Abstract
Chemical coagulants like alum, ferric salts, and polyacrylamide derivatives are helpful in water treatment. However, the long-term detrimental effects of chemical coagulants on humans and the environment require alternative research for natural coagulants. This study used novel leguminous (green beans (GB), pigeon pea (PP)), fruit seeds (Tamarind indica (TI), and date palm (DS)) as coagulants to remove turbidity. The seeds were powdered, and the crude active coagulants were extracted with distilled water and a 1 M NaCl solution. The result showed that PP’s distilled water extract had the highest turbidity removal of 81.12%, while DS had the least performance of 62.54%. The NaCl extract of PP had the highest removal (94.62%), followed by TI (76.08%). This study found the optimum doses for GB, TI, PP, and DS to be 50, 40, 10, and 70 mL/L, with their optimum pH at 3, 1, 3, and 1, respectively. The FTIR spectra confirmed the existence of -OH, -NH, COOH, C = O, C–C, and C-H peaks, indicating the presence of protein-specific functional groups supporting their potential use as coagulants. Therefore, PP would have been used based on turbidity performance; however, due to their nutritional value, TI and DS are suitable seeds for the coagulation-flocculation treatment of turbid water because they are waste materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Turbidity is a visual characteristic of water, characterized by opacity, cloudiness, and unclarity. Water turbidity is often caused by the presence of solid particles, microorganisms, soil, organic matter, colored compounds, decaying matter, plankton, and algae. Turbidity must be removed before effluent discharge to achieve legislated standards and minimize the impact on aquatic ecosystems (Das et al. 2018; Al-Hashimi et al. 2021). In some cases, dissolved and colloidal material (such as dyes, metals, and silica) can also be destabilized and removed (Miranda et al. 2009). It is the most used technique due to its effectiveness and relative economic operational cost.
The use of chemical coagulants in reducing turbidity in water has been established and immensely used globally. However, these chemicals are often expensive and need high dosages; their residues in treated water are harmful to human health (Alzheimer’s disease, neurological, and carcinogenic effects), and sludge discharge is detrimental to the ecosystem (Ben Rebah et al. 2018). These downsides to coagulants incited the need to develop environmental, health-friendly, and economical natural coagulants from plant seeds, roots, leaves, gums, mucilage, chitin, and tannin (Miranda et al. 2013). Using natural organic coagulants has gained a lot of exciting advantages over synthetic chemical coagulants (Al-Saati et al. 2019).
Affordable legumes with the protein content needed for the coagulation process, including soybeans, dal seed, hyacinth bean, and peanut, were reported as potential coagulants; however, pigeon pea and green beans are yet to be studied (Birima et al. 2013). Proteins are the main component that aids coagulation in leguminous seeds (Abidin 2019). Green beans (GB) have a protein content of 21.22–24.06% (Kazydub et al. 2017). Pigeon pea (PP) has a protein and carbohydrate content of 20–22% and 65%, respectively (Sarkar et al. 2006). The presence of a protein that acts as a natural polyelectrolyte that can clarify water through its coagulant and flocculant activity is attributed to the coagulation performance efficiency of natural coagulants (Choudhary and Neogi 2017).
It is impossible to overestimate the importance of coagulation dosage, pH, and cationic/anionic water content on the efficacy of natural coagulants in water treatment (Antunes et al. 2008; Owodunni and Ismail 2021). According to Miranda Carreño et al. (2011) research, coagulants’ charge and structure are key parameters that determine their efficiency under different conditions. Furthermore, the charge and structure of these chemicals vary with the pH and surface charges of the contaminants. As a result, it affects the efficiency of the coagulation-flocculation treatment. At low pH levels, most inorganic particles, such as kaolin, have a point of zero charges and carry a negative charge throughout an extensive pH range (Naceradska et al. 2019). Therefore, the optimum coagulant dose is a critical parameter directly influencing coagulation performances. Overdosing could aid treated wastewater pollution and increase the organic load, turbidity, and sludge volume. Nonetheless, underdosing hinders the effective aggregation of colloidal particles in water; thus, achieving an accurate coagulant dosage control in water treatment should be considered (Alazaiza et al. 2022).
In the study, Lek et al. (2018) used chickpea powder to remove turbidity, TSS, and COD from palm oil mill effluent (POME); this study used the extract as the coagulant. The study reduced 86%, 87%, and 56% of turbidity, TSS, and COD, respectively, from the effluent. In addition, the FTIR analysis of the chickpea powder identified the presence of OH, CH, NH, CC, CO, and CN groups, which contributes to the bridging flocculation mechanism during the coagulation process (Choong Lek et al. 2018). Fruits, including lime, mango (Seghosime et al. 2017), and watermelon (Manyuchi and Chikomo 2016), showed good performance for turbidity reduction. However, this study was the first to investigate Tamarindus indica and date palm seeds.
Impurities have reportedly hindered the performance of natural coagulants. Aside from the active coagulating agents in the powdered seeds, plant tissues rich in organic constituents increase the organic load in the treated water. In addition, other components, including oil, and fats, interfere with their coagulation capabilities. Thus, extracting the active coagulation agents has been studied to improve turbidity reduction and coagulation efficiency. However, the effectiveness of these bio-coagulants in water and wastewater treatment depends on the preparation (extraction) techniques applied (Owodunni and Ismail 2021). The most common and cheapest extraction solvent is distilled water. However, it does not adequately break the protein–protein bonds in the coagulant sources as a salt solution (KCl, MgCl2, NaCl, CaCl2, NaOH), which exhibits excellent coagulation efficiency via the salting-out effect and keeps interfering with hydrophobic materials out of the extracts (Ang et al. 2020).
In studying the effect of extracting solvents for turbidity removal, Megersa et al. (2017) used deionized water and NaCl to extract coagulants from M. subordata tubers and M. stenopetala seeds. It was reported that NaCl extraction of M. subordata and M. stenopetala gave a higher turbidity removal of 96% and 90.3%, respectively, while extraction with deionized water was lower at 71% and 60%, respectively (Megersa et al. 2017). The result informed the use of NaCl as extracting solvent in this study.
Chemical coagulant has been effective in coagulation; however, the long-term toxicity effect leads to exploring natural sources for coagulants. This study investigates the suitability of green beans, pigeon pea, Tamarindus indica, and date palm seeds as coagulants. According to the literature, there has been very little research on using the selected seeds for coagulation in wastewater treatment. Therefore, this study included comparing turbidity removal performance of seed powder, distilled water-crude extract, and NaCl-crude extract of the coagulants.
Materials and methods
Sigma-Aldrich and R&M Chemicals supplied analytical grade kaolin, sodium hydroxide (NaOH), sodium chloride (NaCl), and hydrochloric acid (HCl). The seeds used in this study were obtained locally in Penang, Malaysia, and prepared according to their sources.
Preparation of seed powders and crude active extract coagulants
The prepared seeds were ground into powder, water active extract, and salt solution active extract. Figure 1 depicts the preparation process of the coagulants.
The tamarind and date palm seeds were extracted from the fruits and washed thoroughly with water before air-drying and oven-drying. Pigeon pea and green beans were cleaned, with the impurities removed, and the seeds were oven-dried at 60 °C for 5 h to remove moisture. The dried seeds were ground into a fine powder with an electric laboratory dry mill grinder and sieved using a sieve shaker with a 0.75-mm sieve. The dried seed powders were stored in air-tight containers to avoid mold formation and re-humidification before being used in further experiments (Sivakumar 2013).
The active water extract was prepared using dried seed powders from Tamarindus indica, date palm, pigeon pea, and green beans. Two grams of each seed powder was mixed in 100 mL of distilled water. The solutions were stirred for 45 min with a magnetic stirrer, after which the supernatant was separated for 30 min using an Eppendorf Centrifuge 5702 RH at 4000 rpm. The water supernatant was directly used as the coagulants for the coagulation experiments. Daily preparation of fresh solutions was the order to avoid the aging effects. Also, the replication of the extraction process was done for the salt solution extract of each seed using 1 M of NaCl (Ramamurthy et al. 2012; Camacho et al. 2017).
The analysis of coagulation-specific functional groups of the seeds is to evaluate their potential as coagulants in turbidity removal through the FTIR test using Shimadzu IR Affinity (Shimadzu Corporation, Japan) to comprehend their responsibility to coagulation. The potassium bromide (KBr) pellet method recorded FTIR spectra between the 4000 cm−1 and 400 cm−1 range. The KBr was mixed and ground with the seed powder samples at a 10:1 ratio. A mini-hand compressor was used to prepare the FTIR pellets.
Synthetic wastewater preparation
The study was laboratory-based, using kaolin synthetic wastewater to model the actual wastewater, with modifications according to the method reported by Daverey et al. (2019). Different masses of kaolin powder were mixed with 1 L of distilled water in a beaker. The mixture was stirred for 1 h at 200 rpm and kept at room temperature for 24 h to enable complete kaolin hydration. The resulting suspension was used as a stock solution for the coagulation experiment in preparing the turbid water samples by dilution.
Turbidity removal coagulation test
The coagulants’ turbidity removal was evaluated using a conventional jar test apparatus fitted with six beakers, the Model JLT6, VELP Scientifica. The kaolin synthetic water was diluted to fixed initial turbidity of 250 ± 10 NTU. Each of the six beakers was filled with 500 mL of turbid water. For the coagulation process involving the seed powder coagulant, the dosage of 0.8 g/L was added to the beakers, while 40 mL/L of the active crude extracts were added to the beakers for coagulation experiments involving the distilled water and NaCl solution extracts. The experiments were conducted by setting the apparatus at a rapidly mixing speed of 100 rpm for 2 min, followed by a slower mixing at 25 rpm for 20 min, and allowed to settle for 30 min before withdrawing the samples for turbidity test at 3 cm below the surface with the aid of a pipette (Ramamurthy et al. 2012).
The effect of coagulation dosage on the turbidity removal efficiency of the coagulants (NaCl solution extracts) was evaluated by varying the dosage from 20 mL/L to 70 mL/L at an interval of 10 mL (Yimer and Dame 2021). In studying the effect of pH on the turbidity removal efficiency of the coagulants, the pH of the water was adjusted with 1 M HCl and 1 M NaOH and was measured with a pH meter after stirring (Daud et al. 2014). The turbidity was evaluated using the EUCTH ECTN 100IR turbidity meter and expressed in nephelometric turbidity units (NTU). All experiments were run in triplicate, with the average values presented.
Results and discussion
Functional group analysis of the seed powders
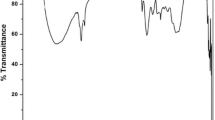
The FTIR spectra analysis of Tamarindus indica, date palm, green bean, and pigeon pea seed powders is presented in Fig. 2. The analysis evaluated the functional groups actively participating in coagulation. The spectra of the seed powders portrayed the presence of hydroxyl, protein, polysaccharides, lignin, and fatty acids, which is similar to the spectra of Moringa oleifera seeds. The samples had strong peaks between 3500–3200 cm−1, 3400–3250 cm−1, and 3300–2500 cm−1, corresponding to O–H stretching, N–H stretching (amines), and COO− carboxylic acid, respectively. It was reported that O–H and N–H and COO− functional groups are a criterion for selecting natural coagulants; the functional groups mainly attribute to the flocculation activity and indicate the coagulant’s effectiveness (Vunain et al. 2019). In using natural coagulants, the hydroxyl group is the most frequently referenced functional group/compound that contributes to the coagulation process, followed by the amine, carboxyl groups, and protein (Kurniawan et al. 2022).
Peaks indicating the presence of C-H stretching at wavelengths 3000–2850 cm−1 were observed in all the seed powders spectra; the alkane functional group was also noted to identify effective natural coagulants as it helps in the coagulation-flocculation process (Kakoi et al. 2017). In addition, the absorbance peaks between 1680–1640 cm−1 and 1640–1450 cm−1 of all sample spectra indicated the presence of C = C alkene ester and N–H bending amine groups. Other functional groups observed in the spectra include C-N stretching aromatic amines (1335–1250 cm−1) and C-O stretching (1320–1300 cm−1). The identified functional groups confirmed the existence of proteins, carbohydrates, and fatty acids, all of which are required to remove contaminants in wastewater.
Effect of seed type on turbidity removal efficiency
The experimental results in Fig. 3 show the turbidity removal efficiencies of the seed powder, distilled water, and NaCl-extracted coagulants. Coagulation with date seed (DS) powder yielded the highest turbidity removal of 52.39%, with tamarind seed powder yielding at least 41.78%. The low turbidity removal caused by the remaining powdered seed particles in the treated water resulted in a decreased coagulation efficiency. Some particles floated on the treated water, which may be attributable to the powder’s low density. The performance, however, is an indication of the turbidity removal potential of the agricultural seeds.
Based on the results obtained from using the seed powders directly as a coagulant and previous works of literature, further improvement of the coagulant in terms of extraction was made. The active coagulant agents in plants, such as proteins, are highly soluble and can be effectively extracted using distilled water (DW) and saline solution (SS) (1 M NaCl) (Ang and Mohammad 2020).
Based on Fig. 3, for extractants with distilled water, pigeon pea seed had the highest turbidity removal of 81.12% and is closely followed by green beans (65.11%), Tamarind indica (64.14%), and date palm (62.54%). Compared with the coagulation performance of the coagulants extracted with NaCl solution, pigeon pea also exhibited the highest turbidity removal of 94.62%, followed by Tamarind indica (76.08%) and green bean (71.47%). Date palm removed 62.98%, showing no significant increase in the performance with its distilled water counterpart. Based on the results displayed, pigeon pea, green bean, and Tamarind indica-based coagulants showed high turbidity removal efficiencies when both distilled water, and NaCl was used as an extraction solvent.
The results showed an improvement in turbidity removal using the extracted coagulants, and it corroborates that the presence of protein in the extracts has contributed to coagulation activity (Taiwo et al. 2020). The salt solution extractives exhibited a better turbidity removal performance when compared with that of the distilled water extracted coagulants, which could be attributed to a higher protein solubility from the seeds. This finding is consistent with earlier research and is linked to the “salting-in effect” (Das et al. 2021). The presence of salt has been reported severally to increase the solubility of proteins as it breaks down the protein–protein bond of the coagulants; however, in excess, precipitation may occur instead of solubility (Kristianto et al. 2019). The protein solubility increases and simultaneously increases the coagulant’s coagulation capacity against the contaminant’s interference (Das et al. 2021).
Effect of coagulant dosage on the turbidity removal efficiency
Coagulation dosage is a factor that significantly influences the coagulation efficiency of natural coagulants. The NaCl extracts were used to study the effect of dosage on turbidity removal due to their performance. Figure 4 shows the effect of NaCl solution-extract dosage on turbidity removal. PP yielded the highest turbidity removal efficiency at a dosage of 20 mL/L when compared with the other coagulants. The optimum dosages for TI, DS, and GB were found to be 40 mL/L (76.08%), 70 mL/L (66.04%), and 50 mL/L (88.34%), respectively. The results from the previous study done by Seghosime et al. (2017) agree with these results. While dosing with other dosages besides the optimum dosages, weaker and less large floc formations were observed.
At doses above the optimum, dispersed suspended particles were re-stabilized, resulting in higher turbidity. It is generally recognized that a minimal quantity of coagulants destabilizes particles and that optimal destabilization corresponds to neutralizing the charge of the particles. Charge reversal, such that the particles become positively charged, resulting in restabilization and higher turbidity levels, is also a result of overdosing. Natural coagulants’ ability to remove turbidity makes them suitable for water coagulation in developing countries’ rural and peri-urban areas.
Effect of coagulation pH on the turbidity removal efficiency
The effect of coagulation pH on the turbidity of the coagulants was monitored by varying the dosage from 1 to 13. This experiment used the optimum coagulation dosages of individual coagulants from Fig. 4. As shown in Fig. 5, at pH1, TI and DS had their highest turbidity removal of 96.28% and 96.71%, respectively.
It is observed from the experiment that larger flocs were formed in this acidic condition. Both GB and PP extracts removed 93.74% and 97.78% at pH3. The best coagulation performances of the coagulants were detected at extreme acidic or alkaline pH, which is similar to the findings of Ramamurthy et al. (2012). The optimum pH results from this study match the high turbidity removal of 96% by Jatropha curcas at pH1-3 (Abidin et al. 2011). The anionic components (carboxyl) in the coagulants are accountable for the turbidity removal at acidic pH, while the cationic protein components (amine) are accountable for the removal at alkaline pH (Ramamurthy et al. 2012; Benalia et al. 2021). Even at the neutral pH, all the coagulants had efficiencies from 55 to 80%, supporting their potential as coagulants for turbidity removal. Larger flocs, quick settling, and clearer water were observed at all points of optimum pH, reiterating the effect of pH in the coagulation-flocculation process.
The result of the turbidity removal from this study was compared with previous research to evaluate its efficiency. This work was majorly compared with the turbidity removal efficiencies obtained by Kumar and Balasundaram and Saritha et al., who studied turbidity removal using 0.8 g/L of Alum (Al2(SO4)3) and ferric sulfate (Fe2(SO4)3) coagulant at pH 7 and 4.5, respectively (Kumar and Balasundaram 2017; Saritha et al. 2017). The results of this comparison are given in Table 1 with a few other similar studies. In their studies, alum and ferric sulfate had a turbidity removal of 94.46% and 82.76%, respectively, which was on par with the natural coagulants’ results. Furthermore, a few studies on turbidity removal using various seed extractions and results from this study are highlighted in Table 1. The results demonstrated that this present investigation is comparable to, and even exceeds, the turbidity reduction effectiveness of the coagulants in work.
Turbid water modeling with kaolin concentration
A calibration curve was drawn by taking the mean values of three readings for each kaolin concentration and plotting them against their respective turbidity, as shown in Fig. 6. To get the linear model, we used the following:
where y is the turbidity in NTU;
and x is the kaolin concentration (g/L).
Conclusion
Natural coagulants can be sourced from seeds and fruit waste which is abundant and renewable, using distilled water or non-toxic inorganic salt, as demonstrated in this study. This technique can make coagulants readily available to impoverished locations, which reduces excess morbidity from waterborne diseases and improves the general quality of life. The effectiveness of the coagulants (Tamarind indica, green beans, date seed, and pigeon pea) was evaluated in treating kaolin synthetic wastewater. Based on the findings, it can be concluded that saline solution extracts of pigeon pea (PP), green bean (GB), and Tamarind indica (TI) have tremendous promise as a turbidity reduction and other wastewater treatment alternative to chemical coagulants. Turbidity removal at optimum coagulation dosages and pH showed high efficiency. At optimum coagulation dosage and pH, PP, DS, TI, and GB remove turbidity at 97.8% and 96.71%, 96.28%, and 93.74%, respectively. The PP gave the best performance; however, DS and TI are recommended for coagulants because they are waste products due to their nutritional value. The turbidity removal using the saline solution (NaCl extract) was higher than both distilled water extracts and seed powder coagulants. The NaCl salt solution is highly potent in extracting active coagulation (soluble proteins) for turbidity removal. Therefore, seeds saline solution extract has been chosen as the best concerning availability and effectiveness in turbidity removal. The result of this study can be adapted for fertilizer, the mining industry, and acidic wastewater treatment.
Data availability
Not applicable.
References
Abidin ZZ (2019) Ecofriendly approach to adsorption of Congo red from aqueous media using chaff powder from Jatropha curcas seed (isotherm and kinetic model). Preprints. https://doi.org/10.20944/preprints201903.0274.v1
Abidin ZZ, Ismail N, Yunus R et al (2011) A preliminary study on Jatropha curcas as coagulant in wastewater treatment. Environ Technol 32:971–977
Alazaiza MY, Albahnasawi A, Ali GA et al (2022) Application of natural coagulants for pharmaceutical removal from water and wastewater: a review. Water 14:140
Al-Hashimi O, Hashim K, Loffill E et al (2021) A comprehensive review for groundwater contamination and remediation: occurrence, migration and adsorption modelling. Molecules 26:5913. https://doi.org/10.3390/molecules26195913
Al-Saati N, Hussein T, Abbas M et al (2019) Statistical modelling of turbidity removal applied to non-toxic natural coagulants in water treatment: a case study. Desalin Water Treat 150:406–412
Ang T-H, Kiatkittipong K, Kiatkittipong W et al (2020) Insight on extraction and characterisation of biopolymers as the green coagulants for microalgae harvesting. Water 12:1388
Ang WL, Mohammad AW (2020) State of the art and sustainability of natural coagulants in water and wastewater treatment. J Clean Prod 262:121267
Antunes E, Garcia FA, Ferreira P et al (2008) Effect of water cationic content on flocculation, flocs resistance and reflocculation capacity of PCC induced by polyelectrolytes. Ind Eng Chem Res 47:6006–6013
Ben Rebah F, Mnif W, Siddeeg SM (2018) Microbial flocculants as an alternative to synthetic polymers for wastewater treatment: a review. Symmetry 10:556
Benalia A, Derbal K, Khalfaoui A et al (2021) Use of Aloe vera as an organic coagulant for improving drinking water quality. Water 13:2024. https://doi.org/10.3390/w13152024
Birima A, Hammad H, Desa M, Muda Z (2013) Extraction of natural coagulant from peanut seeds for treatment of turbid water. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing Ltd., pp 1–4
Camacho FP, Sousa VS, Bergamasco R, Teixeira MR (2017) The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem Eng J 313:226–237
Choong Lek BL, Paul Peter A, Qi Chong KH et al (2018) Treatment of palm oil mill effluent (POME) using chickpea (Cicer arietinum) as a natural coagulant and flocculant: Evaluation, process optimization and characterization of chickpea powder. J Environ Chem Eng 6:6243–6255. https://doi.org/10.1016/j.jece.2018.09.038
Choudhary M, Neogi S (2017) A natural coagulant protein from Moringa oleifera: isolation, characterization, and potential use for water treatment. Mater Res Express 4:105502
Das P, Mondal GC, Singh S, et al (2018) Effluent treatment technologies in the iron and steel industry-a state of the art review: Das et al. Water Environ Res 90:395–408
Das N, Ojha N, Mandal SK (2021) Wastewater treatment using plant-derived bioflocculants: green chemistry approach for safe environment. Water Sci Technol 83:1797–1812
Daud NS, Ghazi TIM, Ahamad IS (2014) Wheat germ as natural coagulant for treatment of palm oil mill effluent (POME). Int J Chem Environ Eng 5:111–115
Daverey A, Tiwari N, Dutta K (2019) Utilization of extracts of Musa paradisica (banana) peels and Dolichos lablab (Indian bean) seeds as low-cost natural coagulants for turbidity removal from water. Environ Sci Pollut Res 26:34177–34183
Igwegbe CA, Onukwuli OD (2019) Removal of total dissolved solids (TDS) from aquaculture wastewater by coagulation-flocculation process using Sesamum indicum extract: effect of operating parameters and coagulation-flocculation kinetics. Pharm Chem J 6:32–45
Jagaba A, Kutty S, Hayder G et al (2020) Sustainable use of natural and chemical coagulants for contaminants removal from palm oil mill effluent: a comparative analysis. Ain Shams Eng J 11:951–960. https://doi.org/10.1016/j.asej.2020.01.018
Kakoi B, Kaluli JW, Ndiba P, Thiong’o G (2017) Optimization of Maerua Decumbent bio-coagulant in paint industry wastewater treatment with response surface methodology. J Clean Prod 164:1124–1134
Kazydub N, Marakayeva T, Kuzmina S et al (2017) Chemical composition of seeds and green beans of common bean varieties, breeded in Omsk State Agrarian University under conditions of southern forest-steppe zone of Western Siberia. Agron Res 15:1918–1927
Kristianto H, Rahman H, Prasetyo S, Sugih AK (2019) Removal of Congo red aqueous solution using Leucaena leucocephala seed’s extract as natural coagulant. Appl Water Sci 9:88
Kumar N, Balasundaram DN (2017) Efficiency of PAC in water treatment plant & disposal of its sludge. 12:10
Kurniawan SB, Imron MF, Chik CENCE et al (2022) What compound inside biocoagulants/bioflocculants is contributing the most to the coagulation and flocculation processes? Sci Total Environ 806:150902
Lek BLC, Peter AP, Chong KHQ et al (2018) Treatment of palm oil mill effluent (POME) using chickpea (Cicer arietinum) as a natural coagulant and flocculant: Evaluation, process optimization and characterization of chickpea powder. J Environ Chem Eng 6(5):6243–6255
Manyuchi MM, Chikomo T (2016) Treatment of water using watermelon (Citrullus lanatus) seeds as organic coagulant and microbial filter. In: Emerging Trends in Chemical Sciences. Flic en Flac, Mauritius
Megersa M, Beyene A, Ambelu A, Triest L (2017) Extraction of natural coagulants from Maerua subcordata and Moringa stenopetala for use in turbid water treatment. Desalination Water Treat 59:127–134
Miranda Carreño R, Blanco Suárez Á, Negro Álvarez CM (2011) Separation of contaminants from deinking process water by dissolved air flotation: effect of flocculant charge density. Ene 13:31
Miranda R, Negro C, Blanco A (2009) Internal treatment of process waters in paper production by dissolved air flotation with newly developed chemicals. 1. Laboratory Tests Ind Eng Chem Res 48:2199–2205
Miranda R, Nicu R, Latour I et al (2013) Efficiency of chitosans for the treatment of papermaking process water by dissolved air flotation. Chem Eng J 231:304–313
Naceradska J, Pivokonska L, Pivokonsky M (2019) On the importance of pH value in coagulation. J Water Supply Res Technol-Aqua 68:222–230. https://doi.org/10.2166/aqua.2019.155
Owodunni AA, Ismail S (2021) Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment—a review. J Water Process Eng 42:102096. https://doi.org/10.1016/j.jwpe.2021.102096
Ramamurthy C, Maheswari MU, Selvaganabathy N et al (2012) Evaluation of eco-friendly coagulant from Trigonella foenum-graecum seed. Adv Biol Chem 2:58
Saritha V, Srinivas N, Srikanth Vuppala NV (2017) Analysis and optimization of coagulation and flocculation process. Appl Water Sci 7:451–460. https://doi.org/10.1007/s13201-014-0262-y
Sarkar B, Chakrabarti P, Vijaykumar A, Kale V (2006) Wastewater treatment in dairy industries- possibility of reuse. Desalination 195:141–152
Seghosime A, Awudza JAM, Buamah R et al (2017) Effect of locally available fruit waste on treatment of water turbidity. Civ Environ Res 9:7–15
Djij S (2013) Adsorption study on municipal solid waste leachate using Moringa oleifera seed. Int J Environ Sci Technol 10:113–124
Sui Kim IT, Sethu V, Arumugasamy SK, Selvarajoo A (2020) Fenugreek seeds and okra for the treatment of palm oil mill effluent (POME) – characterization studies and modeling with backpropagation feedforward neural network (BFNN). J Water Process Eng 37:101500. https://doi.org/10.1016/j.jwpe.2020.101500
Taiwo AS, Adenike K, Aderonke O (2020) Efficacy of a natural coagulant protein from Moringa oleifera (Lam) seeds in treatment of Opa reservoir water, Ile-Ife, Nigeria. Heliyon 6:e03335
Vunain E, Masoamphambe EF, Mpeketula PMG et al (2019) Evaluation of coagulating efficiency and water borne pathogens reduction capacity of Moringa oleifera seed powder for treatment of domestic wastewater from Zomba, Malawi. J Environ Chem Eng 7:103118. https://doi.org/10.1016/j.jece.2019.103118
Yimer A, Dame B (2021) Papaya seed extract as coagulant for potable water treatment in the case of Tulte River for the community of Yekuset district, Ethiopia. Environ Chall 4:100198. https://doi.org/10.1016/j.envc.2021.100198
Acknowledgements
Acknowledgement is given to the Ministry of Higher Education Malaysia for Long Term Research Grant Scheme with Project Code (LRGS/1/2018/USM/01/1/1)(LRGS/2018/USM-UKM/EWS/01) and the Universiti Sains Malaysia for the GRA-Assist Scheme provided.
Funding
This work was supported by the Ministry of Higher Education Malaysia for Long Term Research Grant Scheme with Project Code (LRGS/1/2018/USM/01/1/1)(LRGS/2018/USM-UKM/EWS/01) received by the corresponding author.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Amina A. Owodunni, Suzylawati Ismail, and Niyi G. Olaiya. The first draft of the manuscript was written by Amina A. Owodunni, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable. This manuscript does not involve researching about humans and animals.
Consent to participate
All the authors consented to participate in the drafting of this manuscript.
Consent for publication
All the authors consented to publishing this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Owodunni, A.A., Ismail, S. & Olaiya, N.G. Parametric study of novel plant-based seed coagulant in modeled wastewater turbidity removal. Environ Sci Pollut Res 30, 124677–124685 (2023). https://doi.org/10.1007/s11356-022-21353-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21353-0