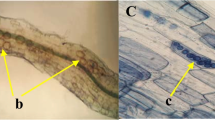
Abstract
Salinity stress is a combination of ionic, osmotic, and oxidative stressors that have a negative impact on crop growth and production. In the present study, experiments were conducted to investigate the role of multi-traits Serratia fonticola (S1T1) on Cucumis sativus L. growing under salinity stress (200 mM). The control plants had stunted growth, while S. fonticola (S1T1) root zone treated plants revealed significantly higher fresh (26.71%) and dry (24.8%) biomass, and improved level of chlorophyll content (25.24%) followed by foliar application of S. fonticola (S1T1) under salt stress. Similarly, increased water potential (15–20%), decreased (14–20%) endogenous abscisic acid (ABA) and lower electrolytic leakage (21–35%) were additional proof of the beneficial impacts of root zone inoculated C. sativus L. under salt stress conditions. Antioxidant analysis revealed a decrease in malondialdehyde (MDA) content (13–31%), H2O2 content (15–36%) and superoxide anion (SOA) (11–32%) while an increase in antioxidant enzymes such as catalase (CAT) (13.2–35.5%) and superoxide dismutase (SOD) (9.61–29.7%). The root zone and foliar application of S. fonticola (S1T1) on cucumber plants improved salt-stress tolerance by up-regulating the transcript accumulation of ion transporter genes HKT1 (2-3-folds), NHX (18.2-folds) and SOS1 (8.2-folds). Conclusively, the symbiotic association of S. fonticola (S1T1) can alleviate the antagonistic effects of salinity stress, improve cucumber plant growth and could be utilized as an eco-friendly biofertilizer or microbial plant biostimulant (MBPs) under salt stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Crop plants are continuously exposed to a variety of stressful events induced by both biotic and abiotic factors. Abiotic stressors such as salinity, drought, waterlogging, temperature extremes, heavy metals, and low soil fertility etc. are generally linked with the climatic and physiographic components of the environment (Bulgari et al. 2019; Kang et al. 2014; Moon and Ali 2022a; Franzoni et al. 2022). Salinity is one of the key issues that has a negative impact on plant growth, productivity, food quality, and the income of rural people that rely on agriculture all over the world (Jiménez-Mejía et al. 2022). According to recent estimates, nearly 20% of agricultural land (1500 million hectares) is damaged by salinity (Giordano et al. 2021) and by 2050, about 50% of arable lands will be affected by some degree of soil salinity due to consistent rise in salinity level all over the world (Jiménez-Mejía et al. 2022; Wang et al. 2003). The accumulation of sodium (Na+) and chloride (Cl−) ions paves the way for saline condition of arable land where different natural and human factors contribute to the deposition of salt and adversely affect the growth and development of various crop plants (Zhao et al. 2020) by causing membrane disorganization, reduction in photosynthesis, metabolic toxicity, formation of reactive oxygen species (ROS) and leads to low level of nutrient acquisition. Similarly, a high concentration of salt (primarily Na+) in plant tissues induce ionic stress conditions which hamper plant absorption of water and nutrients from the rhizospheric soil (Ismail et al. 2014).
Salinity stress is the result of a combination of ionic, osmotic, and oxidative stressors (Ramezani et al. 2013). Generally, plants accumulate Na+ and Cl− in their shoots and lead towards ionic imbalance or ion toxicity and osmotic stress by reducing leaves and tissues water potential under salt stress conditions (Giordano et al. 2021). Whereas, the ability of plants to withstand salt stress varies between and within plant species (Shah et al. 2021). Major staple crops such as rice, wheat and corn are glycophytes and they cannot tolerate salinity stress in their tissues as compared to halophytes (Zhao et al. 2020). Plants respond to salt stress by experiencing a variety of physiological and metabolic changes, and their defensive strategies include a cascade of signals ranging from primary to secondary responses. Primary responses include changes in ionic or osmotic levels and stomatal closer while secondary responses involve the production and modulation of plant hormones and secondary metabolites (Kang et al. 2014; Khan et al. 2019a). Similarly, salt stress has numerous biochemical and molecular effects in plants through the generation of reactive oxygen species (ROS), and higher levels of ROS are harmful to the plants’ normal growth and development. In connection to this, various antioxidant enzymes have been reported for their ability to detoxify high ROS levels in plants and mitigate the stressful conditions (Khan et al. 2019a; Ismail et al. 2014). Similarly, molecular biology techniques are of great importance to trace the genes involved in plant responses to salinity stress. A number of stress-related genes involved in signal transduction, ion transporters, transcription control, and metabolic pathways have been discovered to confer salt stress tolerance in plants (Raza et al. 2019; Ali et al. 2018b; Roy et al. 2021).
In order to address salinity-related challenges and increase crop productivity in the near future, eco-friendly approaches, such as the application of plant growth-promoting rhizobacteria (PGPR), are critical. PGPR form symbiotic association with plants and alleviate the stressful conditions by a number of direct and indirect mechanisms (Moon and Ali 2022a, b). Several PGPR strains have been reported from different environmental conditions for their pragmatic role in abiotic stress mitigation and crop growth improvement. Some of PGPRs belonging to Acinetobacter bereziniae, Alcaligenes faecalis, Arthrobacter nitroguajacolicus, Bacillus subtilis, Burkholderia caryophylli, Enterobacter ludwigii, Flavobacterium pokkalii, Pseudomonas putida, Pseudomonas veronii, Sphingobacterium multivorum and Serratia marcescens are now being used under different abiotic stress conditions with the aim to augment crop growth and yield (Kang et al. 2014; Vives-Peris et al. 2018; Sapre et al. 2022; Safdarian et al. 2019; Singh and Jha 2016; Khan et al. 2021c; Menon et al. 2020). The application of PGPR strains enhance the growth and development of crops under salt stress and some of the recent articles revealed their prolific effects on different agricultural crops such as Triticum aestivum L. (Singh and Jha 2016), Oryza sativa L. (Dabral et al. 2020), Zea mays (Ali et al. 2022a), Solanum lycopersicum (Kapadia et al. 2021), Pisum sativum (Sapre et al. 2022). The application and role of PGPR with one or two plant growth-promoting traits have been reported in different crops, while the role of multi-trait PGPRs have been poorly understood. More recently, we reported S. fonticola (S1T1) as a multi-trait PGPR with 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, indole-3-acetic acid (IAA), siderophore, and phosphate solubilization activities (Moon and Ali 2022b). Moreover, PGPR has now been added to the list of microbial plant biostimulants (MPBs) (Colla and Rouphael 2015; Du Jardin 2015), and routinely applied in the soil, however, the foliar application of MPBs creates an unknown interaction with plant leaf surface (Efthimiadou et al. 2020; Preininger et al. 2018), which needs to be further investigated.
Cucumber (Cucumis sativus L.) is an important horticultural crop belonging to the family Cucurbitaceae. It is an important part of the daily meal in the Republic of Korea, where it is served as a salad (Oi-Muchim) and in traditional Kimchi recipes (Oi-Kimchi and Oi-Sobagi). To improve the quantity and quality of agricultural products, various types of chemical fertilizers are utilized, which not only contaminate and deplete soil fertility but also have an impact on ground water quality (Kubi et al. 2021). However, the use of MPBs, not only improves plant growth and development, but also helps to maintain environmental health and soil fertility (Hamid et al. 2021; Rouphael and Colla 2018). The purpose of the current study was to understand the effect of newly isolated multi-trait PGPR strain Serratia fonticola (S1T1) on cucumber growth under salt stress and to elucidate its effect both in root zone and foliar application. Furthermore, to analyze the efficiency of S. fonticola (S1T1) on the chlorophyll content, relative water content (RWC), electrolyte leakage, enzymatic and non-enzymatic antioxidant, and the expression pattern of salt related genes of cucumber plants under normal and salt stress conditions.
2 Materials and methods
2.1 Inoculum preparation
We have already reported the isolation, evaluation and identification of multi-trait S. fonticola (S1T1) from the coastal sand dune plant species of Pohang beach (Moon and Ali 2022b). The partial sequence of 16 S rRNA gene of the strain was submitted to https://www.ncbi.nlm.nih.gov/ with an accession no. MZ612851 and maintained at 4 °C in equal volume of nutrient broth and 40% glycerol for long term use. Presently, the rhizobacterial strain was inoculated in Luria-Bertani (LB) broth medium and incubated at 28 °C for 24 h.
2.2 Seeds surface sterilization and NaCl stress tolerance
Cucumber seeds (Cucumis sativus L. Asia Seed Korea) were surface-sterilized by soaking in 3% sodium hypochlorite for 90 s, then ethanol (70%) was applied for 90 s and finally washed three times with sterilized distilled water (Tan et al. 2011). NaCl stress tolerance assay was performed by using cucumber seedlings grown up to three weeks in autoclaved soil and application of NaCl concentration (0, 100, 200, 300, and 400 mM) for one week under controlled environment.
2.3 Plant growth conditions and PGPR inoculation
Cucumber plants were grown in pots (12 × 10 cm) containing 210 g of autoclaved soil in the green house (28 ± 2 °C) for three weeks (15 plants per treatment) and irrigated with distilled water or salt water. Similarly, NaCl tolerance growth of S. fonticola (S1T1) was evaluated under different concentrations of NaCl (0, 100, 200, 300, 400 and 500 mM) in LB medium and growth measured at OD600. For the evaluation of the PGPR effect on cucumber plants in the soil and foliar regions, a uniform grown two-week-old seedlings in the trays were selected for further experiment. Before transplantation, autoclaved soil was treated two times with 40 mL of the bacterial isolate (108 CFU/mL) only for root zone treatments. Uniform size seedlings were uprooted and transplanted in bigger pots (12 × 10 cm) containing 210 g of soil, root zone bacterization was performed three times (during transplantation and after transplantation for two weeks). Subsequently, cucumber plants were grown in the green house (28 ± 2 °C) for a total of five weeks and treated with 40 mL of bacterial stain both in root zone and foliar application. The foliar application of bacterial isolate was repeated three times a week and a hand sprayer was used for foliar application. Root colonization is a prerequisite for PGPR activity, therefore, to evaluate the colonization of bacterial isolates in the root zone, soil samples were collected from all treatments and subjected for dilution-plate counting.
2.4 Pot experiment
Our experimental-design consists of six different sets of the plants to evaluate the role of S. fonticola (S1T1) and its interaction in the rhizosphere and phyllosphere regions under salt stress. For this analysis, a pot experiment was conducted (in three replicates) in a complete randomized design (CRD). The experiment consist of six treatments as follow: (i) Control (normal plants without PGPR and NaCl stress); (ii) S1T1 Root Zone Application; (iii) S1T1 Foliar Application; (iv) NaCl Stress; (v) S1T1 Root Zone Application + NaCl Stress; and (vi) S1T1 Foliar Application + NaCl Stress. The fresh culture of S. fonticola (S1T1) was prepared and applied on cucumber plants in a controlled environment in root zone and foliar application to evaluate its interaction with host plant and assess its role in salt stress mitigation.
2.5 Salt stress induction
For the application of salt stress, five-week-old plants were subjected to salt stress and the stress was applied for seven continuous days. For the induction of high salt stress (200 mM) NaCl solution was used, while three replicates were prepared for each treatment. When plants were harvested (seven-week-old plants), growth indicators like shoot and root length and fresh and dry weights were assessed, and the chlorophyll content of fully expanded leaves was evaluated using a SPAD meter (SPAD-502 Minolta, Japan). Similarly, the harvested plants were immediately frozen in liquid nitrogen and transferred to a -80 °C freeze dryer (five plants per treatment) until further analysis such as estimation of antioxidant activities and gene expression analysis. Moreover, after drying the plants at 70 °C for 48 h in an oven, the dry weights of all treatments were calculated.
2.6 Leaf relative water content (LRWC) determination
The detailed method of (Kang et al. 2014) was adopted for determining the percentage of leaf water potential with slight modifications such as imbibition period (24 h). In all treatments, the same type of leaves were removed from the plants (seven-week-old plants) and subjected for weight (fresh weight, FW). After FW, the leaves were placed in distilled water inside a closed container and floated for 24 h to determine the turgid fresh weight (turgid fresh weight, TFW). Blotting paper was used to remove water on the surface of the leaves and then weighed. In order to obtain dry weight (DW), the leaves were placed in a pre-heated oven at 70 °C for 24 h. The values of FW, TW, and DW were used to calculate leaf RWC by using the following formula:
2.7 Measuring of electrolytic leakage from cucumber leaves
The electrolyte leakage from cucumber leaves (seven-week-old plants) was measured according to the detailed method of (Rahim et al. 2022) with slight modifications. To assess electrolyte leakage, fresh leaf samples (200 mg) were cut into 5 mm lengths and put in test tubes containing distilled deionized water (10 mL). In a water bath set at 32 °C, all tubes were sealed. The medium’s initial electrical conductivity (EC1) was measured after two hours using an electrical conductivity meter (HURIBA Twin Cond B-173, Japan). After the samples had been autoclaved at 121 °C for 20 min and cooled to 25 °C, their final electrical conductivity (EC2) was assessed. The following formula was used to determine the amount of electrolyte leakage:
2.8 Estimation of antioxidant activities
For antioxidant analysis, a 400 mg of freeze-dried leaves tissues of seven-week-old plants were ground using chilled mortar and pestle and homogenized in the phosphate buffer (50 mM, pH 7.5) containing polyvinylpyrrolidone (PVP) 1.0% (w/v), EDTA (0.1 mM), and Triton X-100 0.5% (w/v) (Khan et al. 2021b). Superoxide anions (SOAs) were measured according to the detailed procedure of (Gajewska and Skłodowska 2007) at 580 nm wavelength. Hydrogen peroxide (H2O2) levels of different treatments were determined according to previously described methods (Park et al. 2021). Similarly, the detailed method of (Khan et al. 2020) was employed for estimation of malondialdehyde (MDA) content and readings were measured at 532 nm wavelength. The method described in the study by Radhakrishnan and Lee (2013) was used to determine CAT activity, and the resulting absorbance was measured at a wavelength of 240 nm. Superoxide dismutase (SOD) activities were measured by using a spectrophotometer at 560 nm by following the detail method of Marklund and Marklund (1974).
2.9 Endogenous abscisic acid (ABA) analysis
The endogenous ABA content was extracted and quantified using the detail method of (Kang et al. 2014; Qi et al. 1998). Briefly, a freeze-dried sample (0.5 g) of cucumber plants under NaCl stress and bacteria inoculated samples were extracted using an isopropanol and acetic acid solution (95%:5%). Standard ABA (20 ng/mL) and filtrate were added to the mixture. As a result, all extracts were dried and methylated using diazomethane in preparation for GC/MS-SIM analysis (6890 N network GC system, and 5973 network mass selective detector from Agilent Technologies, Palo Alto, CA, USA). The quantification of the responses to the ions of m/e 162 and 190 for Me-ABA and of m/e 166 and 194 for Me-[2H6]-ABA was performed using lab-based data system software (Thermo Quest, UK).
2.10 HKT1, NHX, and SOS1 gene expression analysis using qRT-PCR
Quantitative real-time PCR (qRT-PCR) was used to estimate gene expression following the detail approach of (Rahim et al. 2022; Khan et al. 2021a). Briefly, TRIzol reagent (Invitrogen, USA) was used to extract total RNA from the cucmber leaves (crushed samples 1.5 g) in accordance with the manufacturer’s instructions. The DiaStarTM RT kit (SolGent, Korea) was used to produce complementary DNA (cDNA) in accordance with the manufacturer’s recommendations. For the transcript accumulation analysis, cDNA was utilized as a template in the EcoTM real-time PCR machine (Illumina, USA) together with 100 ng of template DNA, 10 nM of each primer, and a final volume of 20 µL of the 2X Real-time PCR Master Mix (containing SYBR Green I BioFACTTM, Korea). No template control was used as a negative control, which contains only distilled water instead of template DNA. A two-step PCR reaction was established for 40 cycles under the following conditions: polymerase activation at 95 °C for 15 min, denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 30 s. The melting curves were assessed at 60–95 °C for the verification of amplicon specificity for each primer pair, and actin was utilized for the normalized level of relative expression of each gene in each reaction, and the level of expression in control plants in comparison to different treatments of cucumber plants was calculated. The primers used in this study are listed in Supplementary Table 1.
2.11 Statistical analysis
Using SAS version 9.2 software, the Duncan Multiple Range Test (DMRT) was performed on all data, which was collected in triplicates. Additionally, using the Graph-Pad Prism software, the results were presented graphically (Version 6.01, USA).
3 Results
3.1 Effects of Serratia fonticola (S1T1) on plant-growth dynamics under salinity stress
According to our initial screening results of PGPR (Moon and Ali 2022b), S. fonticola (S1T1) demonstrated multi-trait plant growth-promoting activities and can improve plant growth and development by a variety of direct and indirect mechanisms, including production of phytohormones, solubilization of phosphate, production of siderophores and ACC deaminase. Currently, under the influence of NaCl stress, the effect of S. fonticola (S1T1) on root zone and foliar application on the cucumber plant’s growth and related characteristics were evaluated. Our findings demonstrated that, in comparison to foliar treatment, the rhizobacteria applied in the root zone considerably alleviate the negative effects of salinity stress. In comparison to untreated control plants, the results demonstrated that the treatment of S. fonticola (S1T1) considerably improved the root and shoot of cucumber plants. During NaCl-induced stress, the rhizobacterium root zone treatment was much more effective in plant growth promotion than foliar application and control plants (Fig. 1).
The colonization and positive role of S. fonticola (S1T1) on cucumber plants were evaluated during NaCl stress, where S. fonticola (S1T1) revealed the stress alleviation effects on cucumber plants under salt stress (200 mM). The inoculated bacterial isolate efficiently colonized the root zone under both unstressed (1.25 × 107CFU/g to 1.56 × 108CFU/g) and NaCl stressed (5.2 × 106CFU/g to 6.1 × 107CFU/g) conditions and revealed stable colonization. The application and colonization of S. fonticola (S1T1) in the root zone revealed significant results where the shoot fresh biomass and shoot length were 26.71% and 20.7% higher respectively, compared to control plants under normal conditions. Conversely, the shoot fresh biomass was 8.4% higher under foliar application of S. fonticola (S1T1) as compared to control plants under normal conditions, whereas the shoot length was 2.12% higher compared to control plants (Table 1). In comparison to NaCl-stressed plants, the rhizobacterial strain considerably influenced cucumber growth, biomass, and chlorophyll content. It also dramatically mitigated the negative effects of NaCl stress. According to our findings, cucumber plants stressed by 200 mM NaCl had significantly shorter shoots (38.6%) and less fresh biomass (36.3%) than control plants. A little increase was noticed in the chlorophyll content under foliar application of the PGPR compared to untreated control plants. However, a significant increase (10.31%) was calculated under root zone application. Upon salinity stress, the chlorophyll content was 25.24% and 13.35% higher in S. fonticola (S1T1) under zone and foliar application, respectively. The root zone application of S. fonticola (S1T1) significantly enhanced shoot fresh and dry biomass and improved root and shoot length, and chlorophyll content compared to untreated control plants (Table 1; Fig. 1). The application of S. fonticola (S1T1) under root zone significantly ameliorated the adverse effects of NaCl stress compare to untreated control plants and the effects of S. fonticola (S1T1) was more pronounced under root zone application than the foliar application.
3.2 Leaf water potential under S. fonticola (S1T1) and NaCl stress
The water potential of plants is greatly impacted by salt stress. In comparison to plants treated with S. fonticola (S1T1) in the root zone, the non-inoculated control cucumber plants have significantly lower leaf water status. Whereas, the foliar application of S. fonticola (S1T1) revealed no significant difference from non-inoculated control plants (Fig. 2). Leaf relative water content (LRWC) of control plants were 12.6% lower than S. fonticola (S1T1) root zone inoculated cucumber plants while 1.26% lower than the plant under foliar application. On the other hand, this effect was more prominent in non-inoculated control plants under NaCl stress. Upon salt stress, the non-inoculated control plants were significantly deficient in their water potential compared to S. fonticola (S1T1) inoculated plants. The application of S. fonticola (S1T1) on cucumber plants under salt stress resulted in an increased leaf water potential of 24.5% and 20.4% under root zone and foliar application, respectively.
Effect of S. fonticola (S1T1) on the leaf relative water content (LRWC) of cucumber plants under normal and salt stress conditions. With standard error bars, the data represent means of three replicates. According to the results of the DMRT analysis, mean bars labeled with different letters are significantly different
3.3 Effect of S. fonticola (S1T1) on electrolyte leakage and antioxidant activities in cucumber
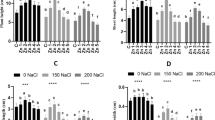
To evaluate the effects of S. fonticola (S1T1) and salt stress on the membrane integrity of cucumber plants, electrolyte leakage and MDA levels were investigated. Our results showed that NaCl stress significantly enhanced electrolyte leakage and MDA contents (Fig. 3). The cucumber plants under NaCl stress revealed higher levels (3-4-fold) of electrolytes leakage from the leaves tissues as compared to control plants. S. fonticola (S1T1) root zone application under NaCl stress decreased the level of electrolyte leakage up to 35% while foliar application of S. fonticola (S1T1) revealed a decrease of 21% in the electrolytes leakage from the leaves tissues of cucumber plants under salt stress. Conclusively, the plants under NaCl stress suffered from more membrane injury compared to plants treated with S. fonticola (S1T1) in the root zone followed by foliar application (Fig. 3 A). Malondialdehyde (MDA) contents were evaluated to assess the extent of lipid peroxidation (LPO). Higher levels of MDA content (136.3%) were observed in cucumber plants treated with salt stress (200 mM) compared to normal control plants (Fig. 3B). However, the application of S. fonticola (S1T1) in root zone and foliar spray on cucumber plants decreased the levels of MDA by 31% and 13.4%, respectively.
Effect of S. fonticola (S1T1) on (A) Leaf electrolyte leakage, (B) MDA contents (lipid peroxidation) (C) H2O2 contents and (D) Superoxide anion in cucumber plants under normal and NaCl (200 mM) stress conditions. Error bars show standard error, while data points represent the mean of the three replicates. According to DMRT analysis, bars with distinct letter combinations are significantly different from each other
Similarly, changes to antioxidant content were evaluated in cucumber plants subjected to salt stress with and without the application of S. fonticola (S1T1). NaCl stress augments the production of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide anion (O− 2) that leads to oxidative stress conditions. The level of hydrogen peroxide (H2O2) content varied in response to salt treatments (Fig. 3 C). The production of H2O2 was significantly inhibited in root zone (36.4%) and foliar (15.2%) applications of S. fonticola (S1T1) in cucumber plants compared to salt stressed plants. Similar trends were observed in superoxide anion (SOA) contents of cucumber plants under salt stress condition (Fig. 3D). However, the production of SOA was significantly decreased in cucumber plants treated with S. fonticola (S1T1) in the root zone (32%) and foliar spray (11%) compared to salt stressed plants.
3.4 Effect of S. fonticola (S1T1) on free radical scavengers
To alleviate the toxic effect of NaCl stress, plants activate antioxidants to control the biosynthesis of reactive oxygen species (ROS). Hence, the activity of enzymes SOD and CAT were also determined in cucumber plants under salt stress conditions with and without the application of S. fonticola (S1T1) (Fig. 4). Our results revealed that the presence of S. fonticola (S1T1) in root zone and foliar application in combination with salt stress augmented the activity of SOD and CAT enzymes as compared to the plants under salt stress that were not inoculated with S. fonticola (S1T1). Under normal conditions, S. fonticola (S1T1) increased CAT biosynthesis by 11.26% and 6.3% in root zone and foliar application, respectively. However, under salt stress the level of CAT increased 35.56% in plants treated with S. fonticola (S1T1) in root zone while the amount of CAT increased 13.72% in plants after foliar application of S. fonticola (S1T1) (Fig. 4 A). Similar trend was observed for SOD in cucumber plants under NaCl stress (Fig. 4B). Under normal conditions, the rhizobacterium S. fonticola (S1T1) enhanced SOD biosynthesis by 9.69% and 7.71% in root zone and foliar application, respectively. On the other hand, SOD activity was increased up to 29.79% in plants treated with S. fonticola (S1T1) in root zone while the level was increased 9.61% in plants with foliar application of S. fonticola (S1T1).
Effect of S. fonticola (S1T1) on (A) Catalase activity and (B) Superoxide dismutase activity in cucumber plants under salt stress (200 mM). With standard error bars, the data represent means of three replicates. According to the results of the DMRT analysis, mean bars labeled with different letters are significantly different
3.5 Regulation of endogenous ABA by S. fonticola (S1T1) under NaCl stress
ABA regulation was assessed during NaCl stress, root zone and foliar applications of S. fonticola (S1T1) on cucumber plants. Our results revealed a significant increase in ABA contents (1.23-fold) in cucumber plants under NaCl stress as compared to normal control plants (Fig. 5). On the other hand, plants treated with S. fonticola (S1T1) and exposed to NaCl stress (200 mM) decrease ABA content up to 20.3% and 14.6% in the root zone and foliar application, respectively. Conclusively, under NaCl stress conditions, ABA contents increased exponentially, whereas S. fonticola (S1T1)-treated plants both in root zone and foliar application did not experience a sharp increase in ABA levels.
3.6 Gene expression under NaCl stress and effect of S. fonticola (S1T1)
Sodium transporters serve a critical function in plant defense under salinity stress. These transporters may be antiporters or symporters, the former expel sodium ion from the root cell and redistribute in different tissues to minimize the adverse effect of salt stress and restore water homeostasis while the later known as HKT1-type transporters (High-affinity K+ transporters-1) is a key regulator of Na+ homeostasis which regulate both uptake and circulation of Na+ and protect shoot from the adverse effects of sodium. Similarly, Na+/H+ (NHX) antiporters have a pivotal role in cellular homeostasis. To study the possible mechanism of salt tolerance in cucumber plants, three candidate genes (HKT1, SOS1, NHX) expression were investigated.
Our results revealed that HKT1 was highly expressed (1.5-2-folds) in cucumber plants exposed to salt stress conditions. Whereas, the expression of HKT1 gene was up-regulated in cucumber plants inoculated with S. fonticola (S1T1) under root zone followed by foliar application during salt stress conditions (Fig. 6 A). The application of S. fonticola (S1T1) in the root zone improved cucumber plants adaptability against salt stress and resulted in a significant increase (2-3-folds) in HKT1 in cucumber plants exposed to salt stress. Whereas, the foliar application of S. fonticola (S1T1) revealed 0.5-fold increase in the expression of HKT1 gene under salt stress condition. Similar to this, cucumber plants exposed to salt stress conditions showed a significant increase (14.15-folds) in the expression of NHX gene (Fig. 6B). However, S. fonticola (S1T1) root zone inoculation dramatically enhanced cucumber resistance to salt stress and augmented the expression of NHX (22-folds) in cucumber plants exposed to salt stress followed by foliar application (18.2-folds). Likewise, a significant increase (8.2-folds) in the transcript accumulation of SOS1 gene was observed in cucumber plants inoculated with S. fonticola (S1T1) in the root zone under salt stress condition (Fig. 6 C). Conversely, the foliar application of S. fonticola (S1T1) on cucumber plants enhanced salt resistance by inducing the transcript accumulation of SOS1 (6.2-folds), as shown in Fig. 6 C.
Gene expression in response to S. fonticola (S1T1) under normal and salt stress (200mM) conditions. Samples were collected from the leaf portion of all treatments. Relative expression of (A)HKT1, (B)NHX and (C)SOS1 genes under normal and salt stress conditions calculated using actin gene expression. Different letters indicate significant differences between the mean values of the three replicates ± standard deviation
4 Discussion
Salt stress has a significant negative impact on plant growth and development, which results in productivity losses of crops globally (Jan et al. 2022). Prolonged salinity in the vicinity of plant roots has detrimental effects on morphological, biochemical, and molecular levels of the plants that results in nutritional imbalance, chlorophyll loss, ROS production, changes in phytohormone biosynthesis, inactivation of antioxidative enzymes, and a decrease in photosynthetic rate (Jan et al. 2022; Roy et al. 2021; Haroon et al. 2022; Ali et al. 2018a). Different strategies have been developed to reduce the negative effects of salt stress on plants, however, some of them are expensive and not environmentally friendly. The association of PGPR with plants have been shown in a number of recent studies to confer a high level of salinity tolerance and to promote plant growth and development in saline conditions (Khan et al. 2019b; Ali et al. 2022b). This work has validated the effectiveness of the rhizobacterium “S. fonticola (S1T1)” isolated from the coastal sand dune plant species of Pohang beach in inducing salinity tolerance by promoting various plant growth-promoting characteristics of cucumber plants.
In our research, we found that the multi-trait S. fonticola (S1T1) colonized and effectively supported in the growth and development of cucumber plants and reduced the negative impacts of salt stress (200 mM). Kang et al. (2014) also reported similar plant growth promoting and salt stress tolerance effects and revealed that the application of Acinetobacter calcoaceticus (SE370), Burkholderia cepacia (SE4), and Promicromonospora sp. (SE188) to C. sativus L. improved plant growth-promoting properties and mitigated the antagonistic effect of salt and drought stress conditions. In another study, Nadeem et al. (2016) examined the role of PGPRs; Pseudomonas fluorescens, Bacillus megaterium, and Variovorax paradoxus in the growth and development of cucumber under various salt stress concentrations. According to Sapre et al. (2022), the PGPR strains Acinetobacter bereziniae, Enterobacter ludwigii, and Alcaligenes faecalis can reduce salinity stress in pea plants. The inoculation of PGPRs enhanced pea seedling development characteristics under salinity stress. PGPR inoculation also reduced salt stress by ‘modulating biochemical parameters such as chlorophyll content, proline content, total soluble sugar, electrolyte leakage, and antioxidant enzyme activity’. Furthermore, in comparison to un-inoculated pea seedlings, PGPR-inoculated plants showed lower levels of electrolyte leakage and H2O2 concentration in saline conditions.
Salinity stress causes a water deficit inside plant tissues, which affects plant growth and development. A reduction in RWC is the first noticeable result of salt stress, and RWC assessment is a useful technique for describing the water condition of the plant (Shabaan et al. 2022). Under salt stress conditions, it was observed that S. fonticola (S1T1) root zone and foliar sprayed plants had higher RWC than control plants. The result of the present work are confirmatory to the studies of Khan et al. (2019b; Singh et al. (2013)) which suggest that the application of rhizobacteria not only alleviates the adverse effects of salt stress conditions, but also assists in obtaining more water. Similarly, salt stress can cause an increase in electrolyte discharge, where the membrane’s permeability is reduced, resulting in a higher efflux of electrolytes inside plant tissue (Ilyas et al. 2020). In the present study, our findings revealed that the plants treated with S. fonticola (S1T1) in the root zone have lower electrolyte concentrations followed by plants under foliar application compared to control plants under salt stress condition. This suggests that the application of S. fonticola (S1T1) helps to maintain the possible integrity and stability of cellular tissues under salt stress conditions.
The protective role of ABA is essential for plant growth because it promotes stomatal closure to minimize water loss and controls stress-related damage by activating a variety of stress-responsive genes, increasing the plant’s overall tolerance to stress. Plant’s ABA levels have been shown to rise in response to abiotic stress conditions (Khan et al. 2019b, 2021b). Interestingly, our findings in the present study revealed significantly lower levels of ABA production in the presence of S. fonticola (S1T1) compared to untreated stressed plants. Although some of the studies reported that the application of microbes can increase the level of ABA accumulation in different parts of the plants (roots and leaves), the effect may fluctuate among different microbes and plant species (Herrera-Medina et al. 2007; Evelin et al. 2009). Various studies reported the low levels of ABA under abiotic stress and application of PGPR (Khan et al. 2019a, b; Kang et al. 2014; Kubi et al. 2021). ABA contents of cucumber plants treated with S. fonticola (S1T1) in root zone significantly lowered compared to foliar application and untreated control plants. In different plant species, ABA inhibits leaf expansion and shoot growth, whereas, plants treated with advantageous rhizobacteria significantly mitigate the negative effects of stressful conditions by considerably expanding leaf area and showed a low level of ABA in plants tissues compared to control plants under stress.
The presence of salt stress leads to oxidative stress in plants; therefore, we also investigated the activities of antioxidant enzymes. Under salt stress, the level of ROS such as superoxide anions and hydrogen peroxide are increased and cause oxidative damage to the cell structure, however, the defense system (antioxidant enzymes) of plant activated and scavenge excessive ROS produced under stressful conditions (Khan et al. 2019b). The finding of our study revealed that salt stress leads to the formation of ROS, consequently, antioxidant enzyme activity could be expected. The plant defense system is activated under stressful conditions consisting of several ROS-scavenging enzymes such as CAT and SOD. These enzymes have the potential to alleviate the levels of free radicals under stressful conditions. Cucumber plants inoculated with S. fonticola (S1T1) exhibited significant elevation in the activities of antioxidant enzymes (CAT and SOD) under salt stress which ultimately reduced the levels of ROS (MDA content, H2O2 content and superoxide anion). Our results are confirmatory to the studies of Khan et al. (2019b; Yasmeen and Shaheed Siddiqui (2017)) where they reported the lowering of ROS formation via the activities of antioxidant enzymes by using microbes under salt stress conditions.
Several microbes are also known to improve salt stress tolerance in plants by regulating the ion transporter genes such as HKT1, NHX and SOS1 (Roy et al. 2021). A number of different stress-related genes involved in signal transduction, ion transporters, transcription control, and metabolic pathways have been found to confer salt stress tolerance in plants. Bharti et al. (2016) reported the protective role of salt tolerant PGPR (Dietzia natronolimnaea), which was able to regulate the expression of potassium ion transporters (HKT), vacuolar transporters (NHX), and salt overly sensitive (SOS) pathway related genes in salt stressed wheat plants. HKT1-type transporters are important regulators of Na+ and K+ homeostasis in plants, and they help to minimize Na+ toxicity. HKT1 transporters have a vital role in salt stress in a variety of plant species, including Arabidopsis, wheat, rice, sorghum, tomato (Ali et al. 2019). In the current study, HKT1 expression was shown to be significantly higher in S. fonticola (S1T1) inoculated plants both under root zone and foliar treatment compared to control plants. Our results are confirmatory to the studies of Bharti et al. (2016), ion transporters are known as terminal determinants of ionic homeostasis, under NaCl stressed conditions. Increased NHX expression has been linked to enhanced salt tolerance. In the present study, a significantly increased levels of NHX were found in leaves of root zone S. fonticola (S1T1) inoculated plants followed by foliar-sprayed cucumber plant as compared to their untreated counterparts. This revealed a possible effect of S. fonticola (S1T1) in modifying the ion transport systems in cucumber plants under salt stress. Similarly, the SOS signaling system is a crucial mechanism for Na+ exclusion and ion homeostasis control at the cellular level. Which is also defined as a signaling pathway essential for the control of ionic homeostasis in plants. SOS1 is a plasma membrane Na+/H+ antiporters involved in salt tolerance; it regulates long-distance Na+ transport from root to shoot (Bharti et al. 2016). Our findings revealed that S. fonticola (S1T1) induce the expression of SOS1 gene both in root zone and foliar application compared to salt stressed untreated plants, implying that they play a role in salt tolerance mechanisms. The improved expression of above genes in S. fonticola (S1T1) inoculated cucumber plants can be correlated with the improvement in plant growth-promoting attributes under salt stress conditions.
5 Conclusion
The findings of the present study are much useful to the horticultural crops for utilizing this multi-trait PGPR strain S. fonticola (S1T1) as a microbial plant biostimulant which can pragmatically improve the stress tolerance and augment the crop growth and development under salinity stress. The application S. fonticola (S1T1) under root zone significantly enhanced plant growth attributes followed by foliar application of S. fonticola S1T1 under salt stress. Similarly, the beneficial effects of S. fonticola (S1T1) on C. sativus L. were assessed by increased water potential, decreased endogenous ABA, lower electrolytic leakage, antioxidant analysis and candidate genes expressions under different treatments. It could be suggested that the symbiotic association of S. fonticola (S1T1) under root zone and foliar application is a fruitful strategy to mitigate salt stress associated damages on cucumber plants and could be utilized as an eco-friendly microbial plant biostimulant.
References
Ali S, Khan MA, Kim W-C (2018a) Pseudomonas veronii KJ mitigates flood stress-associated damage in Sesamum indicum L. Appl Biol Chem 61(5):575–585
Ali S, Park S-K, Kim W-C (2018b) The pragmatic introduction and expression of microbial transgenes in plants. J Microbiol Biotechnol 28(12):1955–1970
Ali A, Maggio A, Bressan RA, Yun D-J (2019) Role and functional differences of HKT1-type transporters in plants under salt stress. Int J Mol Sci 20(5):1059
Ali B, Wang X, Saleem MH, Hafeez A, Afridi MS, Khan S, Ullah I, Amaral Júnior ATd, Alatawi A, Ali S (2022a) PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 11 (3):345
Ali S, Moon Y-S, Hamayun M, Khan MA, Bibi K, Lee I-J (2022b) Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J Plant Interact 17(1):705–718
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6(1):1–16
Bulgari R, Franzoni G, Ferrante A (2019) Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 9(6):306
Colla G, Rouphael Y (2015) Biostimulants in horticulture. Sci Hort 196:1–134
Dabral S, Saxena SC, Choudhary DK, Bandyopadhyay P, Sahoo RK, Tuteja N, Nath M (2020) Synergistic inoculation of Azotobacter vinelandii and Serendipita indica augmented rice growth. Symbiosis 81(2):139–148
Du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hort 196:3–14
Efthimiadou A, Katsenios N, Chanioti S, Giannoglou M, Djordjevic N, Katsaros G (2020) Effect of foliar and soil application of plant growth promoting bacteria on growth, physiology, yield and seed quality of maize under Mediterranean conditions. Sci Rep 10(1):1–11
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Botany 104(7):1263–1280
Franzoni G, Cocetta G, Prinsi B, Ferrante A, Espen L (2022) Biostimulants on crops: their impact under Abiotic stress conditions. Horticulturae 8(3):189
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20(1):27–36
Giordano M, Petropoulos SA, Rouphael Y (2021) Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 11(5):463
Hamid B, Zaman M, Farooq S, Fatima S, Sayyed RZ, Baba ZA, Sheikh TA, Reddy MS, El Enshasy H, Gafur A (2021) Bacterial plant biostimulants: a sustainable way towards improving growth, productivity, and health of crops. Sustainability 13(5):2856
Haroon U, Khizar M, Liaquat F, Ali M, Akbar M, Tahir K, Batool SS, Kamal A, Chaudhary HJ, Munis MFH (2022) Halotolerant plant growth-promoting rhizobacteria induce salinity tolerance in wheat by enhancing the expression of SOS genes. J Plant Growth Regul 41(6):2435–2448
Herrera-Medina MJ, Steinkellner S, Vierheilig H, Ocampo Bote JA, Garcia Garrido J (2007) Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol 175(3):554–564
Ilyas N, Mazhar R, Yasmin H, Khan W, Iqbal S, Enshasy HE, Dailin DJ (2020) Rhizobacteria isolated from saline soil induce systemic tolerance in wheat (Triticum aestivum L.) against salinity stress. Agronomy 10(7):989
Ismail A, Takeda S, Nick P (2014) Life and death under salt stress: same players, different timing? J Exp Bot 65(12):2963–2979
Jan FG, Hamayun M, Moon Y-S, Jan G, Shafique M, Ali S (2022) Endophytic aspergillus oryzae reprograms Abelmoschus esculentus L. to higher growth under salt stress via regulation of physiochemical attributes and antioxidant system. Biologia:1–14
Jiménez-Mejía R, Medina-Estrada RI, Carballar-Hernández S, Orozco-Mosqueda MdC, Santoyo G, Loeza-Lara PD (2022) Teamwork to survive in hostile soils: Use of Plant Growth-Promoting Bacteria to ameliorate soil salinity stress in crops. Microorganisms 10(1):150
Kang S-M, Khan AL, Waqas M, You Y-H, Kim J-H, Kim J-G, Hamayun M, Lee I-J (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9(1):673–682
Kapadia C, Sayyed RZ, El Enshasy HA, Vaidya H, Sharma D, Patel N, Malek RA, Syed A, Elgorban AM, Ahmad K (2021) Halotolerant microbial consortia for sustainable mitigation of salinity stress, growth promotion, and mineral uptake in tomato plants and soil nutrient enrichment. Sustainability 13(15):8369
Khan MA, Asaf S, Khan AL, Adhikari A, Jan R, Ali S, Imran M, Kim K-M, Lee I-J (2019a) Halotolerant rhizobacterial strains mitigate the adverse effects of NaCl stress in soybean seedlings. BioMed research international 2019
Khan MA, Ullah I, Waqas M, Hamayun M, Khan AL, Asaf S, Kang S-M, Kim K-M, Jan R, Lee I-J (2019b) Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 77(1):9–21
Khan MA, Asaf S, Khan AL, Jan R, Kang S-M, Kim K-M, Lee I-J (2020) Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 15(4):e0232228
Khan M, Al Azawi TNI, Pande A, Mun B-G, Lee D-S, Hussain A, Lee B-H, Yun B-W (2021a) The role of nitric oxide-induced ATILL6 in growth and disease resistance in Arabidopsis thaliana. Front Plant Sci 12:685156
Khan MA, Hamayun M, Asaf S, Khan M, Yun B-W, Kang S-M, Lee I-J (2021b) Rhizospheric bacillus spp. rescues plant growth under salinity stress via regulating gene expression, endogenous hormones, and antioxidant system of Oryza sativa L. Front Plant Sci 12:1145
Khan N, Ali S, Shahid MA, Mustafa A, Sayyed RZ, Curá JA (2021c) Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells 10(6):1551
Kubi HAA, Khan MA, Adhikari A, Imran M, Kang S-M, Hamayun M, Lee I-J (2021) Silicon and plant growth-promoting Rhizobacteria Pseudomonas psychrotolerans CS51 mitigates salt stress in Zea mays L. Agriculture 11(3):272
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Menon RR, Kumari S, Viver T, Rameshkumar NJMR (2020) Flavobacterium pokkalii sp. nov., a novel plant growth promoting native rhizobacteria isolated from pokkali rice grown in coastal saline affected agricultural regions of southern India. Kerala 240:126533
Moon Y-S, Ali S (2022a) A fruitful decade of bacterial ACC deaminase biotechnology: a pragmatic approach towards abiotic stress relief in plants.Theoretical and Experimental Plant Physiology:1–21
Moon Y-S, Ali S (2022b) Isolation and identification of multi-trait plant growth–promoting rhizobacteria from coastal sand dune plant species of Pohang beach. Folia Microbiol 67(3):523–533
Nadeem SM, Ahmad M, Naveed M, Imran M, Zahir ZA, Crowley DE (2016) Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch Microbiol 198(4):379–387
Park H-S, Kazerooni EA, Kang S-M, Al-Sadi AM, Lee I-J (2021) Melatonin enhances the tolerance and recovery mechanisms in Brassica juncea (L.) Czern. Under saline conditions. Front Plant Sci 12:593717
Preininger C, Sauer U, Bejarano A, Berninger T (2018) Concepts and applications of foliar spray for microbial inoculants. Appl Microbiol Biotechnol 102(17):7265–7282
Qi Q, Rose PA, Abrams GD, Taylor DC, Abrams SR, Cutler AJ (1998) (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol 117(3):979–987
Radhakrishnan R, Lee I-J (2013) Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J Plant Growth Regul 32(1):22–30
Rahim W, Khan M, Al Azzawi TNI, Pande A, Methela NJ, Ali S, Imran M, Lee D-S, Lee G-M, Mun B-G (2022) Exogenously Applied Sodium Nitroprusside mitigates lead toxicity in Rice by regulating antioxidants and metal stress-related transcripts. Int J Mol Sci 23(17):9729
Ramezani A, Niazi A, Abolimoghadam AA, Zamani Babgohari M, Deihimi T, Ebrahimi M, Akhtardanesh H, Ebrahimie E (2013) Quantitative expression analysis of TaSOS1 and TaSOS4 genes in cultivated and wild wheat plants under salt stress. Mol Biotechnol 53(2):189–197
Raza A, Mehmood SS, Shah T, Zou X, Yan L, Zhang X, Khan RSA (2019) Applications of molecular markers to develop resistance against abiotic stresses in wheat. Wheat production in changing environments. Springer, pp 393–420
Rouphael Y, Colla G (2018) Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front Plant Sci 9:1655
Roy S, Chakraborty AP, Chakraborty R (2021) Understanding the potential of root microbiome influencing salt-tolerance in plants and mechanisms involved at the transcriptional and translational level. Physiol Plant 173(4):1657–1681
Safdarian M, Askari H, Shariati JV, Nematzadeh G (2019) Transcriptional responses of wheat roots inoculated with Arthrobacter nitroguajacolicus to salt stress. Sci Rep 9(1):1–12
Sapre S, Gontia-Mishra I, Tiwari S (2022) Plant growth-promoting rhizobacteria ameliorates salinity stress in pea (Pisum sativum). J Plant Growth Regul 41(2):647–656
Shabaan M, Asghar HN, Zahir ZA, Zhang X, Sardar MF, Li H (2022) Salt-Tolerant PGPR Confer Salt Tolerance to maize through enhanced soil Biological Health, enzymatic activities, nutrient uptake and antioxidant defense. Front Microbiol 13:901865
Shah AN, Tanveer M, Abbas A, Fahad S, Baloch MS, Ahmad MI, Saud S, Song Y (2021) Targeting salt stress coping mechanisms for stress tolerance in Brassica: a research perspective. Plant Physiol Biochem 158:53–64
Singh R, Soni SK, Patel RP, Kalra A (2013) Technology for improving essential oil yield of Ocimum basilicum L.(sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind Crops Prod 45:335–342
Singh RP, Jha PN (2016) The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 11(6):e0155026
Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM (2018) Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep 37(11):1557–1569
Tan, H., Zhou, S., Deng, Z., He, M., & Cao, L. (2011). Ribosomal-sequence-directed selection for endophytic streptomycete strains antagonistic to Ralstonia solanacearum to control tomato bacterial wilt. Biological Control, 59(2):245–254.
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218(1):1–14
Yasmeen R, Shaheed Siddiqui Z (2017) Physiological responses of crop plants against Trichoderma harzianum in saline environment. Acta Bot Croatica 76(2):154–162
Zhao C, Zhang H, Song C, Zhu J-K, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. The innovation 1(1):100017
Funding
The authors have no financial conflict of interest to declare.
Author information
Authors and Affiliations
Contributions
SA, and YSM conceived and designed the experiments. SA, MK, and MAK performed the experiments. SA, MAK and MK analyzed the data and interpretation. SA and YSM contributed reagents/materials/analysis tools. SA, and YSM wrote the paper. YSM and SA contributed equally to this work and have the right to list their names first in their CVs. All authors have read and agreed to publish this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moon, YS., Khan, M., Khan, M.A. et al. Ameliorative symbiosis of Serratia fonticola (S1T1) under salt stress condition enhance growth-promoting attributes of Cucumis sativus L. Symbiosis 89, 283–297 (2023). https://doi.org/10.1007/s13199-023-00897-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-023-00897-w