Abstract
The association between the ciliate Paramecium bursaria and symbiotic Chlorella spp. is mutually beneficial. However, this relationship is facultative mutualism because both the host and the symbiotic algae can grow by themselves. This association is easily re-established by mixing the two species together. Following algal mixing, some algae become enclosed in the digestive vacuole membrane of the paramecia to which both acidosomes and lysosomes fuse. To establish endosymbiosis, some algae acquire temporal resistance to the host lysosomal enzymes in the digestive vacuoles. We examined whether the algae influence the differentiation of the host digestive process using LysoSensor staining to evaluate the acidification of the digestive vacuoles. Furthermore, to assess lysosomal fusion with the digestive vacuole, Gomori’s staining was conducted. Acidification and lysosomal fusion occurred later in digestive vacuoles containing living algae than in those containing boiled algae or latex spheres. This phenomenon was observed when the living algae were maintained under a constant light condition. These results suggest that the algae release some unknown factor in response to light exposure, and the factor may be associated with the alteration of the host digestive process, indicating that the living algae can influence the host digestive processes during early algal infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Endosymbiosis between the ciliate Paramecium bursaria and Chlorella spp. is a mutualistic relationship (Kodama and Fujishima 2010; Reisser 1986). The host paramecia supply the symbiotic algae with nitrogen components and CO2 (Albers et al. 1982; Albers and Wiessner 1985; Reisser 1976a; Reisser 1976b). In addition, the symbiotic algae are protected from infection by the Chlorella virus (Kawakami and Kawakami 1978; Reisser et al. 1988; Van Etten et al. 1991; Yamada et al. 2006), and the algal carbon fixation is enhanced (Kamako and Imamura 2006; Kato and Imamura 2009) inside the host cells. In return, the algae supply the host with photosynthetic products, particularly maltose and oxygen (Brown and Nielsen 1974; Reisser 1976a; Reisser 1976b; Reisser 1980; Reisser 1986).
Despite this mutually beneficial relationship, both P. bursaria and the symbiotic algae have retained the ability to grow without a partner. However, endosymbiosis is easily induced by mixing alga-free P. bursaria cells with isolated symbiotic algae (Karakashian 1975; Kodama and Fujishima 2005; Siegel and Karakashian 1959). Therefore, the symbiotic association between these species provides an excellent model for studying cell-to-cell interactions and the evolution of eukaryotic cells through secondary endosymbiosis.
Details of the algal infection process have recently been elucidated (Kodama 2013; Kodama and Fujishima 2005; Kodama and Fujishima 2007; Kodama and Fujishima 2009a; Kodama and Fujishima 2009b; Kodama and Fujishima 2010; Kodama and Fujishima 2011; Kodama and Fujishima 2012a; Kodama and Fujishima 2012b). Shortly after being mixed with alga-free P. bursaria cells, one or several green algae pass through the host cytopharynx and are pinched off and the vacuole comes into digestive vacuole (DV)-I. Acidified and condensed DV-II appears 0.5–1 min after mixing as a result of acidosomal fusion, with a concurrent reduction in the intravacuolar pH to 2.4–3.0. Swollen DV-III appears at 2–3 min as a result of lysosomal fusion, which is accompanied by an increase in the intravacuolar pH to ≥6.5, and partially digested yellow algae also appear in DV-III. Condensed DV-IV appears at 20–30 min, at which stage the digested algae are become brown in color and have an extremely small diameter because of their digestion. A single green Chlorella sp. (SGC) appears as a result of the budding of the DV-IV membrane at 30 min. Then, SGCs start to localize beneath the host cell cortex. The localized alga finally starts to undergo cell division, after which it can establish endosymbiosis with the alga-free cells.
During the algal infection process, the first hurdle for the algae is the acquisition of resistance to the host’s lysosomal enzymes in DV-III (Kodama and Fujishima 2005). It is known that the majority of algae are hardly digested if ingested into a large DV (Karakashian 1975; Kodama et al. 2007). Karakashian and Karakashian (1973) found that the digestion of dead boiled algae is delayed when they are enclosed in the same DV as live algae, which is a clear indication that live algae can influence the host’s digestive processes. This influence may be due to the prevention or delay of acidification and lysosomal fusion with the DVs. Recently, we found that when symbiotic algae isolated from algae-bearing paramecia are maintained under constant-dark (DD) conditions for 24 h before being mixed with alga-free paramecia, almost all of the algae are digested in the host DVs (Kodama and Fujishima 2014). This finding suggests that some unknown factor produced in response to light is a prerequisite for algal resistance to the host’s lysosomal enzymes (Kodama and Fujishima 2014). However, the exact stage of the host DV differentiation or algal infection that influences this was not clear because the detailed processes were unknown at that time. Therefore, in this study, we aimed to clarify the effects of live algae on the DV differentiation process or the algal infection process using the following methods: 1) lysosomal fusion with the DVs was detected using Gomori’s staining method (Gomori 1952); 2) the pH inside the DVs was determined using LysoSensor Yellow/Blue DND-160 (LysoSensor); 3) to manipulate DV size, we used different concentrations of living symbiotic algae; and 4) to test the sensitivity of the process to the contents within the DV, we added different types of inocula, i.e., latex beads, boiled symbiotic algae, and algal cells grown under different light environments to the alga-free P. bursaria cells.
2 Materials and methods
2.1 Strains and cultures
The alga-free P. bursaria strain Yad1w was produced from cells of the Chlorella sp.-bearing P. bursaria strain Yad1g, as described in our previous papers (Kodama and Fujishima 2009a; Kodama and Fujishima 2009b). The Yad1g1N strain was produced by infecting cloned symbiotic Chlorella variabilis strain 1 N cells with the Yad1w cells (Kodama and Fujishima 2011). Symbiotic algae were isolated from the Yad1g1N cells. The culture medium was red pea (Pisum sativum) extract culture medium (Tsukii et al. 1995) in modified Dryl’s solution (Dryl 1959) (KH2PO4 was used instead of NaH2PO4·2H2O), which was inoculated with a non-pathogenic strain of Klebsiella pneumoniae 1 day before use (Fujishima et al. 1990). In ordinary cultures, several hundred cells were inoculated into 2 ml of culture medium, and then 2 ml of fresh culture medium were added on each of the next 12 days. One day after the final feeding, the cultures were in the early stationary phase of growth, and all cells used in this study were of this phase. Cultivation was performed at 25 ± 1 °C. Algae-bearing cells were cultured under fluorescent lighting (20–30 μmol photons m−2 s−1) using an incandescent lamp. The Paramecium strains used in this study were provided by Yamaguchi University, Japan, with support in part from the National Bio-Resource Project of the Japan Agency for Medical Research and Development.
2.2 Pulse labeling and chasing with symbiotic algae
Symbiotic algae were isolated from algae-bearing P. bursaria using previously described methods (Kodama and Fujishima 2005; Kodama et al. 2007). To evaluate the relationship between DV diameter and acidification of the DV, we needed to control the number of ingested algae. It has previously been reported that the algal reinfection ratio is roughly proportional to the algal concentration and the exposure time of alga-free P. bursaria cells to the algae (Weis and Ayala 1979). Furthermore, only small DVs were formed when a small quantity of algae was added to the alga-free P. bursaria (Kodama and Fujishima, unpubl. Data). Consequently, we adjusted the ratio of alga-free P. bursaria cells relative to the algae in a stepwise manner (alga-free cells:algae =1:10, 1:100, 1:1000, 1:10,000). Cell density was adjusted using a hemocytometer. In some experiments, the isolated algae were incubated under constant-light (LL) or DD conditions for 24 h at 25 ± 1 °C, as outlined previously (Kodama and Fujishima 2014). In addition, some algae were boiled for 10 min, as described previously (Kodama and Fujishima 2005). The alga-free P. bursaria cells were mixed with the treated symbiotic algae at densities of 5000 paramecia/ml and 5 × 104–5 × 107 algae/ml for 1.5 min, washed, and chased, as outlined previously (Kodama and Fujishima 2005; Kodama and Fujishima 2007; Kodama et al. 2007). In this study, the algal reinfection ratio was defined as the ratio of P. bursaria cells containing SGCs beneath the host cell cortex 24 h after algal mixing, as outlined in our previous study (Kodama and Fujishima 2005). The cells were observed under a differential-interference contrast (DIC) and fluorescence microscope (BX51; Olympus Corp.). Part of the cell image was captured digitally using an Olympus DP73 camera system (Olympus Corp.). Within 15 min after mixing with the algae, the diameter of the alga(e)-containing DVs was measured using ImageJ software (NIH).
2.3 Gomori’s staining of the pulse-labeled P. bursaria cells
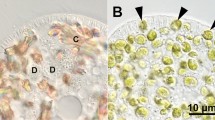
To detect lysosomal acid phosphatase (AcPase) activity, a 500-μl aliquot of the cell suspension was fixed 3 min after mixing it with the algae by adding an equal volume of 4.0 % glutaraldehyde in 0.2 M cacodylate buffer containing 16.0 % sucrose (pH 7.2) for 30 min at 4 °C. The fixed cells were washed three times with the cacodylate buffer, after which the cells were incubated in 1 ml of Gomori’s staining solution (Gomori 1952). AcPase activity was detected under a DIC microscope, as described previously (Kodama and Fujishima 2008; Kodama and Fujishima 2009b). AcPase activity indicated by a black precipitate in DVs demonstrates that lysosomal fusion to the DVs has occurred. In this study, DVs were classified into three different types according to the localization of their AcPase activity, based on our previous study (Kodama and Fujishima 2009b), as follows: AcPase-negative DVs (Fig. 1a-D and d-D, arrow); DVs with a partial AcPase-positive area near the DV membrane (Fig. 1b-D and e-D); and entirely AcPase-positive black DVs (Fig. 1c-D and f-D).
Photomicrographs of alga-free Paramecium cells that have ingested live or boiled algae. Three minutes after mixing with live (a–c) or boiled (d–f) algae, acid phosphatase (AcPase) activity was detected using Gomori’s staining. In both cases, all three types of digestive vacuole (DV)s classified according to the localization of their AcPase activity were observed: AcPase-negative DVs (a-D and d-D, arrow); DVs with a partial AcPase-positive area near the DV membrane (b-D and e-D); and entirely AcPase-positive black DVs (c-D and f-D). D shows a DV containing algae. The reproducibility of these results was confirmed eight times. Bar, 20 μm
2.4 LysoSensor yellow/blue DND-160 staining of the pulse-labeled P. bursaria cells
To measure the pH inside the DVs, a 500-μl aliquot of the cell suspension was stained with LysoSensor Yellow/Blue DND-160 (LysoSensor, Molecular Probes Inc.) for 10 min under DD conditions at various times after mixing with algae, as described in our previous study (Kodama and Fujishima 2013). Within 15 min after mixing, the cells were observed under a fluorescence microscope (BX51; Olympus Corp.) to determine the pH inside the DVs.
2.5 Pulse labeling and chasing with latex spheres
The alga-free P. bursaria cells were mixed with polystyrene latex spheres (Difco; diameter, 3.00 μm) at densities of 5000 paramecia/ml and 5 × 107 latex spheres/ml for 1.5 min, washed, and chased, as outlined previously (Kodama and Fujishima 2005; Kodama and Fujishima 2007; Kodama et al. 2007). The cells were stained using LysoSensor for 10 min under the DD condition and observed under a fluorescence microscope as shown above.
3 Results and discussion
3.1 AcPase activity of DVs containing live or boiled algae
Three minutes after mixing with live or boiled algae, all three types of DVs were observed in alga-free Paramecium cells (Fig. 1). When live algae were ingested by the Paramecium cells, few AcPase-positive DVs were observed, whereas when boiled algae were added to the Paramecium cells, few AcPase-negative DVs were observed, with nearly all DVs exhibiting AcPase activity. The percentage of each type of DV that was detected 3 min after mixing with live or boiled algae is shown in Fig. 2. When live algae were ingested, approximately 70 % of DVs were AcPase-negative, as shown in Fig. 1a-D, and approximately 10 % of DVs displayed AcPase activity, as shown in Fig. 1c-D. By contrast, when boiled algae were ingested, the percentage of AcPase-negative DVs was approximately 25 %, with nearly all DVs exhibiting either partial AcPase activity (approximately 55 %) or full AcPase activity (approximately 25 %). Our previous study revealed that even when live algae were ingested, the majority of DVs became AcPase-positive within 30 min of mixing (Kodama and Fujishima 2009b). The results from the present study indicate that AcPase activity appears earlier in DVs that contain boiled algae than in those that contain live algae. There are two possible explanations for this finding: either lysosomal fusion occurs earlier in DVs containing boiled algae, or live algae suppress AcPase activity 3 min after mixing with the algae. We also found no correlation between the diameter of DVs and the intensity of AcPase activity (data not shown).
Percentage of each type of DV 3 min after mixing Paramecium bursaria with live or boiled algae. Note that most of the digestive vacuole (DV)s containing live algae exhibited no acid phosphatase (AcPase) activity, as shown in Fig. 1 a-D, whereas the majority of DVs containing boiled algae displayed AcPase activity, as shown in Fig. 1 e-D and f-D. Bar, 90 % confidence limit. The reproducibility of these results was confirmed five times. For each experiment, more than 150 cells were counted. A statistically significant difference was found between live algae and boiled algae in three types of DVs (p < 0.01 by Fisher’s exact test)
3.2 pH inside DVs containing live or boiled algae and latex spheres
Fok et al. (1987) demonstrated that the use of ionophores, weak bases, and cytochalasin B inhibited acidosome-DV fusion or reduced both the acidification rate and the pH of the DVs, which in turn inhibited lysosome-DV fusion (Fok et al. 1987). These results revealed that acidosomal fusion is needed for lysosomes to fuse with the DVs. Therefore, to confirm whether acidosomal fusion had occurred, the pH of DVs containing live algae, boiled algae, or latex spheres was measured before lysosomal fusion to the DVs using LysoSensor. In live cells, LysoSensor accumulates in acidic organelles or components and exhibits yellow fluorescence, whereas it displays blue fluorescence in less acidic conditions.
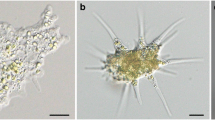
One hour after mixing alga-free P. bursaria with live algae, boiled algae, or latex spheres, the cells were stained with LysoSensor for 10 min and then observed within 10 min with no fixation (Fig. 3). When live algae were added to the alga-free P. bursaria, some cells formed large DVs, as shown in Fig. 3a (arrowhead). No yellow or blue fluorescence was observed in such large DVs, as only red autofluorescence was noted in the algal chloroplast. By contrast, the small DVs (Fig. 3a, arrow) displayed yellow fluorescence. These results suggest that acidification of the small DVs, which contained only a few algae, occurred earlier than that of the large DVs, which contained more than 10 algae. When boiled algae were added to the alga-free P. bursaria, large DVs also exhibited yellow fluorescence (Fig. 3b, arrow). When latex spheres were added, large DVs were rarely formed for some unknown reason; however, the small DVs (Fig. 3c, arrow) displayed yellow fluorescence. Although latex spheres are excreted without digestion, this result illustrates that acidosomal fusion to the DVs containing latex spheres occurred normally. Indeed, Karakashian (1975) previously revealed that AcPase activity appears in DVs containing bacteria or inert particles such as carmine, Celkate®, or latex spheres (Karakashian 1975).
Fluorescence photomicrographs of LysoSensor-treated alga-free Paramecium bursaria cells. Alga-free P. bursaria cells were mixed with living algae (a), boiled algae (b), or latex spheres (c) for 1.5 min. One hour after mixing, the cells were stained with LysoSensor for 10 min and observed under a fluorescence microscope. A large digestive vacuole (DV) containing many living algae exhibited no fluorescence (a, arrowhead), whereas a small DV containing a few algae displayed yellow fluorescence (a, arrow). By contrast, DVs containing boiled algae (b, arrow) or latex spheres (c, arrow) exhibited yellow fluorescence irrespective of their diameter. Ma, macronucleus. The reproducibility of these results was confirmed three times. More than 50 cells and 200 DVs were observed in each experiment
3.3 Algal reinfection ratio at varying cell densities
As shown in Fig. 3a (arrowhead), no yellow or blue fluorescence was observed in large DVs. By contrast, the small DVs (Fig. 3a, arrow) exhibited yellow fluorescence. These observations suggest that there may be some relationship between the diameter of DVs and their pH. To evaluate the relationship between DV diameter and acidification, we added symbiotic algae in a stepwise manner to alga-free P. bursaria. As shown in Fig. 4, when the algae were extremely rare (alga-free cells:algae =1:10), the algal reinfection ratio was 0 %; in addition, only alga(e)-containing DVs with extremely small diameters were formed, and some paramecia did not have any DVs (data not shown). When the algae were rare (alga-free cells:algae =1:100), the algal reinfection ratio was approximately 5 %, and alga(e)-containing DVs with both large and small diameters were observed (data not shown). By contrast, when the algae were abundant (alga-free cells:algae =1:1000 or 1:10,000), the algal reinfection ratio was approximately 50 % or 80 %, respectively, and only DVs with large diameters that contained many green algae were observed (data not shown). Consequently, we adjusted the ratio of alga-free P. bursaria cells and algae to a ratio of 1:100 for observation of alga(e)-containing DVs with both small and large diameters during the algal reinfection process.
The relationship between the algal reinfection ratio and the ratio of alga-free cells to algae. Alga-free Paramecium bursaria cells were mixed with the living algae at the densities of 5000 paramecia/ml and 5 × 104–5 × 107 algae/ml for 1.5 min, washed, and chased. The algal reinfection ratio was examined 24 h after the algal mixing by observing the percentage of cells with single green Chlorella sp. beneath the cell cortex. The algal reinfection ratio was roughly proportional to the concentration of alga-free P. bursaria cells to algae, as shown in a preliminary study (Weis and Ayala 1979). Error bars represent the standard deviation from the mean (n = 3). More than 500 cells were observed in each experiment
3.4 Relationship between the pH and mean diameter of the DVs
As shown in Fig. 5a, some DVs exhibited yellow fluorescence (arrow), whereas others had no fluorescence (arrowhead). It should also be noted that few large DVs (see Fig. 3a, arrowhead) were observed. The mean diameters and associated fluorescent colors are shown in Fig. 5b. The mean diameter of DVs with yellow fluorescence was 5.2 ± 1.9 μm (n = 249), whereas that of DVs with no fluorescence was 7.7 ± 4.0 μm (n = 64). In a previous study, we observed that all DVs containing living or boiled algae exhibit AcPase activity 30 min after mixing with each alga and with alga-free P. bursaria cells (Kodama and Fujishima 2009b). Accordingly, this result reveals that lysosomes fused to all DVs within 30 min. Fok et al. (1987) examined whether the acidification of the DVs is required for lysosome-DV(s) fusion in Paramecium as noted previously, and they concluded that inhibition of acidosome-DV fusion or a reduction in both the acidification rate and pH of the DVs would inhibit lysosome-DV(s) fusion. Taken together, these results suggest that all DVs underwent acidification during the digestive process. Our results indicate that acidification tends to occur earlier in small DVs than in larger DVs. Because acidification of the small DVs was observed even when living algae were ingested, it can also be argued that it is not simply that the acidification of DVs containing boiled algae occurred earlier than that of DVs containing living algae. Therefore, it is possible that the living algae may secrete some inhibitor of DV acidification when large numbers of algae are ingested by one DV membrane.
Relationship between the pH inside the digestive vacuole (DV)s and their diameter. a Fluorescence was observed in DVs when alga-free Paramecium bursaria cells (5000 cells/ml) were added to 5 × 105-cells/ml living algae. Some DVs displayed no fluorescence (arrowhead), whereas others exhibited yellow fluorescence (arrow). b The relationship between the fluorescent colors inside DVs and their mean diameter. Note that small DVs displayed yellow fluorescence, whereas large DVs exhibited no fluorescence. Ma, macronucleus. The reproducibility of these results was confirmed three times
3.5 pH inside DVs containing LL- or DD-incubated algae
How can the living algae influence acidification of the host’s DVs? Our previous study suggested that symbiotic algae that are incubated under DD conditions for 24 h lose the ability to avoid digestion by the host’s lysosomal enzymes inside the DVs (Kodama and Fujishima 2014). Therefore, in the present study, the isolated symbiotic algae were incubated under LL or DD conditions and then mixed with alga-free P. bursaria cells. Immediately after algal mixing, fluorescence of the LysoSensor was observed in the DVs containing LL- or DD-incubated algae (Fig. 6). Because the ratio of alga-free P. bursaria cells and LL- or DD-incubated algae in this experiment was 1:100, the diameter of the most of the DVs containing LL- or DD-incubated algae were small and they showed yellow fluorescence as shown by Fig. 6, arrows. When LL-incubated algae were ingested, we observed more than 200 DVs and about 80 % of them showed yellow fluorescence (arrow, Fig. 6). About 20 % of the DVs showed no fluorescence (arrowhead, Fig. 6a). However, when DD-incubated algae were ingested, we observed more than 200 DVs and all of them displayed yellow fluorescence (arrow, Fig. 6b). This result suggests that some unknown factor that is produced in response to light may be influence acidification of the host’s DVs.
Fluorescence of the digestive vacuole (DV)s of alga-free Paramecium bursaria cells after mixing with algae that were incubated under constant-light (LL) (a) or constant-dark (DD) (b) conditions for 24 h. As shown in (a), red (no LysoSensor fluorescence, arrowhead) or yellow (arrow) fluorescence was observed inside the DVs when the alga-free P. bursaria cells ingested LL-incubated algae. By contrast, all of the DVs exhibited yellow fluorescence (arrow) when the alga-free P. bursaria cells ingested DD-incubated algae. Ma, macronucleus. The reproducibility of this result was confirmed twice. For each experiment, more than 50 cells and 200 DVs were observed
3.6 Summary
The results from this study and our previous studies are summarized in Fig. 7. We found that when boiled algae or latex spheres were ingested by alga-free P. bursaria cells, acidification of the DVs occurred irrespective of their diameter. As explained in the Introduction, the appearance of SGCs from the DV membrane is an indispensable step in the establishment of endosymbiosis between alga-free P. bursaria and isolated living symbiotic algal cells. To date, we have clarified that budding from the DV membranes is also induced by boiled algae or 3.00-μm-diameter latex spheres (Kodama and Fujishima 2005; Kodama and Fujishima 2009a; Kodama and Fujishima 2009b; Kodama and Fujishima 2012b). Late DVs that contained single boiled or single latex spheres were observed 30 min after mixing with alga-free P. bursaria cells (Kodama and Fujishima 2005; Kodama and Fujishima 2012b). Boiled algae inside DV are digested, and their undigested cell walls are excreted by the host cytoproct. Conversely, latex spheres inside DV are excreted without digestion (data not shown). Such DVs showed yellow fluorescence (Fig. 7III and IV). By contrast, when living algae were ingested, acidification occurred inside the DVs, and this occurred earlier inside small DVs than large DVs. DVs containing both SGCs and single digested Chlorella sp. (SDC)s were observed 30 min after mixing living algae with alga-free P. bursaria cells, as shown in our previous study (Kodama and Fujishima 2005). Yellow fluorescence was only observed inside DVs containing SDCs (Fig. 7II); however, DVs containing SGCs that were localized beneath the host cell cortex only exhibited algal chloroplastic red fluorescence, as shown in Fig. 8, arrowhead and Fig. 7I. The perialgal vacuole (PV) membrane differentiates from the DV membrane soon after the alga appears from the DV membrane (Kodama and Fujishima 2009b). The PV membrane differs from the DV membrane in the following manners: (1) the PV membrane encloses only a single alga (Gu et al. 2002; Karakashian and Rudzinska 1981); (2) the gap separating the algal cell wall and the PV membrane is approximately 0.05 μm, and thus, the PV membrane can be observed using a transmission electron microscope (Reisser 1986); (3) the PV diameter does not vary greatly (2.5–4.5 μm), except during algal cell division (Reisser 1992); (4) the PV does not participate in cyclosis, but rather, it localizes beneath the host cell cortex (Kodama and Fujishima 2005; Reisser 1986); and (5) the density and distribution of particles in the PV membrane do not indicate any endocytotic or exocytotic activity, which can be observed in the DV membrane (Meier et al. 1984). Because LysoSensor fluorescence was only observed inside the DV membrane (Fig. 8, arrow) and not inside the PV membrane (Fig. 8 arrowhead), the timing of the disappearance of this fluorescence should be considered an indicator of the timing of differentiation of the PV membrane from the DV membrane. The findings shown in Fig. 6 indicate that it is possible that some photosynthetic product(s) produced by the algae may be involved in the alteration of the DV differentiation process. Further study is required to identify the exact nature of the material that controls the differentiation of the host’s DV.
Summary of the findings of this study and our previous studies. When living algae, boiled algae, and latex spheres were added to the alga-free Paramecium bursaria cells, digestive vacuole (DV)s of varying diameter were formed. Only DVs that contained many living algae exhibited delayed acidification. Note that the vacuoles containing one living alga that were observed 30 min after mixing with alga-free P. bursaria cells (late DV) displayed no yellow fluorescence (I)
Fluorescence photomicrographs of LysoSensor-stained alga-free Paramecium bursaria 2.5 h after mixing with algae. Alga-free P. bursaria cells and symbiotic algae were mixed, washed, and observed 2.5 h after mixing by staining with LysoSensor. Arrowhead shows single green alga localized beneath the host cell cortex. These algae started to increase in number via cell division and established endosymbiosis. Yellow fluorescence was observed only in the digestive vacuoles (arrows) and was not observed in the perialgal vacuoles (arrowhead). The reproducibility of this result was confirmed more than 5 times. For each experiment, more than 20 cells were observed
4 Conclusions
We examined whether the symbiotic Chlorella sp. of the ciliate Paramecium bursaria can influence the host digestion process. Acidification and lysosomal fusion occurred later in DVs that contained living algae than in those containing boiled algae or latex spheres. These results suggest that some unknown factor in the algae that is produced in response to light may be associated with alteration of the host digestive process and indicate that the living algae can influence the host digestive processes during the early stage of algal infection.
References
Albers D, Wiessner W (1985) Nitrogen nutrition of endosymbiotic Chlorella Spec. Endocytobio Cell Res 2:55–64
Albers D, Reisser W, Wiessner W (1982) Studies of the nitrogen supply of endosymbiotic chlorellae in green Paramecium bursaria. Plant Sci Lett 25:85–90
Brown JA, Nielsen PJ (1974) Transfer of photosynthetically produced carbohydrate from endosymbiotic Chlorellae to Paramecium bursaria. J Protozool 21:569–570
Dryl S (1959) Antigenic transformation in Paramecium aurelia after homologous antiserum treatment during autogamy and conjugation. J Protozool 6:25
Fok AK, Ueno MS, Azada EA, Allen RD (1987) Phagosomal acidification in Paramecium: effects on lysosomal fusion. Eur J Cell Biol 43:412–420
Fujishima M, Nagahara K, Kojima Y (1990) Changes in morphology, buoyant density and protein composition in differentiation from the reproductive short form to the infectious long form of Holospora obtusa, a macronucleus-specific symbiont of the ciliate Paramecium caudatum. Zool Sci 7:849–860
Gomori G (1952) Microscopic Histochemistry. University of Chicago Press, Chicago, Principles and Practice
Gu F, Chen L, Ni B, Zhang X (2002) A comparative study on the electron microscopic enzymo-cytochemistry of Paramecium bursaria from light and dark cultures. Europ J Protistol 38:267–278. doi:10.1078/0932-4739-00875
Kamako S, Imamura N (2006) Effect of Japanese Paramecium bursaria extract on photosynthetic carbon fixation of symbiotic algae. J Eukaryot Microbiol 53:136–141. doi:10.1111/j.1550-7408.2005.00084.x
Karakashian MW (1975) Symbiosis in Paramecium bursaria. Symp Soc Exp Biol 29:145–173
Karakashian MW, Karakashian SJ (1973) Intracellular digestion and symbiosis in Paramecium bursaria. Exp Cell Res 81:111–119
Karakashian SJ, Rudzinska MA (1981) Inhibition of lysosomal fusion with symbiont-containing vacuoles in Paramecium bursaria. Exp Cell Res 131:387–393
Kato Y, Imamura N (2009) Metabolic control between the symbiotic Chlorella and the host Paramecium. In: Fujishima M (ed) Endosymbionts in Paramecium, Microbiology Monographs, vol 12. Springer, Berlin Heidelberg, pp. 57–82. doi:10.1007/978-3-540-92677-1_3
Kawakami H, Kawakami N (1978) Behavior of a virus in a symbiotic system, Paramecium bursaria—zoochlorella. J Protozool 25:217–225
Kodama Y (2013) Localization of attachment area of the symbiotic Chlorella variabilis of the ciliate Paramecium bursaria during the algal removal and reinfection. Symbiosis 60:25–36. doi:10.1007/s13199-013-0233-3
Kodama Y, Fujishima M (2005) Symbiotic Chlorella sp. of the ciliate Paramecium bursaria Do not prevent acidification and lysosomal fusion of host digestive vacuoles during infection. Protoplasma 225:191–203. doi:10.1007/s00709-005-0087-5
Kodama Y, Fujishima M (2007) Infectivity of Chlorella species for the ciliate Paramecium bursaria is not based on sugar residues of their cell wall components, but on their ability to localize beneath the host cell membrane after escaping from the host digestive vacuole in the early infection process. Protoplasma 231:55–63. doi:10.1007/s00709-006-0241-8
Kodama Y, Fujishima M (2008) Cycloheximide induces synchronous swelling of perialgal vacuoles enclosing symbiotic Chlorella vulgaris and digestion of the algae in the ciliate Paramecium bursaria. Protist 159:483–494. doi:10.1016/j.protis.2008.02.005
Kodama Y, Fujishima M (2009a) Localization of perialgal vacuoles beneath the host cell surface is not a prerequisite phenomenon for protection from the host’s lysosomal fusion in the ciliate Paramecium bursaria. Protist 160:319–329. doi:10.1016/j.protis.2008.11.003
Kodama Y, Fujishima M (2009b) Timing of perialgal vacuole membrane differentiation from digestive vacuole membrane in infection of symbiotic algae Chlorella vulgaris of the ciliate Paramecium bursaria. Protist 160:65–74. doi:10.1016/j.protis.2008.06.001
Kodama Y, Fujishima M (2010) Secondary symbiosis between Paramecium and Chlorella cells. In: Jeon KW (ed) Int Rev Cell Mol Biol, vol 279. Elsevier Inc., pp 33–77. doi:10.1016/s1937-6448(10)79002-x
Kodama Y, Fujishima M (2011) Endosymbiosis of Chlorella species to the ciliate Paramecium bursaria alters the distribution of the host's trichocysts beneath the host cell cortex. Protoplasma 248:325–337. doi:10.1007/s00709-010-0175-z
Kodama Y, Fujishima M (2012a) Cell division and density of symbiotic Chlorella variabilis of the ciliate Paramecium bursaria is controlled by the hosts nutritional conditions during early infection process. Environ Microbiol 14:2800–2811. doi:10.1111/j.1462-2920.2012.02793.x
Kodama Y, Fujishima M (2012b) Characteristics of the digestive vacuole membrane of the alga-bearing ciliate Paramecium bursaria. Protist 163:658–670. doi:10.1016/j.protis.2011.10.004
Kodama Y, Fujishima M (2013) Synchronous induction of detachment and reattachment of symbiotic Chlorella spp. from the cell cortex of the host Paramecium bursaria. Protist 164:660–672. doi:10.1016/j.protis.2013.07.001
Kodama Y, Fujishima M (2014) Symbiotic Chlorella variabilis incubated under constant dark conditions for 24 hours loses the ability to avoid digestion by host lysosomal enzymes in digestive vacuoles of host ciliate Paramecium bursaria. FEMS Microbiol Ecol 90:946–955. doi:10.1111/1574-6941.12448
Kodama Y, Nakahara M, Fujishima M (2007) Symbiotic alga Chlorella vulgaris of the ciliate Paramecium bursaria shows temporary resistance to host lysosomal enzymes during the early infection process. Protoplasma 230:61–67. doi:10.1007/s00709-006-0193-z
Meier R, Lefort-Tran M, Pouphile M, Reisser W, Wiessner W (1984) Comparative freeze-fracture study of perialgal and digestive vacuoles in Paramecium bursaria. J Cell Sci 71:121–140
Reisser W (1976a) The metabolic interactions between Paramecium bursaria Ehrbg. And Chlorella Spec. In the Paramecium bursaria-symbiosis. II. Symbiosis-specific properties of the physiology and the cytology of the symbiotic unit and their regulation (author's transl). Arch Microbiol 111:161–170
Reisser W (1976b) The metabolic interactions between Paramecium bursaria Ehrbg. And Chlorella Spec. In the Paramecium bursaria-symbiosis. I. The nitrogen and the carbon metabolism. Arch Microbiol 107:357–360
Reisser W (1980) The metabolic interactions between Paramecium bursaria Ehrbg. And Chlorella Spec. In the Paramecium bursaria-symbiosis. III. The influence of different CO2-concentrations and of glucose on the photosynthetic and respiratory capacity of the symbiotic unit. Arch Microbiol 125:291–293. doi:10.1007/BF00446890
Reisser W (1986) Endosymbiotic associations of freshwater protozoa and algae. In: Corliss JO, Patterson DJ (eds) Progress in Protistology, vol 1. Biopress Ltd., Bristol, pp. 195–214
Reisser W (1992) Endosymbiotic associations of algae with freshwater protozoa and invertebrates. In: Reisser W (ed) Algae and symbioses: plants, animals, fungi, viruses, interactions explored, vol 1.1. Biopress, Bristol, pp. 1–19
Reisser W, Burbank DE, Meints SM, Meints RH, Becker B, Van Etten JL (1988) A comparison of viruses infecting two different Chlorella-like green algae. Virology 167:143–149
Siegel R, Karakashian SJ (1959) Dissociation and restoration of endocellular symbiosis in Paramecium bursaria. Anat Rec 134:639
Tsukii Y, Harumoto T, Yazaki K (1995) Evidence for a viral Macronuclear Endosymbiont in Paramecium caudatum. J Euk Microbiol 42:109–115. doi:10.1111/j.1550-7408.1995.tb01550.x
Van Etten JL, Lane LC, Meints RH (1991) Viruses and viruslike particles of eukaryotic algae. Microbiol Rev 55:586–620
Weis DS, Ayala A (1979) Effect of exposure period and algal concentration on the frequency of infection of aposymbiotic ciliates by symbiotic algae from Paramecium bursaria. J Protozool 26:245–248
Yamada T, Onimatsu H, Van Etten JL (2006) Chlorella Viruses. In: Karl M, Aaron JS (eds) Adv. Virus Res., vol Volume 66. Academic Press, pp 293–336. doi:10.1016/S0065-3527(06)66006-5
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (B) [Number 26840119] from the Japan Society for the Promotion of Science to Y. Kodama. The authors thank the Faculty of Life and Environmental Sciences in Shimane University for financial support for publishing this report and Enago (www.enago.jp) for the English language review.
Author contributions
Conceived and designed the experiments: YK. Performed the experiments: YK MN AT. Wrote the paper: YK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yuuki Kodama, Miyuki Nagase and Akane Takahama declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kodama, Y., Nagase, M. & Takahama, A. Symbiotic Chlorella variabilis strain, 1 N, can influence the digestive process in the host Paramecium bursaria during early infection. Symbiosis 71, 47–55 (2017). https://doi.org/10.1007/s13199-016-0411-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-016-0411-1