2023). The okra (Abelmoschus esculentus) plant from the Malvaceae family is known as gumbo or lady’s fingers (Sayyad et al. 2024). The plant has a straight stem, covered with webs and a height of 0.5 m, sometimes 2 m. Okra seed is rich in carbohydrates, tanen and contains a large amount of water-soluble polysaccharides that can create very high viscosity in low concentrations (Yu et al. 2024). Okra polysaccharide is an acidic polysaccharide and consists of galactose, rhamnose and galactonic acid. The polysaccharide content in okra seed extract is responsible for its viscous texture and exhibits promising coagulation properties. This suggests the presence of active sites capable of effectively adsorbing colloids during the coagulation-flocculation process (Agarwal et al. 2001). These features render it a highly appealing choice for the removal of diverse pollutants, including MPs, from aqueous solutions (Chung et al. 2018; Lanan et al. 2021). In a study, okra polysaccharide removed 87% of MPs from water samples (Bhuju 2020). Okra seed is an effective coagulant to remove turbidity (Fahmi et al. 2014). The ability of okra polysaccharides to form strong bonds with particles makes them a promising natural and sustainable solution for various particle removal applications, including wastewater treatment (Oladoja 2015).
Considering the numerous benefits of okra, it becomes crucial to utilize okra seeds for the removal of MPs such as PS and PVC. The main goal is divided into two objectives: (1) to clarify the underlying coagulation mechanisms that facilitate the efficient removal of PVC and PS MPs, and (2) to thoroughly investigate the impact of various operational factors, such as pH, coagulant dosage, MP concentration, and EC, on the overall removal efficiency of these contaminants.
Materials and methods
Chemicals and reagents
Chemicals with a purity level of 95%, including sodium hydroxide, hydrochloric acid, sodium chloride, and sodium sulfate, were procured from Merck, Germany. Additionally, Whatman filter paper with a pore size of 0.45 microns was sourced from Merck, Germany.
Preparation of microplastics
The PS MP used in this experiment was purchased in the form of transparent granules from Mashhad Tos Polymer Company. The PS granules were ground and sieved to obtain particles smaller than 100 µm. PVC MPs with a size of less than 85 µm were obtained from Mashhad Tos Polymer Company. To minimize potential interference and ensure proper contact between okra particles and MPs, the raw okra seeds were washed with distilled water. Subsequently, the materials were dried for 12 h at 60 °C. The resulting MPs were then stored in a sealed container, protected from moisture and light, in a dark environment.
Preparation of okra seed
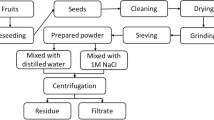
Okra was bought from a market in Mashhad. Iran. The seeds were manually removed from the pods. The seeds underwent a thorough cleansing process using laboratory-distilled water to eliminate contaminants such as stones, plant debris, and dust, which may affect their integrity and quality. Following the washing step, the cleaned seeds were subsequently dried in an oven at 60 °C for 6 h (Fig. 1). After drying, the seeds were ground into a powder and were passed through a 400 µm sieve for granulation.
Coagulation experiments
Various parameters were considered to evaluate the removal efficiency of PVC and PS MPs by okra seeds. These parameters included the initial concentration of MPs (20, 50, 100 mg/L), the dose of okra seed (10, 40, 70 mg/L), the pH levels (3, 7, 10), and EC (0.05, 2500–5000, > 5000 mS/cm). The removal process was conducted using the jar test method (Richmond, VA 23228) at room temperature (25 ± 2 °C). The stirring speed was set to 150 rpm for 3 min, followed by 20 rpm for 20 min. Afterward, the suspension was transferred to a decanter funnel, and the formed flocs were allowed to settle undisturbed for a duration of 1 h. Following the settling period, the liquid above the settled flocs was filtered, and the filtrate was subsequently dried at 60 °C for a period of 24 h. The final weight of the filter paper was subtracted from the initial weight to determine the weight of the remaining MPs. The removal efficiency of MPs was calculated using the following formula:
where M1 represents the initial weight of MP before the removal process, M2 represents the weight of MP on a Whatman filter after the removal process, and W represents the mass of MP (Eydi Gabrabad et al. 2024).
Characteristics and measurements
The MPs and flocs that precipitated in the lower layer were collected for characterization. FTIR (FTIR/NIR FRONTIER model) was used to identify the functional groups present on the surfaces of okra seeds, MPs, and the bio-based flocculants. FESEM (Carl Zeiss model, Germany) was employed to investigate the surface morphology of the samples under study. ZP (Oxford model connected to a JEOL-JSM-5600 SEM) was used to measure the surface charge and the amount of repulsion or attraction of the samples under study.
Statistical data analysis
The number of tests was determined employing a single-factor experimental design. Within each trial, one variable was altered while the remaining variables were held constant at their optimal levels. Subsequent data analysis was conducted utilizing SPSS version 22.0. ANOVA was executed to evaluate sample differences, applying a minimal significant difference criterion (P-Value < 0.05).s
Result and discussion
Effect of double-layer compression
To elucidate the coagulation mechanism, it is imperative to investigate the process of charge neutralization during coagulation. ZP assesses the electrostatic dispersion process and serves as an indicator of particle stability. It is influenced by several parameters, such as pH, solution conductivity, and particle concentration. Table 1 displays the ZP values of the MPs utilized in the coagulation experiments, as well as the ZP of the supernatant following the reaction. ZP values for PVC and PS were obtained as − 79.6 and − 78 mV, respectively. After coagulation, the ZP of PVC at alkaline pH was − 52.4 mV, whereas under acidic conditions, the ZP of PS was approximately − 54.5 mV. The addition of okra seeds into the suspension diminishes the repulsive force acting between the particles, facilitating the formation of bio-based flocculants. As a result, these flocs gradually settle due to their heightened density, indicating the potential of okra seeds to impact the settling and subsequent removal of PVC and PS particles from the environmen (Zhang et al. 2021). The charge of the colloidal particles and the absolute value of the ZP of the MPs both decreased as a result of this process, indicating charge neutralization and the compression of the electrical double layer of the MPs (Zhang et al. 2021). The final ZP in the PVC-okra seed system was closer to zero potential than that in the PS-okra seed system, illustrating that the charge neutralization in the PVC-okra seed system was more effective.
The presence of electrostatic repulsion between the MPs and the negatively charged coagulant indicates that charge neutralization is not the sole dominant mechanism at play. Most polyelectrolytes, like okra seeds, have hydrogen atoms that are covalently attached to a more electronegative atom or group such as carboxylic, amide, amine, and hydroxyl and can form hydrogen bonds with other electronegative atoms that have alone pair of electrons (Tosif et al. 2021). The availability of a large number of hydroxyl groups (polar agent) in the galactone chain increases the absorption of these polymers on the surface of the pollutant particles and also increases the bridging action between the pollutant (Koul et al. 2022). In addition, a mixture of polysaccharides such as galactomannan and galactan isolated from tannin seeds, cactus, Nirmali and Strychnos potatorum have the ability to reduce turbidity (Dwarapureddi and Saritha 2016; La Mer 1966). Furthermore, the decrease in electrophoretic mobility after the reaction in Table 1, can be related to the efficiency of the coagulation processes for MP removal (Martic et al. 2022). An efficient coagulation process can effectively agglomerate MPs and lead to a decrease in their electrophoretic mobility (Azizi et al. 2023).
Effect of adsorption
In addition to charge neutralization and bridging, adsorption plays a crucial role in the coagulation process. As shown in Fig. 2, the joint morphology of MPs and loaded flocs in the PVC + okra seed system was analyzed by FESEM. Based on Fig. 2a, it can be observed that PVC exhibits a non-uniform surface with spherical chains, along with significant valleys and grooves. These surface characteristics of PVC are advantageous (Suganya et al. 2016), as they provide a substantial surface area and active sites for adsorption. This enables PVC to effectively carry other particles and pollutants (Ren et al. 2021). Yu et al. (2020) demonstrated that PVC may possess a higher number of adsorption sites compared to other MPs due to its rough surface and internal wrinkles (Yu et al. 2020).
As shown in Fig. 2b, the okra structure looks spongy and compact, and this porosity is caused by transverse connections. This porous structure prepares minimal matrix space, enabling the ingredients to be incorporated more efficiently into the tablet (Zaharuddin et al. 2014). As depicted in Fig. 2c, the flocs formed after the elimination process consist of a combination of okra seeds and PVC particles. The entanglement and accumulation of MPs and okra seeds are evident, signifying the adsorption and bridging of MPs by okra seed cells (Khan et al. 2023). The interaction between the pores and gaps present in okra seed particles and the uneven surface of MPs results in the formation of larger flocs. Consequently, these flocs settle at an accelerated rate due to their increased size and weight (Fahmi et al. 2014). In conclusion, the adsorption of PVC onto the okra particles as well as the bridging effect of the okra seed contributes to the effective coagulation and sedimentation of the MPs.
From Fig. 3d, it can be observed that PS particles have irregular cracks on their surfaces (Kurniawan et al. 2023; Zhou et al. 2021). Figure 3f presents the FESEM image depicting the coagulation of PS particles post-treatment with okra seeds. The image clearly demonstrates the entanglement and accumulation of the MP particles and okra. This visual evidence indicates the effective adsorption and bridging of the PS MPs by the okra seed (Zhou et al. 2021). The interaction between the porous and spongy structure of the okra seed and the uneven surface of the PS MPs appears to be a key factor driving the coagulation process. Also Kurniawan et al. (2023) stated that the irregular cracks of PS particles lead to an increase in the contact surface with polymer particles. (Kurniawan et al. 2023; Wang et al. 2024).
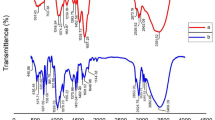
FTIR analysis was used to investigate the chemical composition and interactions between the PVC and the okra seeds. According to Fig. 4, the FTIR spectrum of the okra seeds reveals the presence of several functional groups that contribute to the coagulation mechanism. The broad peak observed at 3423.140 cm−1 indicates the presence of aromatic sugar groups with O–H as the main functional group (Zaharuddin et al. 2014). These hydrophilic O–H groups act as active sites, facilitating the binding of colloidal particles and metal ions (Di Bernardo and Dantas 1993). The peak at 2927.64 cm−1 corresponds to the C–H stretching vibrations of the methyl and methylene groups present in the cellulose and hemicellulose components, such as galactose and rhamnose (Zaharuddin et al. 2014). Additionally, the peak at 1631.102 cm−1 is attributed to the C=O stretching vibrations of carboxylic acids, esters, and amides, indicating the possible attachment of N, N-methylene bisacrylamide to the okra structure (Rahman et al. 2018). The presence of these functional groups suggests that the okra seeds possesses the necessary characteristics to effectively interact with and coagulate the PVC MPs (Wang et al. 2023).
In the infrared spectrum related to PVC in Fig. 4, a prominent peak is observed at 3669.81 cm−1, which corresponds to the stretching vibrations of the O–H hydrogen bond (Lu et al. 2022). Additionally, peaks at 2974.57 and 2912.97 cm−1 are attributed to the C–H stretching vibrations, while the peak at 1331.18 cm−1 is associated with the C–Cl stretching vibrations (Wu et al. 2014). Figure 4 indicates that the peak corresponding to the O–H groups decreased to 3408.73 cm1. Furthermore, the CH2 asymmetric stretch exhibits a significant decrease, measuring 2918.183 cm−1, compared to the peak observed prior to the removal process (He et al. 2023). The peak at 1639.183 cm−1 is related to amine groups in okra, which reduce the repulsive force between PVC particles. The peak corresponding to the O–H group decreased from 1446.121 to 1434.48 cm−1. This reduction may be attributed to the formation of flocs caused by the adsorption of PVC on okra. When okra is adsorbed on PVC, the molecules interact with chlorine atoms in PVC, leading to a peak shift from 833.11 to 827.24 cm−1. The appearance of C–H and O–H groups after the adsorption process shows that okra molecules are connected to PVC through hydrogen bonding (Atugoda et al. 2020). Hydrophilic groups, the cellulose and hemicellulose components, and the carbonyl amide groups in the okra seed play a crucial role in the coagulation of the PVC MPs (Kim et al. 2020). Amide, amine, carbonyl, and methylene groups play a crucial role in charge neutralization and point flocculation mechanisms during coagulation-flocculation processes (Chum 2020; Magalhães et al. 2021).
The FTIR spectrum of the PS in Fig. 5 shows prominent peaks around 3438.10 and 2915.9 cm−1 are attributed to –OH and C–H stretching vibration, respectively. The peak in the region of 1745.143 cm−1 is related to the C=O group (da Silva et al. 2008). The peak at 1602.169 cm−1 was attributed to the C=C stretching band. The presence of absorption peaks at 906.45, 753.91, and 533.89 cm−1 is related to C–H bending band, C–H stretching band, and C–H stretching band, respectively. The peaks at around 841.91 and 753.91 cm−1 corresponded to the substitution of the benzene ring (Fang et al. 2010; Zhou et al. 2021). After the elimination process in Fig. 5, the peak corresponding to O–H groups in Fig. 5 was reduced to 3426.33 cm−1. In the obtained spectrum, the asymmetric stretching of CH2 has increased to 2936.44 cm−1, indicating a significant change compared to the peak observed prior to the removal process. Additionally, the peak associated with the C=O group has decreased from 1493.08 to 1449.44 cm−1. This reduction in intensity could be attributed to the formation of clots resulting from the adsorption of PS on okra. The peak at 1631.02 cm−1 is related to the amine groups in okra, which can reduce the repulsive force between the PS particles and contribute to the bridging between the particles, which is responsible for the coagulation-flocculation process (Ma et al. 2022). The benzene ring’s substituted group, which is characteristic of PS, exhibited weak absorption peaks at 752.46 and 829.49 cm−1. These peaks decreased following the coagulation process (Zhou et al. 2021). Also, the presence of polysaccharides in okra has been shown to help in bridging between particles, which is responsible for the coagulation-clotting process (Kim et al. 2020). Functional groups like the hydroxyl, the carbonyl, and the amine in the okra seed play a crucial role in the coagulation of the PS. Fard et al. (2021) highlighted that polysaccharides, proteins, carbonyl, carboxyl, and hydroxyl groups facilitate the bridging mechanism in coagulation processes (Fard et al. 2021). Similarly, another study emphasized the importance of amine, carboxyl, and hydroxyl groups in the charge neutralization mechanism during coagulation processes (Igwegbe et al. 2021).
Effect of effective factors on coagulation
Effects of pH on removal efficiency
After investigating the coagulation mechanism, the study also examined the impact of experimental conditions on the removal efficiency of MPs. The pH of the solution has a critical effect on the removal of MPs through coagulation and controls the surface charge of the particles (Han et al. 2020). Hence, it is crucial to elucidate the influence of pH on the removal efficiency. Figure 6 illustrates the removal efficiencies of PVC and PS MPs by the okra seed at various pH levels. In the PVC-okra seed system when the initial pH increased from 3 to 10, the removal efficiency of PVC was increased to 63.45% and the removal efficiency of PS is at an initial pH level of 3 about 59.8%, while those in the control groups without okra seed were 32.1 and 23.6%, respectively. These indicated that okra seed played an important role in the MPs removal. Throughout all stages of testing, the removal efficiency of PVC consistently outperformed that of PS. Studies have shown that PVC is more susceptible to electrochemical deposition and flotation, with lower electrical resistivity, making settling easier (Wu et al. 2014). Statistical analysis demonstrated a significant correlation between the removal efficiency of MPs and pH for both PVC and PS (P-value < 0.003 and P-value < 0.02, respectively). Theoretically, at low pH levels, H + ions compete with organic ligands and active compounds from okra, thereby limiting their ability to establish bonds with the MPs (Kim et al. 2020). Conversely, at pH values above the isoelectric point, where surface charges are neutralized, the net charge of the MPs becomes negative, causing the polymer chains to expand. This expansion facilitates a bridging mechanism between the particles, promoting their removal. In general, the swelling of okra seed mucilage increases as the pH level rises. This phenomenon can be attributed to the enhanced ionization of the -COOH groups present in the mucilage at higher pH values. Consequently, there is a slight improvement in the removal efficiency of MPs in alkaline conditions compared to acidic conditions (Elkhalifa et al. 2021).
Moreover, it has been observed that the average size of flocs formed in alkaline conditions is larger compared to acidic conditions (Khan et al. 2023). This larger floc size is more favorable for effective sweep and sedimentation processes. Azizi et al. (2023) found that the rate of PS MPs removal by coagulants at pH 3 was substantially higher compared to other pH levels (Azizi et al. 2023).
Effects of microplastic concentration on removal efficiency
MP concentration is another factor that affects the removal efficiency. Figure 7 shows that as the concentration of PVC and PS increases from 20 to 100 mg/L, the removal efficiency decreases. The removal rate of PVC and PS in the control group without okra seed was 33% and 25.09%. Furthermore, a statistically significant difference was observed between the removal efficiency and MP dosage for both PVC and PS, respectively (P-value < 0.03, P-value < 0.04). In a study, it was confirmed that the removal efficiency of polyethylene MPs using Chlorella vulgaris algae decreased by increasing the concentration of PE from 250 to 400 mg/L (Nasrabadi et al. 2023a). The findings suggest that the removal efficiency of MPs is maximized within an optimal concentration range. However, beyond this range, the removal efficiency starts to decrease. At higher concentrations, the MPs can form a protective layer around themselves, reducing the contact between the MPs and okra seeds. Additionally, the repulsive forces between the particles at high concentrations contribute to their stability, making their removal more challenging (Nasrabadi et al. 2023a; Tang et al. 2022). Ziembowicz et al. (2023) discovered that during the coagulation of polyethylene and PVC in water using aluminum salt, an increase in MPs concentrations resulted in a slight decrease in removal efficiency (Ziembowicz et al. 2023).
Effects of coagulant concentration on removal efficiency
The effect of varying the okra seed coagulant dosage from 10 to 70 mg/L on the removal of PVC and PS MPs was evaluated. The results presented in Fig. 8 demonstrate a direct relationship between the dose of the coagulant and the removal efficiency.
By decreasing the coagulant dose to 10 mg/L, the removal efficiency of PVC and PS reached its lowest level of 54.62 and 40.14%, respectively. On the other hand, when the okra seed dose was increased to 70 mg/L, the removal efficiency significantly improved, reaching 64.76% for PS and 80.11% for PVC while those in the control groups without okra seeds are 36 and 32.6%, respectively. Statistical analysis revealed a statistically significant difference between the removal efficiency and coagulant dosage for PS (P-value < 0.01), but not for PVC (P-value < 0.726). These findings can be attributed to the mechanism of coagulation using the okra seed coagulant. At low coagulant doses, the formation of smaller and less dense flocs occurs, leading to poor sedimentation of the MPs. As shown in Fig. 4, the FTIR analysis of okra confirms the presence of carbonyl and hydroxyl groups, which are major components of carbohydrate molecules (Fard et al. 2021). These functional groups can be adsorbed on the surface of suspended colloids, allowing for the formation of bridges between the particles. In systems where the bridging mechanism is responsible for coagulation, increasing the dose of the coagulant generally improves the coagulation process (Kim et al. 2020). Therefore, the increase in okra seed coagulant dosage led to the formation of larger and more densely packed flocs (Jones and Bridgeman 2016), resulting in enhanced sedimentation and increased removal efficiency for both PVC and PS. Zhou et al. (2021) found that charge neutralization was dependent on coagulant and that removal efficiency increased with coagulant dosage because positive coagulant charges gradually decreased the ZP of MPs (Zhou et al. 2021).
Effects of electrical conductivity changes on removal efficiency
The study investigated the effects of various ions present in water samples on the removal efficiency of PVC and PS MPs. As shown in Fig. 9, the presence of NaCl being the most common compound in aquatic environments had little influence on the removal efficiency of MPs. This finding is consistent with a previous study (Ma et al. 2019a), in which the effect of inorganic salts on the coagulation process is influenced by the charge of the ions present in the solution (Jia et al. 2017). In contrast, the results indicate that SO42- had a certain inhibitory effect on the removal efficiency of MPs (Duan and Gregory 2003). This is likely due to the fact that SO42 -is not useful for the binding of coagulant hydrolyzates and MPs (Zhou et al. 2021). When the combination of Na2SO4 and NaCl was present, the removal efficiency of MPs was further lowered. As the salinity increases, Na+ cations can easily attach to the negatively charged MPs through electrostatic interaction, preventing the adsorption of okra by the MPs (Perren et al. 2018). Statistically, a significant difference was found between the removal efficiency and EC for both PVC and PS MPs (P-value < 0.01 and P-value < 0.03, respectively). In summary, the findings of the study indicate that NaCl exhibits minimal impact on the removal efficiency of MPs. However, the presence of SO42- and the combination of Na2SO4 and NaCl demonstrate inhibitory effects on the removal process of MPs.
Future prospective
We propose the integration of waste-to-energy technology, specifically pyrolysis, as a promising method for addressing the disposal of MPs. Pyrolysis offers a transformative approach by converting MPs waste into valuable products, such as oils and gases, while minimizing environmental impacts. By incorporating pyrolysis into the waste management process, we can effectively address the issue of MPs disposal, contributing to a more sustainable approach while simulta neously harnessing energy from the process. This indicates a promising direction for future research and development in the field of MPs waste management.
Conclusion
In summary, this research elucidates the removal performance and mechanism of PVC and PS MPs through the coagulation process utilizing okra seed. The study reveals that okra seed exhibits a notable removal performance on both types of MP and with PVC displaying a higher removal efficiency compared to PS. The coagulation process involves charge neutralization and bridging phenomena. According to the FESEM images, agglomeration adsorption was observed in the system. Furthermore, the FTIR spectra indicate the formation of new bonds during the interaction between the MPs and coagulants. The removal efficiencies of PVC and PS were the largest at pH levels of 10 and 3, respectively. The increase in MP concentration was conducive to the decreased removal performance. The increase in coagulant dos was conducive to the improved removal performance of MPs. NaCl and SO42− had inhibitory and promoting effects on the removal efficiency of MPs, respectively.
Data availability
All necessary data are included in the document.
Abbreviations
- PS:
-
Polystyrene
- PVC:
-
Polyvinyl chloride
- MPs:
-
Microplastics
- EC:
-
Electrical conductivity
- ZP:
-
Zeta potential
- FESEM:
-
Field emission scanning electron microscopy
- FTIR:
-
Fourier transform infrared
- NOAA:
-
National oceanic and atmospheric administration
- PAHs:
-
Polycyclic aromatic hydrocarbons
References
Agarwal M, Srinivasan R, Mishra A (2001) Study on flocculation efficiency of okra gum in sewage waste water. Macromol Mater Eng 286:560–563
Almujally NA, Khan D, Al Mudawi N, Alonazi M, Alazeb A, Algarni A, Jalal A, Liu H (2024) Biosensor-driven IoT wearables for accurate body motion tracking and localization. Sensors 24:3032
Anbarani MZ, Nourbakhsh S, Toolabi A and Bonyadi Z (2023) Biodegradation of crystal violet dye by Saccharomyces cerevisiae in aqueous medium. Heliyon 9
Atugoda T, Wijesekara H, Werellagama D, Jinadasa K, Bolan NS, Vithanage M (2020) Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: implications for vector transport in water. Environ Technol Innov 19:100971
Azizi N, Pirsaheb M, Jaafarzadeh N and Nodehi RN (2023) Microplastics removal from aquatic environment by coagulation: selecting the best coagulant based on variables determined from a systematic review. Heliyon
Badawi AK, Salama RS, Mostafa MMM (2023) Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: a review of recent developments. RSC Adv 13:19335–19355
Bai B, Bai F, Li X, Nie Q, Jia X, Wu H (2022) The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ Technol Innov 28:102944
Bai B, Chen J, Bai F, Nie Q, Jia X (2024) Corrosion effect of acid/alkali on cementitious red mud-fly ash materials containing heavy metal residues. Environ Technol Innov 33:103485
Bajt O (2021) From plastics to microplastics and organisms. FEBS Open Bio 11:954–966
Barari F, Bonyadi Z (2023) Evaluation of the leaching of microplastics from discarded medical masks in aquatic environments: a case study of Mashhad city. Appl Water Sci 13:229
Barari F, Gabrabad ME and Bonyadi Z (2024) Recent progress on the toxic effects of microplastics on Chlorella sp. in aquatic environments. Heliyon
Bayo J, López-Castellanos J, Olmos S (2020) Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar Pollut Bull 156:111211
Behmanesh M, Chamani A, Chavoshi E (2023) Sedimentary abundance and major determinants of river microplastic contamination in the central arid part of Iran. Appl Water Sci 13:239
Bhuju R (2020) Removal of microplastics from fresh water using plant derived polysaccharides. Tarleton State University, Washington
Bonyadi Z, Dehghan A, Sadeghi A (2012) Determination of sonochemical technology efficiency for cyanide removal from aqueous solutions. World Appl Sci J 18:425–429
Botterell ZL, Beaumont N, Dorrington T, Steinke M, Thompson RC, Lindeque PK (2019) Bioavailability and effects of microplastics on marine zooplankton: a review. Environ Pollut 245:98–110
Chaudhry AK, Sachdeva P (2021) Microplastics’ origin, distribution, and rising hazard to aquatic organisms and human health: Socio-economic insinuations and management solutions. Reg Stud Mar Sci 48:102018
Chum C (2020) Treatment of pulp and paper mill wastewater using moringa oleifera via coagulation-flocculation treatment process, UTAR
Chung CY, Selvarajoo A, Sethu V, Koyande AK, Arputhan A, Lim ZC (2018) Treatment of palm oil mill effluent (POME) by coagulation flocculation process using peanut–okra and wheat germ–okra. Clean Technol Environ Pol 20:1951–1970
Couch RL, Price JT, Fatehi P (2016) Production of flocculant from thermomechanical pulping lignin via nitric acid treatment. ACS Sustainable Chem Eng 4:1954–1962
Di Bernardo L and Dantas ADB (1993) Methods and techniques of water treatment, Abes Rio de Janeiro
Donuma KU, Ma L, Bu C, George L-Y, Gashau M, Suleiman AO (2024) Environmental and human health risks of indiscriminate disposal of plastic waste and sachet water bags in Maiduguri. Borno State Nigeria Waste Manag Bull 2:130–139
Duan J, Gregory J (2003) Coagulation by hydrolysing metal salts. Adv Colloid Interface Sci 100:475–502
da Silva FF, Aquino KADS, Araújo ES (2008) Effects of gamma irradiation on poly (vinyl chloride)/polystyrene blends: Investigation of radiolytic stabilization and miscibility of the mixture. Polym Degr Stab 93(12):2199–2203
Egbeocha CO, Malek S, Emenike CU, Milow P (2018) Feasting on microplastics: ingestion by and effects on marine organisms. Aquat Biol 27:93–106
Elkhalifa AEO, Al-Shammari E, Adnan M, Alcantara JC, Mehmood K, Eltoum NE, Awadelkareem AM, Khan MA, Ashraf SA (2021) Development and characterization of novel biopolymer derived from Abelmoschus esculentus L. extract and its antidiabetic potential. Molecules 26:3609
Enfrin M, Lee J, Gibert Y, Basheer F, Kong L, Dumée LF (2020) Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J Hazard Mater 384:121393
Esmaeili Nasrabadi A, Zahmatkesh Anbarani M, Bonyadi Z (2023) Investigating the efficiency of oak powder as a new natural coagulant for eliminating polystyrene microplastics from aqueous solutions. Sci Rep 13:20402
Esmaili Z, Barikbin B, Shams M, Alidadi H, Al-Musawi TJ, Bonyadi Z (2023) Biosorption of metronidazole using Spirulina platensis microalgae: process modeling, kinetic, thermodynamic, and isotherm studies. Appl Water Sci 13:63
Eydi Gabrabad M, Yari M, Bonyadi Z (2024) Using Spirulina platensis as a natural biocoagulant for polystyrene removal from aqueous medium: performance, optimization, and modeling. Sci Rep 14:2506
Eydi M and Bonyadi Z (2023) Utilizing Chlorella vulgaris algae as an eco-friendly coagulant for efficient removal of polyethylene microplastics from aquatic environments. Heliyon 9
Fahmi MR, Hamidin N, Abidin CZA, Fazara U, Ali M, Hatim M (2014) Performance evaluation of okra (Abelmoschus esculentus) as coagulant for turbidity removal in water treatment. Trans Tech Publ
Fang J, Xuan Y, Li Q (2010) Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci China Technol Sci 53:3088–3093
Fard MB, Hamidi D, Yetilmezsoy K, Alavi J, Hosseinpour F (2021) Utilization of Alyssum mucilage as a natural coagulant in oily-saline wastewater treatment. J Water Process Eng 40:101763
Feizi F, Hamidian AH, Akhbarizadeh R, Jonoobi M (2022) Study on presence of microplastic pollution in the wastewater treatment plant of district 22 of Tehran. J Nat Environ 75:1–6
Fernández-González V, Andrade-Garda JM, López-Mahía P, Muniategui-Lorenzo S (2022) Misidentification of PVC microplastics in marine environmental samples. TrAC Trends Anal Chem 153:116649
Han X et al (2020) The role of in situ Fenton coagulation on the removal of benzoic acid. Chemosphere 238:124632
He J et al (2023) Unveiling interactions of norfloxacin with microplastic in surface water by 2D FTIR correlation spectroscopy and X-ray photoelectron spectroscopy analyses. Ecotoxicol Environ Saf 251:114521
Ibarra-Rodríguez D et al (2017) Capacity of ‘nopal’pectin as a dual coagulant-flocculant agent for heavy metals removal. J Chem Eng 323:19–28
Igwegbe CA et al (2021) Bio-coagulation-flocculation (BCF) of municipal solid waste leachate using picralima nitida extract: RSM and ANN modelling. Curr Res Green Sustain Chem 4:100078
Jia D et al (2017) Effect of basicity and sodium ions on stability of polymeric ferric sulfate as coagulants. Colloids Surf A: Physicochem Eng Asp 512:111–117
Khan MT et al (2023) Microplastic removal by coagulation: a review of optimizing the reaction conditions and mechanisms
Kim ITS et al (2020) Fenugreek seeds and okra for the treatment of palm oil mill effluent (POME)– characterization studies and modeling with backpropagation feedforward neural network (BFNN). J Water Process Eng 37:101500
Kim JH et al (2021) Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: a review. J Hazard Mater 413:125423
Klein S et al (2018) Analysis, occurrence, and degradation of microplastics in the aqueous environment. In: Freshwater microplastics: emerging environmental contaminants? pp 51–67
Koul B et al (2022) Application of natural coagulants in water treatment: a sustainable alternative to chemicals. Water 14(22):3751
Kurniawan TA et al (2023) Source, occurrence, distribution, fate, and implications of microplastic pollutants in freshwater on environment: a critical review and way forward. Chemosphere 325:138367
Lanan FABM et al (2021) Utilisation of natural plant-based fenugreek (Trigonella foenum-graecum) coagulant and okra (Abelmoschus escluentus) flocculant for palm oil mill effluent (POME) treatment. J Environ Chem Eng 9(1):104667
Lee HS et al (2021) Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem Toxicol 154:112356
Li C, Busquets R, Moruzzi RB, Campos LC (2021) Preliminary study on low-density polystyrene microplastics bead removal from drinking water by coagulation-flocculation and sedimentation. J Water Process Eng 44:102346
Li J, Zhang K, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467
Liu H, Gamboa H, Schultz T (2022) Sensor-based human activity and behavior research: where advanced sensing and recognition technologies meet. MDPI Sensors 23:125
Lotfi Golsefidi F, Zahmatkesh Anbarani M, Bonyadi Z (2023) Removal of metronidazole antibiotic by modified red mud from aqueous solutions: process modeling, kinetic, and isotherm studies. Appl Water Sci 13:202
Lu Y-H, Chen Z-L, Lu Y-W (2022) Synthesis, characterization and thermal behavior of plasticized poly(vinyl chloride) doped with folic acid-modified titanium dioxide. Sci Rep 12:3379
Ma J, Qiu Y, Zhao J, Ouyang X, Zhao Y, Weng L, Yasir MD, A., Chen, Y. and Li, Y. (2022) Effect of agricultural organic inputs on nanoplastics transport in saturated goethite-coated porous media: particle size selectivity and role of dissolved organic matter. Environ Sci Technol 56:3524–3534
Ma B, Xue W, Ding Y, Hu C, Liu H, Qu J (2019a) Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J Environ Sci 78:267–275
Ma B, Xue W, Hu C, Liu H, Qu J, Li L (2019b) Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem Eng J 359:159–167
Magalhães ERB, de Menezes NNF, Silva FL, Garrido JWA, Sousa MADSB, dos Santos ES (2021) Effect of oil extraction on the composition, structure, and coagulant effect of Moringa oleifera seeds. J Clean Prod 279:123902
Martic S, Tabobondung M, Gao S, Lewis T (2022) Emerging electrochemical tools for microplastics remediation and sensing. Front Sens 3:958633
Mazloomi S, Bonyadi Z, Haghighat GA, Nourmoradi H, Soori MM, Eslami F (2021) Removal of methylene blue by Saccharomyces cerevisiae: process modeling and optimization. Desalin Water Treat 236:318–325
Mishra S, Singh RP, Rout PK and Das AP (2022) Membrane bioreactor (MBR) as an advanced wastewater treatment technology for removal of synthetic microplastics. Dev. Wastewater Treat Res Process 45–60
Nasoudari E, Ameri M, Shams M, Ghavami V, Bonyadi Z (2023) The biosorption of Alizarin Red S by Spirulina platensis; process modeling, optimization, kinetic and isotherm studies. Int J Environ Anal Chem 103:633–647
Nasrabadi AE, Ramavandi B, Bonyadi Z (2023b) Recent progress in biodegradation of microplastics by Aspergillus sp. in aquatic environments. Colloid Interface Sci Commun 57:100754
Nasrabadi AE, Eydi M and Bonyadi Z (2023) Utilizing Chlorella vulgaris algae as an eco-friendly coagulant for efficient removal of polyethylene microplastics from aquatic environments. Heliyon 9
Oladoja NA (2015) Headway on natural polymeric coagulants in water and wastewater treatment operations. J Water Process Eng 6:174–192
Padervand M, Lichtfouse E, Robert D, Wang C (2020) Removal of microplastics from the environment. A Rev Environ Chem Lett 18:807–828
Pathak N, Tran VH, Merenda A, Johir M, Phuntsho S, Shon H (2020) Removal of organic micro-pollutants by conventional membrane bioreactors and high-retention membrane bioreactors. Appl Sci 10:2969
Perren W, Wojtasik A, Cai Q (2018) Removal of microbeads from wastewater using electrocoagulation. ACS Omega 3:3357–3364
Pirsaheb M, Khodadadi T, Bonyadi Z, Sharafi K, Khosravi T (2013) Evaluation of pesticide residues 2, 4-D, Atrazine and Alachlor concentration in drinking water well of Mahidasht district-Kermanshah, Iran, 2010–2011. World Appl Sci J 23:1530–1537
Rahman MM, Maniruzzaman M, Islam MR, Rahman MS (2018) Synthesis of nano-cellulose from okra fiber and ftir as well as morphological studies on it. Am J Polym Sci Tech 4:42–52
Rami Y, Shoshtari-Yeganeh B, Ebrahimi A, Ebrahimpour K (2023) Occurrence and characteristics of microplastics in surface water and sediment of Zayandeh-rud river. Iran Environ Health Eng Manage J 10:207–216
Ren X, Zhang X, Guo R, Li X, Peng Y, Zhao X, Pu X (2021) Hollow mesoporous g-C3N4/Ag2CrO4 photocatalysis with direct Z-scheme: Excellent degradation performance for antibiotics and dyes. Sep Purif Technol 270:118797
Sayyad IM, Ganjiwale RO, Gandhare BR, & Kediya AS (2024) A review on bioactive components, validated pharmacological application and technological applications of Abelmoschus Esculentus Linn.
Shahadat M, Teng TT, Rafatullah M, Shaikh Z, Sreekrishnan T, Ali SW (2017) Bacterial bioflocculants: a review of recent advances and perspectives. Chem Eng J 328:1139–1152
Shi W, Zhou C, Zhang Y, Li K, Ren X, Liu H, Ye X (2023) Hybrid modeling on reconstitution of continuous arterial blood pressure using finger photoplethysmography. Biomed Signal Process Control 85:104972
Suganya A, Shanmugavelayutham G, Rodríguez CS (2016) Study on structural, morphological and thermal properties of surface modified polyvinyl chloride (PVC) film under air, argon and oxygen discharge plasma. Mater Res Express 3:095302
Tang W, Li H, Fei L, Wei B, Zhou T, Zhang H (2022) The removal of microplastics from water by coagulation: a comprehensive review. Sci Total Environ 851:158224
Tosif MM, Najda A, Bains A, Kaushik R, Dhull SB, Chawla P, Walasek-Janusz M (2021) A comprehensive review on plant-derived mucilage: characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 13:1066
Wang X, Liu X, Shi N, Zhang Z, Chen Y, Yan M, Li Y (2023) Response surface methodology optimization and HPLC-ESI-QTOF-MS/MS Analysis on ultrasonic-assisted extraction of phenolic compounds from okra (Abelmoschus esculentus) and their antioxidant activity. Food Chem 405:134966
Wang Z, Yang P, He X, Yu Q (2024) Preparation of intercalated MXene by TPAOH and its adsorption characteristics towards U (VI). J Radioanal Nucl Chem 333:1999–2014
Wu J, Chen T, Luo X, Han D, Wang Z, Wu J (2014) TG/FTIR analysis on co-pyrolysis behavior of PE. PVC and PS Waste Manag 34:676–682
Yang J, Cang L, Sun Q, Dong G, Ata-Ul-Karim ST, Zhou D (2019) Effects of soil environmental factors and UV aging on Cu 2+ adsorption on microplastics. Environ Sci Pollut Res 26:23027–23036
Yari M, Bonyadi Z, Najafpoor A, Barikbin B (2024) Polyethylene microplastics as adsorbent of diazinon in aqueous environments: optimization, and modeling, isotherm, kinetics, and thermodynamic studies. Appl Water Sci 14:1–13
Yu F, Li Y, Huang G, Yang C, Chen C, Zhou T, Zhao Y, Ma J (2020) Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere 260:127650
Yu X, Mao W, Gao W, Liu J, Wang B, Zhang X, Liu X, Wang D, Lyu Y (2024) Antioxidant properties of wheat bran FOs prepared by Bacillus amyloliquefaciens IT-45 fermentation. Int J Food Sci Tech 59:816–828
Yu H-Y, Zhang D-Z, Lu F-F, Yao J (2016) New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustain Chem Eng 4:2632–2643
Zafarzadeh A, Taghani JM, Toomaj MA, Ramavandi B, Bonyadi Z, Sillanpää M (2021) Assessment of the health risk and geo-accumulation of toxic metals in agricultural soil and wheat, northern Iran. Environ Monit Assess 193:1–10
Zaharuddin ND, Noordin MI, Kadivar A (2014) The use of Hibiscus esculentus (Okra) gum in sustaining the release of propranolol hydrochloride in a solid oral dosage form. BioMed Res Int 2014(1):735891
Zahmatkesh Anbarani M, Esmaeili Nasrabadi A, Bonyadi Z (2023a) Use of Saccharomyces cerevisiae as a new technique to remove polystyrene from aqueous medium: modeling, optimization, and performance. Appl Water Sci 13:166
Zahmatkesh Anbarani M, Esmaeili Nasrabadi A, Bonyadi Z (2024) Aging effect on the adsorption behavior of microfibers obtained from cigarette butts in aqueous solutions. Appl Water Sci 14:30
Zahmatkesh Anbarani M, Najafpoor A, Barikbin B, Bonyadi Z (2023b) Adsorption of tetracycline on polyvinyl chloride microplastics in aqueous environments. Sci Rep 13:17989
Zhang Y, Zhou G, Yue J, Xing X, Yang Z, Wang X, Wang Q, Zhang J (2021) Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride. Coagulation with three typical coagulant aids. Sci Total Environ 800:149589
Zhou R, Bai B, Cai G, Chen X (2024) Thermo-Hydro-Mechanic-chemical coupling model for hydrate-bearing sediment within a unified granular thermodynamic theory. Comput Geotech 167:106057
Zhou G, Wang Q, Li J, Li Q, Xu H, Ye Q, Wang Y, Shu S, Zhang J (2021) Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: performance and mechanism. Sci Total Environ 752:141837
Zhu H, Zhang Y, Yang X, Liu H, Shao L, Zhang X, Yao J (2015) One-step green synthesis of non-hazardous dicarboxyl cellulose flocculant and its flocculation activity evaluation. J Hazard Mater 296:1–8
Ziembowicz S, Kida M, Koszelnik P (2023) Elimination of a mixture of microplastics using conventional and detergent-assisted coagulation. Mater 16:4070
Acknowledgements
Not applicable.
Funding
The authors would like to thank the financial support provided by the Mashhad University of Medical Sciences (Iran) through the Grant number of 1402045.
Author information
Authors and Affiliations
Contributions
M.E.G. contributed to methodology and writing—original draft, Z.B. contributed to investigation and methodology, M.D. contributed to investigation and methodology, and B.B. contributed to investigation, methodology, review and editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Eydi Gabrabad, M., Bonyadi, Z., Davoudi, M. et al. Microplastic removal using Okra (Abelmoschus esculentus) seed from aqueous solutions. Appl Water Sci 14, 217 (2024). https://doi.org/10.1007/s13201-024-02249-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02249-5