Abstract
Phosphate solubilization, 1-aminocyclopropane-1-carboxylic acid (ACC)-deaminase activity and production of siderophores and indole acetic acid (IAA) are well-known traits of plant growth-promoting rhizobacteria (PGPR). Here we investigated the expression of these traits as affected by salinity for three PGPR strains (Pseudomonas fluorescens, Bacillus megaterium and Variovorax paradoxus) at two salinity levels [2 and 5 % NaCl (w/v)]. Among the three strains, growth of B. megaterium was the least affected by high salinity. However, P. fluorescens was the best strain for maintaining ACC-deaminase activity, siderophore and IAA production under stressed conditions. V. paradoxus was the least tolerant to salts and had minimal growth and low PGPR trait expression under salt stress. Results of experiment examining the impact of bacterial inoculation on cucumber growth at three salinity levels [1 (normal), 7 and 10 dS m−1] revealed that P. fluorescens also had good rhizosphere competence and was the most effective for alleviating the negative impacts of salinity on cucumber growth. The results suggest that in addition to screening the PGPR regarding their effect on growth under salinity, PGPR trait expression is also an important aspect that may be useful for selecting the most promising PGPR bacterial strains for improving plant tolerance to salinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the abiotic stresses that affect plant growth, soil salinity is one of the most serious problems in irrigated agriculture (Hu and Schmidhalter 2005). A number of approaches are used to deal with the negative impacts of salinity including gypsum applications, organic matter amendments and optimization of irrigation practices to limit the quantities of salts applied and to effectively leach salts from the root zone (Ahmad and Qamar 2003). On the biological side, relevant practices to mitigate salinity include the planting of salt tolerant crop varieties and the use of soil management practices that promote microbial activity and high population densities of plant growth-promoting rhizobacteria (PGPR).
PGPR include diverse bacterial taxa that facilitate plant growth through phytohormones production, disease suppression, phosphate solubilization and stress ethylene suppression, which otherwise inhibits root growth in saline soils and other stress conditions. The management of PGPR populations in soils is currently one of the most difficult challenges in agriculture and has only recently been facilitated by development of methods to characterize soil microbial communities. PGPR include a large number of diverse taxa that express at least one, but typically two or multiple traits that affect plant growth (Kloepper et al. 1989; Glick et al. 1995; Saharan and Nehra 2011; Baig et al. 2014). Another term “plant stress homeoregulating bacteria” coined by Cassan et al. (2009) likewise refers to PGPR strains that specifically are able to improve plant growth under stress conditions.
One of the current practices for improving the population densities of high effective PGPR in the rhizosphere of crop plants is to introduce selected strains as seed or soil inoculants. Candidate strains are identified using culture based methods to determine which traits they carry and quantify their potential activity in different laboratory culture media. Candidate strains should also have the potential to tolerate adverse conditions (Sandhya et al. 2009). There are a number of reports that demonstrate the efficacy of PGPR for promoting plant growth under both normal conditions as well as in saline soils and other stressed environments (Egamberdieva 2009; Zahir et al. 2004, 2009; Glick et al. 2007; Saharan and Nehra 2011; Nadeem et al. 2012). With respect to soil salinity, bacteria carrying traits for phosphate solubilization, synthesis of siderophores, exopolysaccharides and indole acetic acid (IAA) and with high ACC-deaminase enzyme activity were shown to be effective PGPR (Nadeem et al. 2010; Upadhyay et al. 2011; Glick 2014). Bacteria with ACC activity degrade ACC into ammonia and α-ketobutyrate for use as a nitrogen source. Therefore, inoculation of plants with these strains can provide one method to increase the population density in the rhizosphere and lower the accumulation of stress ethylene (Mayak et al. 2004; Glick et al. 2007).
Current research in this field is aimed at identification of which combinations traits are most important and the extent to which measurements of PGPR trait expression in pure culture can be correlated with improved plant growth or specific plant growth parameters. For example, IAA production levels in pure culture can serve as indicator for potential efficacy of a strain for reducing the effects of osmotic stress on plant growth (Boiero et al. 2006). Likewise, exopolysaccharide production at high levels by certain bacteria enables plants to better withstand stressful environments (Upadhyay et al. 2011; Qurashi and Sabri 2012). With respect to improved root growth facilitated by ACC-deaminase activity, it is hypothesized that measurements of bacterial ACC-deaminase expression under stress conditions may be a better indicator of potential efficacy than measurements taken for cells grown under optimal conditions with low salinity.
In selecting PGPR strains for possible use as soil or plant inoculants, there are several open questions as to the best methods and most relevant traits to use to compare the potential efficacy of different strains. Individual PGPR strains carry different combinations of traits that are expressed at different levels. Siddikee et al. (2012) studied the effect of PGPR containing ACC-deaminase activity on red pepper (Capsicum annuum L.) under salinity stress. They found that among three strains (Brevibacterium iodinum, Bacillus licheniformis and Zhihengliuella alba), B. iodinum was the most efficient for reducing the negative impact of stress-induced ethylene. They also observed that although Z. alba had low ACC-deaminase activity compared to B. licheniformis, it performed better in promoting plant growth. Based on their findings, they suggested that this might be due to difference in rhizosphere competence or failure of B. licheniformis to express genes relevant to plant growth promotion under nutrient limitations and elevated environmental stress in soil. These results are in line with observations that microorganisms isolated from drought-stressed soil show higher adaptability and tolerance to stress conditions compared to strains isolated from unstressed sites (Marulanda et al. 2009). In this manner, characterization of candidate PGPR strains for use as soil inoculants may be improved by screening their trait expression levels as affected by specific environmental factors such as salinity level. The present study was carried out to determine changes in PGPR trait expression at different salinity levels for three bacterial strains that vary in salinity tolerance. We then further compare their efficacy for alleviating negative impacts of salinity on cucumber growth.
Materials and methods
Characterization of rhizobacteria
Three pre-isolated rhizobacterial strains, i.e., Variovorax paradoxus (JN858091), Pseudomonas fluorescens (JN858088) and Bacillus megaterium (JN858098) (Nadeem et al. 2012), were characterized for salinity tolerance, ACC-deaminase activity, exopolysaccharide production, phosphate solubilization and indole acetic acid and siderophore production under normal and saline conditions. Measurement of each activity was carried out using three replicates.
The ability of the strains to tolerate high salt concentrations was evaluated by growing the strains on tryptic soy broth (TSB) medium containing 0, 2 and 5 % NaCl (w/v). For this purpose, TSB media containing certain amount of NaCl was autoclaved and after cooling inoculated with particular strain. Inoculum was prepared from cell cultures adjusted to an optical density of 0.5 at 535 nm by dilution to maintain a uniform cell density [108–109 colony-forming units (cfu) mL]. The flasks were placed in a shaking incubator for 72 h at 28 ± 2 °C, and cell growth was monitored by turbidity (A535 nm). Strains were categorized as low, medium and high salt tolerant on the basis of their OD value.
The ACC-deaminase activities of the selected strains were measured by the method described by Penrose and Glick (2003) except that the medium was supplemented with 0, 2 and 5 % NaCl. ACC-deaminase activity was measured by determining the amount of α-ketobutyrate produced when the enzyme cleaved ACC into ammonia and α-ketobutyrate. All measurements were carried out in triplicate and ACC-deaminase activity was calculated as µmol α-ketobutyrate mg−1 protein h−1. Exopolysaccharide production was measured qualitatively according to Nicolaus et al. (1999) with slight modification. The strains were grown in culture medium supplemented with 0, 2 and 5 % NaCl. Supernatants were collected by centrifuging at 1000 rev min−1. Cold absolute ethanol (threefold) was added drop wise into the supernatant under stirring. The formation of precipitates was an indication of the production of exopolysaccharides.
Phosphate solubilization was measured qualitatively by using National Botanical Research Institute (NBRI) growth medium (Mehta and Nautiyal 2001) amended with NaCl (0, 2 and 5 %). Individual bacterial isolates were cultured and spot inoculated in the center of the agar plates. Plates were incubated at 28 °C for 5 days after which phosphate solubilization was quantified. Colonies showing formation of clear zones were considered positive for phosphate solubilization, and diameter of clear halo zone was measured to evaluate the relative efficacy of these strains for phosphate solubilization.
Siderophore production of the strains was assayed qualitatively according to the universal method of Schwyn and Neilands (1987) except that the medium was amended with NaCl. Fresh cultures of each strain were inoculated into low iron medium (modified MM9 medium) and incubated at 28 °C for 48 h. The cultures were centrifuged and the supernatant from each culture was then mixed with chrome azurol S (CAS) solution. The change of color from blue to orange indicated the presence of siderophores in the solution.
Indole acetic acid production of the selected isolates was determined colorimetrically. For this purpose, 5 mL of filter-sterilized 0.5 % l-tryptophan solution was added to the autoclaved, cooled medium containing 0, 2 and 5 % NaCl. Each flask was inoculated with particular strain and after plugging incubated at 28 °C for 48 h on a horizontal shaker at 100 rev min−1. Un-inoculated control was also kept for comparison. After incubation period, the contents were filtered through Whatman filter paper No. 2. Three milliliters of filtrate was mixed with 2.0 mL of Salkowski reagent as described by Sarwar et al. (1992) and allowed to stand for 30 min for color development. Auxin compounds expressed as IAA equivalents were determined by spectrophotometer (model T80).
Evaluation of potential of PGPR strains to promote plant growth under salinity stress
The ability of three selected PGPR strains to promote plant growth was evaluated under axenic conditions using cucumber as a test plant. The experiment was performed under completely controlled conditions in a growth chamber. For seed inoculation, inoculum was prepared without agar. For this purpose, 250-mL Erlenmeyer flasks containing 60 mL broth were inoculated with the selected strains and placed on a shaking (100 rev min−1) incubator at 28 °C for 72 h. Cucumber seeds were surface-sterilized by dipping in 95 % ethanol solution for 2 min, followed by immersion in a 0.2 % HgCl2 solution for 3 min. The seeds were then rinsed with sterile deionized water. Surface-sterilized seeds were inoculated with each respective bacterial strain (dipping in broth inoculated with bacterial strain for 5 min), and then, three seeds were sown in plastic tubes containing 250 g sand. After germination, the plants were thinned to one plant per tube. To obtain desired levels of salinity in sand, i.e., normal, 7 and 10 dS m−1, the required amount of NaCl was dissolved in deionized water and added to each tube according to soil saturation percentage before sowing. There were five replications of each treatment. Half-strength Hoagland solution was applied to fulfill nutrient requirements at the first irrigation, after which the plants were watered with distilled water. After 35 days, growth parameters for the cucumber plants were measured and recorded.
Root colonization assay
To quantify root colonization by the selected strains, root samples with adhering soil were agitated in a sterile saline solution (0.85 % NaCl) with vigorous shaking for 1 h. Serial dilutions of bacterial suspensions were prepared in phosphate buffer and spread on already prepared tryptic soya agar plates. The plates were placed in incubator at 28 °C for 48 h. All treatments in the colonization experiment were replicated three times, and the average number of bacterial colony-forming units was calculated and presented as mean cfu g−1 root biomass.
Statistical analysis
The data were analyzed by analysis of variance (ANOVA) by using IBM SPSS program version 19. Significant differences (p > 0.05) between treatment means were determined by Duncan’s multiple range tests.
Results
Characterization of PGPR strains under salinity stress
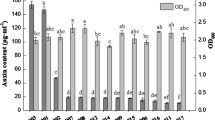
Data regarding biochemical characterization viz. salt tolerance/growth inhibition, IAA production, siderophore production, phosphate solubilization, exopolysaccharide production and ACC-deaminase activity under normal and saline conditions are presented in Table 1. Among the three strains tested here, B. megaterium was the most salt tolerant, whereas V. paradoxus was the most sensitive. Growth of P. fluorescens, B. megaterium and V. paradoxus was reduced by up to 34, 10 and 35 %, respectively, in medium containing 5 % NaCl.
The results of IAA production assay showed that all three strains produced IAA and that under normal conditions (no NaCl), the IAA production was highest with B. megaterium followed by P. fluorescens and V. paradoxus. The maximum reduction (38 %) in IAA production was recorded in B. megaterium when subjected to 5 % salinity. Furthermore, it was observed that P. fluorescens was the most tolerant to salinity with respect to IAA production, which increased slightly in cultures with 5 % NaCl. Siderophore production also varied among the selected rhizobacteria depending on the salinity level. All three strains were similar in siderophore production under normal conditions. Siderophores production for V. paradoxus was reduced at 2 % NaCl and completely inhibited at 5 % NaCl. The other two strains P. fluorescens and B. megaterium produced equal amount of siderophores at 2 % NaCl; however, less siderophores were produced at the highest salinity level (5 % NaCl) as compared to nonsaline conditions. With respect to phosphate solubilization, P. fluorescens performed well at all salinity levels followed by B. megaterium. In contrast, V. paradoxus was negative for phosphate solubilization under both normal and saline conditions. The diameter of the halo zones indicating phosphate solubilization was reduced by approximately 10 % for P. fluorescens at the highest salinity level followed by B. megaterium. Data on exopolysaccharides production showed that both P. fluorescens and B. megaterium were positive for exopolysaccharides production and that production levels were not affected by high salt concentrations. In contrast V. paradoxus was unable to produce exopolysaccharides under both normal and saline conditions. The maximum ACC-deaminase activity was observed for P. fluorescens followed by V. paradoxus and B. megaterium under normal conditions. ACC-deaminase activities of P. fluorescens and B. megaterium were not changed significantly by salt concentrations, but in V. paradoxus, the activity was substantially reduced.
Root colonization assay
Data regarding root colonization showed that inoculation with PGPR strains increased the number of bacteria on the root surface of cucumber plants under normal as well as saline conditions compared to the un-inoculated control (Table 2). Under normal conditions, inoculation increased the bacterial cfu g−1 root biomass compared to un-inoculated plants. The highest root colonization was observed with B. megaterium followed by P. fluorescens. Under salt-stressed conditions, P. fluorescens was the most tolerant as maximum bacterial cfu g−1 root biomass was observed by this strain in soil having 10 dS m−1 salinity level. Furthermore, at this salinity level, reduction in initial population (no salt) was also less than observed for the other two strains. B. megaterium showed better results in case of root colonization under salt-stressed conditions than V. paradoxus.
Plant growth promotion under salinity stress
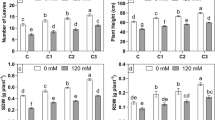
Results of the greenhouse experiments showed that cucumber growth was significantly (p < 0.05) reduced with increasing salinity levels, for both inoculated and un-inoculated plants. Reduction in growth was less for inoculated plants as compared to un-inoculated plants at all salinity levels. The reduction in shoot length of un-inoculated cucumber plants at 10 dS m−1 NaCl level was 36 % as compared to normal conditions (Table 3). All three PGPR strains significantly improved the shoot length of cucumber as compared to un-inoculated control under normal as well as salt-stressed conditions. P. fluorescens produced higher shoot length under both normal and saline conditions compared to other strains. It is also evident form Fig. 1a that inoculation with P. fluorescens significantly reduced the negative impact of salinity stress on cucumber shoot growth. At 7 and 10 dS m−1, the increase was 24 and 55 %, respectively, when compared to the respective un-inoculated controls at the same salinity levels. As observed for shoot length, root length was decreased for both inoculated and un-inoculated plants when subjected to 7 and 10 dS m−1 NaCl salinity (Table 4). Inoculation of plants with P. fluorescens, B. megaterium and V. paradoxus in nonsaline soil increased root length by up to 39, 29 and 41 %, respectively, as compared to unstressed control. At 7 dS m−1, inoculation with P. fluorescens, B. megaterium and V. paradoxus, respectively, resulted in 36, 12 and 26 % increases in root length as compared to control plants at this salinity level. The benefit of PGPR inoculation was most evident for plants grown at 10 dS m−1, where inoculation increased total root lengths by 67, 26 and 46 %, respectively, for plants inoculated with P. fluorescens, B. megaterium and V. paradoxus. The results showed better performance of P. fluorescens for enhancing root growth at high level of salinity as it also depicted from Fig. 1b under salt-stressed conditions.
As expected based on the results for shoot and root lengths, the effects of inoculation with PGPR strains were also manifested by improved shoot and root biomass values for plants grown under salinity stress (Tables 5, 6). Plants inoculated with P. fluorescens had the highest shoot and root dry weights as compared to those inoculated with B. megaterium and V. paradoxus at all salinity levels. Inoculation with P. fluorescens resulted in 86, 60 and 73 % increases in shoot weight over the respective un-inoculated controls grown at 1, 7 and 10 dS m−1 NaCl salinity levels, respectively. The increase in root weight due to P. fluorescens inoculation over the un-inoculated control was 97, 68 and 87 % at 1, 7 and 10 dS m−1 NaCl salinity levels, respectively. Maximum total biomass (4.73 g) was observed in case of inoculation with P. fluorescens under normal conditions while the maximum improvement in total biomass (118 %) as compared to respective un-inoculated control was observed by the same strain at 7 dS m−1 NaCl salinity level (Table 7). However, at 10 dS m−1, the highest biomass values were recorded for plants inoculated with either B. megaterium or P. fluorescens. Inoculation with P. fluorescens, B. megaterium and V. paradoxus also improved the chlorophyll contents in leaves of cucumber grown under salt-stressed conditions (Table 8). At 7 dS m−1, B. megaterium was significantly better than other two strains for improving plant chlorophyll content, whereas, at the highest salinity level (10 dS m−1), P. fluorescens inoculated plants had significantly higher chlorophyll contents.
Discussion
There is now a large body of research that has examined the mechanisms by which PGPR affect plant growth through IAA production, phosphate solubilization, siderophore production and ACC-deaminase activity (Zahir et al. 2009; Ahmad et al. 2011; Saharan and Nehra 2011; Baig et al. 2014). Nonetheless, PGPR strains vary in their field performance and there is still a gap in our knowledge on how environmental factors affect both the rhizosphere competence (growth) and PGPR trait expression after they are introduced into soil. The present study was conducted to examine whether in vitro screening of salinity tolerance may predict performance of PGPR strains for improving plant growth in a salinized soil. Three bacterial strains (P. fluorescens, B. megaterium and V. paradoxus) were tested and found to be highly salt tolerant. All of the strains exhibited prolific growth on agar media containing up to 5 % NaCl, which is equivalent to a salinity level of approximately 80 dS m−1. Among the three strains, B. megaterium was the most tolerant, while V. paradoxus showed the least growth under salt stress. Previously, Tank and Saraf (2010) also observed variability in PGPR strains regarding their ability to tolerate salinity. Differences in the tolerance of bacteria to salinity are thought to involve differences in the production of osmolytes and stress resistant compounds (Sandhya et al. 2009; Qurashi and Sabri 2012). Here, the ability of Pseudomonas and Bacillus to tolerate high salt concentration was very likely due to the production of exopolysaccharides which were produced in pure cultures irrespective of salinity level by these bacterial strains. Conversely, salt sensitive V. paradoxus did not produce exopolysaccharides under the experimental conditions used here. Exopolysaccharides are also proposed to decrease the availability of Na+ for plant uptake (Ashraf et al., 2006; Dodd and Perez-Alfocea 2012).
Prior research has shown that ACC-deaminase containing rhizobacteria can mitigate the negative impact of stress-induced ethylene on root growth by degrading its immediate precursor ACC into ammonia and α-ketobutyrate (Glick et al. 1998; Mayak et al. 2004; Shaharoona et al. 2011). The results of the present study showed that salinity significantly reduced the seedling growth (shoot length, root length, shoot biomass, root biomass) of cucumber and that inoculation with PGPR improved plant growth by reducing the deleterious effects of salinity. Salt inhibits plant growth, by causing both ion toxicity and osmotic stress (Zhang et al. 2010; Tavakkoli et al. 2011), which in turn increases stress ethylene production. Under such conditions, elevated levels of ethylene cause negative impacts on root growth (Mattoo and Suttle 1991; Belimov et al. 2009), which ultimately reduces overall plant growth due to water and nutrient limitations.
In the present study, all three strains were able to use ACC as a sole source of nitrogen under normal and 5 % salinity stress conditions. P. fluorescens had the highest ACC utilization rate followed by V. paradoxus and B. megaterium. At the highest salinity level with 5 % NaCl, the strains showed an expected decrease in growth and decreased degradation of ACC, the precursor molecule that leads to stress ethylene accumulation in the rhizosphere. The consistent high ACC-deaminase activity of P. fluorescens thus indicates its potential for plant growth promotion over a range of conditions that would result in stress-induced ethylene accumulation (Zahir et al. 2009). These results are in general agreement with results of previous studies (Nadeem et al. 2010; Ahmad et al. 2011; Tank and Saraf 2010) regarding ACC-deaminase activity under salinity stress.
In addition to maintaining the highest level of ACC-deaminase activity, P. fluorescens also had the highest IAA production levels (5.41–5.47 mg kg−1) as compared to V. paradoxus and B. megaterium. Difference in IAA production ability among bacteria under normal conditions has been reported (Anjum et al. 2011; Ahmad et al. 2013). In the present study, the IAA production ability of B. megaterium and V. paradoxus was both substantially lowered with increasing salt, whereas P. fluorescens actually produced slightly more IAA under salt stress conditions. Kerkar et al. (2012) likewise found that IAA production by PGPR is generally reduced under saline conditions. They reported that IAA production was salinity dependent and varied with the type of bacteria. This argument is further supported from the work of Nakbanpote et al. (2014), who found that among three bacterial strains (two Serratia spp. and one Pseudomonas sp.), IAA production by Serratia spp. was reduced while IAA production by Pseudomonas sp. increased under salinity stress.
All three strains examined here varied for phosphate solubilization and siderophores synthesis under normal culture conditions and their potential regarding these traits was variably affected by salinity stress. P. fluorescens was the most effective for solubilizing phosphorus under saline conditions. The maintenance of phosphate solubilization by P. fluorescens under salinity stress could be a great advantage for improving plant growth in saline soils. As reported by others, phosphate solubilization is related to both organic acid production and phosphatase activity (Tank and Saraf 2010). Some organic acids lower the rhizosphere pH and therefore dissolve calcium phosphates. Anionic organic acids also can displace phosphate from the anion exchange complex, and some organic acids also form complexes with iron and aluminum that then results in further dissolution of iron and aluminum phosphate minerals to liberate more phosphate into solutions. Many bacteria also produce phosphatases that release phosphorus during the decomposition of soil organic matter. Altogether, these processes aid solubilization of inorganic phosphorus in the plant rhizosphere. The efficacy of P. fluorescens for solubilization of phosphorus has already been observed in earlier studies (Tank and Saraf 2010). PGPR strains isolated from same environment have been shown to vary in this and other PGPR traits (Nadeem et al. 2012).
One of the main objectives of the present study was to examine the relationship between PGPR trait activity levels in pure culture and PGPR performance when inoculated on to plant roots. Overall, this hypothesis was supported. P. fluorescens, which showed the highest ACC-deaminase activity under salinity stress in liquid medium, was also the most effective for increasing root length and root biomass in the presence of salts. Variovorax paradoxus which also has relatively high ACC-deaminase activity also caused significant increases in root length and root biomass as compared to un-inoculated control plants. Auxins also play role in plant growth promotion under stress conditions by enhancing plant root growth (Liu et al. 2014). The growth enhancement of cucumber with P. fluorescens also was likely due to its ability to maintain its IAA production ability under salinity stress. In addition to this, enhancement of phosphate solubilization and siderophore production could also contribute to better root growth. Here, inoculation with the selected strains also increased the chlorophyll content of cucumber under salinity stress, suggesting greater plant uptake of nitrogen that contributed to chlorophyll formation. This result is in agreement with Stefan et al. (2013) who also observed enhanced chlorophyll content following PGPR inoculation.
Although B. megaterium showed more resistance to salinity in terms of cell growth, its performance was relatively poor compared to P. fluorescens. Although not examined here, both the population density of the inoculant in the rhizosphere and level of PGPR trait expression are both likely to affect plant growth; i.e., strains with high rhizosphere competence but low levels of PGPR trait expression may provide similar growth enhancement as strains with higher levels of trait expression that maintain lower population densities in the rhizosphere. Ideal inoculants will have both good rhizosphere competence and high levels of PGPR trait expression. Here the excellent performance of P. fluorescens may be attributed to its good ability to colonize the plant roots under salinity stress compared to other two strains and ability to maintain high levels of PGPR trait expression under salinity stress.
Conclusions
PGPR strains have potential to mitigate negative impacts of salinity stress on plant growth, but with variable efficacy. Results of the present study suggest that this is due to both differences in rhizosphere competence and differences in the ability of different PGPR strains to express growth-promoting traits under salinity stress conditions environment. Measurements of variability in the expression of plant growth-promoting traits in normal and stress conditions may thus provide a useful indicator for prediction of PGPR efficacy for plant growth promotion under saline conditions.
References
Ahmad M, Qamar I (2003) Productive rehabilitation and use of salt-affected land through afforestation (a review). Sci Vis 9:1–14
Ahmad M, Zahir ZA, Asghar HN, Asghar M (2011) Inducing salt tolerance in mung bean through co-inoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate-deaminase. Can J Microbiol 57:578–589
Ahmad M, Zahir ZA, Nadeem SM, Nazli F, Jamil M, Khalid M (2013) Field evaluation of Rhizobium and Pseudomonas strains to improve growth, nodulation and yield of mung bean under salt-affected conditions. Soil Environ 32:158–165
Anjum MA, Zahir ZA, Arshad M, Ashraf M (2011) Isolation and screening of rhizobia for auxin biosynthesis and growth promotion of mung bean (Vigna radiata L.) seedlings under axenic conditions. Soil Environ 30:18–26
Ashraf M, Hasnain S, Berge O (2006) Effect of exo-polysaccharides producing bacterial inoculation on growth of roots of wheat (Triticum aestivum L.) plants grown in a salt affected soil. Int J Environ Sci Technol 3:43–51
Baig KS, Arshad M, Khalid A, Hussain S, Abbas MN, Imran M (2014) Improving growth and yield of maize through bioinoculants carrying auxin production and phosphate solubilizing activity. Soil Environ 33:159–168
Belimov AA, Dodd IC, Safronova VI, Davies WJ (2009) ACC-deaminase-containing rhizobacteria improve vegetative development and yield of potato plants grown under water-limited conditions. Asp Appl Biol 98:163–169
Boiero L, Perrig D, Masciarelli O, Penna C, Cassan F, Luna V (2006) Phytohormone production by three strains of Bradyrhizobium japonicum, and possible physiological and technological implications. Appl Microbiol Biotechnol 74:874–880
Cassan F, Perrig D, Sgroy V, Masciarelli O, Penna C, Luna V (2009) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur J Soil Biol 45:28–35
Dodd IC, Perez-Alfocea F (2012) Microbial alleviation of crop salinity. J Exp Bot 63:3415–3428
Egamberdieva D (2009) Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol Plant 31:861–864
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Glick BR, Karaturovic DM, Newell PC (1995) A novel procedure for rapid isolation of plant growth promoting Pseudomonas. Can J Microbiol 41:533–536
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth promoting rhizobacteria. J Theor Biol 190:63–68
Glick BR, Cheng Z, Czarny J, Cheng Z, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339
Hu Y, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
Kerkar S, Raiker L, Tiwari A, Mayilraj S, Dastager S (2012) Biofilm associated indole acetic acid producing bacteria and their impact in the proliferation of biofilm mats in solar salterns. Biologia 67:454–460
Kloepper JW, Lifshitz R, Zablotowicz RM (1989) Free living bacterial inocula for enhancing crop productivity. Trends Biotechnol 7:39–44
Liu L, Guo G, Wang Z, Ji H, Mu F, Li X (2014) Auxin in plant growth and stress responses. In: Tran LSP, Pal S (eds) Phytohormones: a window to metabolism, signaling and biotechnological applications. Springer, New York, pp 1–35
Marulanda A, Barea JM, Azcon R (2009) Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: mechanisms related to bacterial effectiveness. J Plant Growth Regul 28:115–124
Mattoo AK, Suttle CS (1991) The plant hormone ethylene. CRS Press, Boca Raton
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate solubilizing bacteria. Curr Microbiol 43:57–58
Nadeem SM, Zahir ZA, Naveed M, Asghar HN, Arshad M (2010) Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci Soc Am J 74:533–542
Nadeem SM, Shaharoona B, Arshad M, Crowley DE (2012) Population density and functional diversity of plant growth promoting rhizobacteria associated with avocado trees in saline soils. Appl Soil Ecol 62:147–154
Nakbanpote W, Panitlurtumpai N, Sangdee A, Sakulpone N, Sirisom P, Pimthong A (2014) Salt-tolerant and plant growth-promoting bacteria isolated from Zn/Cd contaminated soil: identification and effect on rice under saline conditions. J Plant Interact 9:379–387
Nicolaus BL, Esposito LE, Manca MC, Improta R, Bellitti MR, Duckworth AW, Grant WD, Gambacorta A (1999) Haloarcula spp. able to bio-synthesize exo-endopolymers. J Ind Microbiol Biotechnol 23:489–496
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase containing plant growth promoting rhizobacteria. Physiol Planta 118:10–15
Qurashi AW, Sabri AN (2012) Bacterial exopolysaccharides and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz J Microbiol 43:1183–1191
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:1–30
Sandhya V, Ali SKZ, Grover M, Reddy G, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils 46:17–26
Sarwar M, Arshad M, Martens DA, Frankenberger WT Jr (1992) Tryptophan dependent biosynthesis of auxins in soil. Plant Soil 147:207–215
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shaharoona B, Imran M, Arshad M, Khalid A (2011) Manipulation of ethylene synthesis in roots through bacterial ACC deaminase for improving nodulation in legumes. Crit Rev Plant Sci 30:279–291
Siddikee MA, Chauhan PS, Sa T (2012) Regulation of ethylene biosynthesis under salt stress in red pepper (Capsicum annuum L.) by 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase-producing halotolerant bacteria. J Plant Growth Regul 31:265–272
Stefan M, Munteanu N, Stoleru V, Mihasan M (2013) Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Romanian Biotechnol Lett 18:8132–8143
Tank N, Saraf M (2010) Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact 5:51–58
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK (2011) Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J Exp Bot 62:2189–2203
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth promoting rhizobacteria under salinity condition. Pedosphere 21:214–222
Zahir ZA, Arshad M, Frankenberger WT Jr (2004) Plant growth promoting rhizobacteria application and perspectives in agriculture. Adv Agron 81:96–168
Zahir ZA, Ghani U, Naveed M, Nadeem SM, Asghar HN (2009) Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch Microbiol 191:415–424
Zhang JL, Flowers TJ, Wang SM (2010) Mechanisms of sodium uptake by roots of higher plants. Plant Soil 326:45–60
Acknowledgments
The authors gratefully acknowledge the Higher Education Commission (HEC) of Pakistan for the financial support for this research. The authors also gratefully acknowledge support provided by the Kearney Foundation of Soil Science, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Nadeem, S.M., Ahmad, M., Naveed, M. et al. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch Microbiol 198, 379–387 (2016). https://doi.org/10.1007/s00203-016-1197-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1197-5