Abstract
Ingazeira (Inga vera Willd.), a plant native to Brazil is commonly used by Brazilians for its medicinal properties and the value of its wood. Various plants with therapeutic properties and economic importance benefit from mycorrhizal inoculation, which produces larger quantities of therapeutic compounds. However, the effects of mycorrhizal inoculation on ingazeira have not yet been studied. The objective of this paper is to evaluate the effectiveness of arbuscular mycorrhizal fungi (AMF) on the growth of seedlings and production of primary and secondary metabolites, and to determine the total foliar antioxidant activity in ingazeira seedlings. Soil-inoculum was applied to the root region of ingazeira plantlets, which were transplanted into sacs containing 1.2 kg of soil and 10 % vermicompost (100 g vermicompost kg−1 soil). The inoculum consisted of 200 glomerospores per pot of each AMF: Gigaspora albida N.C. Schenck & G.S. Sm. (UFPE 01), Acaulospora longula Spain & N.C. Schenck (UFPE 21), or Claroideoglomus etunicatum (W. N. Becker & Gerd.) C. Walker & A. Schussler (UFPE 06). After 140 days in a greenhouse, growth variables, primary and secondary metabolite content, and total foliar antioxidant activity were determined. AMF optimized the growth and production of secondary metabolites. Mycorrhizal symbiosis can maximize growth and phytochemical production in ingazeira seedlings, thus providing an alternative to the installation of sustainable crops of this leguminous plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Inga vera Willd., which belongs to the Fabaceae family, is a fruiting and medicinal plant (Siqueira-Filho et al. 2009) often found at the margins of the São Francisco river in northeast Brazil. The nutritional value of this plant, as well as its use in apiculture and as combustible fuel is well-known (Rondon-Neto et al. 2010; Ubessi-Macarini et al. 2011); therapeutic cataplasms or compresses from the stem bark are used as astringents (Rodrigues and Carvalho 2001). In the research on foliar extracts from I. vera, antioxidant activity was detected (Vivot et al. 2001). This property was related to the production of bioactive substances, especially phenolic compounds, such as ellagic acid, gallic acid, and tannins (Vivot et al. 2001).
Studies on I. vera have demonstrated its importance for the economic development of the Brazilian semi-arid region because the species is being used for multiple purposes, especially in the wood and phytotherapeutic industries (Rodrigues and Carvalho 2001; Rondon-Neto et al. 2010; Ubessi-Macarini et al. 2011). However, overharvesting has contributed to the disappearance of the species (Silva et al. 2012). Therefore, it is necessary to use technology that favors the production of seedlings through the installation of plantations and thereby reduce the pressure of local extractivism.
The application of arbuscular mycorrhizal fungi (AMF) favors plant growth (Cavalcante et al. 2002) and results in plants with a higher concentration of therapeutically important compounds (Manoharan et al. 2010; Ratti et al. 2010; Wu et al. 2011; Zubek et al. 2012). These benefits have been primarily attributed to improved absorption of water and nutrients, which is the result of an extensive mycelial network that increases the radicular absorption zone and volume of explored soil (Smith and Read 2008).
Various medicinal plants benefit from inoculation with AMF, which increases the production of phytochemicals (Krishna et al. 2005; Manoharan et al. 2010; Ratti et al. 2010), and includes species from the Caatinga (Oliveira et al. 2013; Pedone-Bonfim et al. 2013). The symbiosis formed by such microorganisms also optimizes the growth of seedlings of native arboreal plants from the Caatinga biome, such as Prosopis juliflora (sw) DC (Aguiar et al. 2004), Anadenanthera macrocarpa (Benth.) Brenan (Santos et al. 2008; Sugai et al. 2011), and Anadenanthera colubrina (vell.) Brenan (Pedone-Bonfim et al. 2013). However, there is no data available about the effects of AMF on I. vera.
The objectives of this study are to determine the effect of AMF inoculation on the growth of seedlings and production of primary and secondary metabolites, measure the total foliar antioxidant activity of I. vera seedlings after mycorrhizal inoculation, and test the hypothesis that the benefits depends on the type of AMF isolate used for inoculation.
2 Materials and methods
The experiment was carried out in a greenhouse at the University of Pernambuco (UPE) Campus Petrolina. Native soil (Latosoil) was used as a substrate for cultivation, which was collected in an area of the local Caatinga (savanna-like vegetation in Petrolina-PE, Brazil). 10 % vermicompost (100 g vermicompost kg−1 soil) was added to the soil. Analysis of mix, yielded the following results: pH, 5.20 (H2O – 1:2.5); organic matter, 3.21 g kg−1; electric conductivity, 3.53 dS m−1; P, 12.68 mg dm−3; Al, 0.05 cmolc dm−3; Na, 0.49 cmolc dm−3; Ca, 2.70 cmolc dm−3; Mg, 1.80 cmolc dm−3; K, 0.26 cmolc dm−3(EMBRAPA 2011).
2.1 Reagents and standards
The following reagents were used: the Folin-Ciocalteu reagent (Merck®); sodium carbonate, methyl alcohol (99.8 % purity and 790 g L−1 concentration), ethyl alcohol (99.3 % purity and 787 g L−1 concentration), sulfuric acid (98 % purity and 1840 g L−1 concentration), and glacial acetic acid (F. Maia Ltda.); phosphoric acid (85 % purity and 1700 g L−1 concentration), pyridine, aluminum chloride, phenol (99 % purity and 1071 g L−1 concentration), Coomassie blue G-250, glucose, tannic acid, and casein (Vetec Ltda.); Bovine Serum Albumin (BSA), rutin, and 2.2-Diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich®).
2.2 Plant material
Seeds from I. vera were obtained in an area of the Caatinga in Petrolina, Brazil and were disinfected with sodium hypochlorite NaClO (20 mL L−1) for 2 min, washed with distilled water, and germinated in trays containing soil that had previously been sterilized in an autoclave (121 °C for 30 min for 2 consecutive days). The seedlings were considered ready for transplantation when one pair of definite leaves (non-cotyledonary) was observed.
2.3 Arbuscular mycorrhizal fungi
The following AMF isolates were used in the experiment: Gigaspora albida N.C. Schenck & G.S. Sm. (UFPE 01), Claroideoglomus etunicatum (W. N. Becker & Gerd.) C. Walker & A. Schussler (UFPE 06), and Acaulospora longula Spain & N.C. Schenck (UFPE 21). The isolates were harvested from multiplication vases in sterilized soil and organic compost (900 mL L−1) associated with Panicum miliaceum L. (Silva 2006). The cultivated AMF were granted by the Universidade Federal de Pernambuco, Brazil.
2.4 Mycorrhizal inoculation and plant growth conditions
I. vera plantlets were transplanted to bags with a capacity of 1.2 kg of substrate (1080 g soil plus 120 g vermicompost) and received soil-inoculum that consisted of 200 spores, hyphae, and colonized roots from either of the three AMF isolates: G. albida, A. longula, and C. etunicatum. The control did not receive soil-inoculum. Seedlings were kept in a greenhouse for 140 days, under ambient light conditions, with minimum and maximum temperatures of 18 °C and 30 °C, respectively, and a minimum and maximum relative air humidity of 34.14 % and 84.23 %, respectively.
2.5 Experimental design
The experimental design was randomized with four inoculation treatments and five repetitions. The treatments included the following: 1) Non-inoculated control; 2) Inoculated with A. longula; 3) Inoculated with G. albida; 4) Inoculated with C. etunicatum, totaling 20 experimental units.
2.6 Evaluation of plant growth and AMF analysis
140 days after transplantation, the following parameters were evaluated: plant height, number of leaves, stem diameter, levels of chlorophyll a, b, and total chlorophyll, fresh and dry matter of the aerial and subterranean plant parts, and mycorrhizal colonization.
2.6.1 Plant growth
The levels of chlorophyll a and, b and total chlorophyll were determined by using an electronic chlorophyll meter (ClorofiLOG, Falker) and the values were expressed based on the Falker Chlorophyll Index (FCI). After harvesting, the aerial part and radicular system of the plants was separated and weighed on an analytic scale (Bel Engineering, Italy) to determine fresh matter. To determine dry matter of the aerial and radicular parts, the fresh matter was placed in an air circulation oven (BIOPAR, Brazil) at 45 °C until a constant weight was obtained.
2.6.2 Evaluation of the AMF
To determine the amount of mycorrhizal colonization, roots were removed from the soil, washed, and clarified with KOH (100 g L−1) and H2O2 (333 mL L−1), acidified with HCl (27 mL L−1), and stained with Trypan blue in lactoglycerol (0.05 g L−1) (Phillips and Hayman 1970). The colonization was evaluated by means of the intersection of quadrants method (Giovannetti and Mosse 1980).
2.7 Evaluation of primary and secondary metabolites
2.7.1 Preparation of plant extract
500 mg of leaves that were dried in an air circulation oven (45 °C) were punctured and transferred to amber flasks (100 mL) to which 20 mL of ethanol (950 mL L−1) was added. After maceration for 12 days at 25 °C, the extract was filtered with gauze, refiltered with qualitative filter paper, and stored in amber flasks (20 mL) at - 4 °C (Brito et al. 2008). Biomolecule contents was determined by measuring the leaf dry matter.
2.7.2 Primary metabolites
Analysis of soluble carbohydrates and total proteins
Soluble carbohydrates were determined through the Dubois et al. (1956) methodology. The analyzed mixture consisted of 50 μL of extracts, 95 μL of distilled water, and 50 μL of phenol (800 g L−1) placed in test tubes and homogenized in a shaker (Vortex Vision, Korea). Subsequently, 2 mL of sulfuric acid was added and after 10 min, the quantification was carried out using a spectrophotometer (Spectrum, China) at 490 nm. Glucose was used for the preparation of the standard curve.
Total proteins contents in extract samples was determined by measuring the absorbance in a spectrophotometer set at 595 nm according to the methodology proposed by Bradford (1976). The analyte mixture consisted of 50 μL of extracts and 2.5 mL of the Bradford reagent placed in test tubes and homogenized in a Vortex shaker. After allowing to stand for 5 min, the analyte was read in the spectrophotomete. Bovine Serum Albumin was used for the preparation of the standard curve.
2.7.3 Secondary metabolites
Analysis of total phenols, flavonoids, and tannins
Total phenols were quantified by the Folin-Ciocalteu method, in samples of the prepared extract, which consisted of a mixture of 2 mL of extract, 5 mL of Folin-Ciocalteu reagent (100 mL L−1), and 10 mL of sodium carbonate (75 g L−1) held a volumetric flask. The volume was completed with 100 mL of distilled water and the resulting solution was stirred and allowed to stand for 30 min in the dark. After allowing to stand, readings were taken to determine absorbance in a spectrophotometer set at 760 nm. Tannic acid was used for the preparation of the standard curve (Monteiro et al. 2006).
Total flavonoids were determined with samples of the plant extract by reading absorbance levels in a spectrophotometer at 420 nm. Rutin was used for the standard curve. The analyzed mixture consisted of 1 mL of the extract, 0.6 mL of glacial acetic acid, 10 mL of 200 mL L−1 pyridine solution in methanol, and a solution of 2.5 mL with 50 g L−1 aluminum chloride in absolute methanol placed in volumetric flasks. The volume was completed to 25 mL with distilled water and the resulting solution was stirred, allowed to stand for 30 min, and spectrophotometric readings were taken subsequently (Araújo et al. 2008).
Total tannins were quantified by means of the methodology proposed by Monteiro et al. (2006); 6 mL of the plant extract was transferred to amber flasks, followed by the addition of 1 g of casein. The mixture was shaken (160 rpm) for 3 h at 25 °C, filtered with qualitative paper, and the resulting volume from the filtering was completed to 25 mL with distilled water. The remaining phenols were quantified by means of the Folin-Ciocalteu method. A spectrophotometric reading was taken at 760 nm, using tannic acid for the standard curve. The quantity of tannins corresponded to the difference between the values found in this final analysis and those obtained by the determination of total phenols.
2.8 Determination of the total antioxidant activity (TAA)
Total antioxidant activity was determined by means of the DPPH radical scavenging assay (2.2-Diphenyl-1-picrylhydrazyl); in the dark, 0.1 mL of the extract was transferred to test tubes with 3.9 mL of the DPPH radical (0.06 mM) and homogenized in a Vortex shaker. After allowing to stand for 30 min, the absorbance was read in a spectrophotometer (515 nm). The DPPH radical was used for the preparation of the standard curve (Rufino et al. 2007).
2.9 Statistical analysis
The data were subjected to analysis of variance (ANOVA) and the means were compared by the Tukey test (p < 0.05), using the Assistat (2011) software.
3 Results and discussion
The used treatments were found to have an effect on some of the analyzed variables (Tables 1 and 2). Inoculation with AMF increased plant growth, considering the increased fresh and dry matter of the aerial part and the radicular system. I. vera seedlings that were inoculated with A. longula showed an increase of 16 % and an increase of 13 % of fresh matter (FMAP) and dry matter (DMAP) of the aerial parts, respectively, as compared to the control (Table 1). Similar results related to this growth variable have been reported in other studies (Nell et al. 2009; Samarão et al. 2011; Sugai et al. 2011; Coelho et al. 2012; Pedone-Bonfim et al. 2013). In general, mycorrhizal plants absorb more nutrients, especially those with low mobility and produce more biomass (Krishna et al. 2005; Rasouli-Sadaghiani et al. 2010; Ratti et al. 2010).
I. vera seedlings that were inoculated with A. longula had a higher fresh root matter (FRM) weight and dry root matter (DRM) weight by 32 % and 13 % respectively, as compared to the control (Table 1). Similar results have been reported in other studies (Toussaint et al. 2008; Samarão et al. 2011; Sugai et al. 2011; Coelho et al. 2012; Karagiannidis et al. 2012). These results are likely associated with nutritional status, which was supplied by AMF to the host plant (Santos et al. 2008; Smith and Read 2008).
With regard to plant height, stem diameter, number of leaves, and levels of chlorophyll a and, b and total chlorophyll, the inoculation did not result in any benefits (Table 1). Other studies, however, have shown that mycorrhizal symbiosis promotes an increase in plant height (Santos et al. 2008; Sugai et al. 2011), stem diameter (Samarão et al. 2011; Coelho et al. 2012), number of leaves (Cavalcante et al. 2002; Aguiar et al. 2004), and levels of chlorophyll (Manoharan et al. 2010; Rahmaty and Khara 2011).
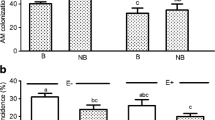
The radicular cortex of I. vera seedlings were colonized more by A. longula and G. albida as compared to the non-inoculated control (Table 2). The highest colonization rate was observed for A. longula and resulted in the highest plant growth (Tables 1 and 2), indicating that the benefit of symbiosis varies with the tested AMF, confirming the initial working hypothesis. Similar colonization results have been reported in other studies (Cavalcante et al. 2002; Karagiannidis et al. 2012).
Inoculation with AMF did not increase production of primary metabolites (Table 2). On the other hand, in other studies, mycorrhizal inoculation has been found to promote production of carbohydrates and proteins (Thamizhiniyan et al. 2009; Ratti et al. 2010; Baslam et al. 2011; Wu et al. 2011).
The arbuscular mycorrhizal symbiosis increased the production of total phenols, flavonoids, and tannins (Table 2), however, increases in these components varied with the AMF isolate used. I. vera seedlings that were inoculated with A. longula showed an increase of 13 %, 22 %, and 13 % for total phenols, flavonoids, and tannins, respectively, as compared to the control treatment (Table 2). Similar results have been reported in other studies (Araim et al. 2009; Lee and Scagel 2009; Ceccarelli et al. 2010; Oliveira et al. 2013; Pedone-Bonfim et al. 2013).
The increase in production of secondary metabolites in the plants that formed symbiosis with A. longula may be related to the increased nutrient supply by AMF to the host plant (Zubek et al. 2012), which stimulated the activity of the metabolic pathways (Lohse et al. 2005; Mandal et al. 2013). The mycorrhizal colonization likely increased activity of phenylalanine ammonia-lyase (PAL, EC 4.3.1.5) and chalcone synthase (CHS, EC 2.3.1.74) enzymes, which are responsible for the biosynthesis of phenolic compounds (Bonanomi et al. 2001; Vermerris and Nicholson 2006).
Various mechanisms are involved with an increased production of secondary metabolites in inoculated plants (Toussaint 2007). The increased nutritional status of the inoculated plants was apparent; however, there were no differences between the levels of chlorophyll after inoculation (Table 1). Therefore, additional research is required in order to clarify the various mechanisms involved.
I. vera seedlings that were inoculated with A. longula showed an increase in the total foliar antioxidant activity (Table 2). The increased antioxidant activity is likely related to the increase of total levels of phenolic compounds (Degáspari and Waszczynskyj 2004), molecules that are known for their antioxidant power. In other studies, the arbuscular mycorrhizal symbiosis has also been found to increase the total antioxidant activity (Ceccarelli et al. 2010; Hernández-Ortega et al. 2012).
The inoculated I. vera seedlings showed an increase in the production of phenolic compounds, which could create an additional market for this species because phenolic compounds are in high demand commercially (Degáspari and Waszczynskyj 2004) for their antioxidant, anti-inflammatory, antifungal, antiviral, and anti-allergic properties (Santos and Mello 2003; Zuanazi and Montanha 2003). The phytomass is therefore more attractive to the phytotherapeutic industry. Furthermore, the obtained benefits do not include phosphate fertilization of the cultivation substrate, which reduces the production cost of the plants. Future studies should include evaluation of the main compounds for High Performance Liquid Chromatography (HPLC) and experiments under field conditions.
4 Conclusions
-
1.
Inoculation with A. longula constitutes a useful biotechnological tool for the production of I. vera seedlings with a large accumulation of phytomass.
-
2.
The production of total phenols, flavonoids, tannins, and antioxidant activity is maximized by the arbuscular mycorrhizal symbiosis formed by A. longula in I. vera seedlings.
-
3.
Mycorrhizal symbiosis can be an alternative biotechnological to increase the production of biomolecules of therapeutic importance in I. vera seedlings, making the phytomass more attractive to the phytotherapeutic industry.
References
Aguiar RLF, Maia LC, Salcedo IH, Sampaio EVSB (2004) Interação entre fungos micorrízicos arbusculares e fósforo no desenvolvimento da algaroba [Prosopis juliflora (Sw) DC]. Rev Arvore 28:589–598
Araim G, Saleem A, Arnason JT, Charest C (2009) Root colonization by an arbuscular mycorrhizal (AM) fungus increases growth and secondary metabolism of purple coneflower, Echinacea purpurea (L.) Moench. J Agric Food Chem 57:2255–2258
Araújo TAS, Alencar NL, Amorim ELC, Albuquerque UP (2008) A new approach to study medicinal plants with tannins and flavonoids contents from de local knowledge. J Ethnopharmacol 120:72–80
Assistat (2011) [Programa de Computador]. Versão 7.6 Beta: Assistência Estatística
Baslam M, Garmendia I, Goicoechea N (2011) Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown Lettuce. J Agric Food Chem 59:5504–5515
Bonanomi A, Oetiker JH, Guggenheim R, Boller T, Wiemken A, Vögeli-Lange R (2001) Arbuscular mycorrhiza in mini-mycorrhizotrons: first contact of Medicago truncatula roots with Glomus intraradices induces chalcone synthase. New Phytol 150:573–582
Bradford MM (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brito HO, Noronha EP, França LM, Brito LMO, Prado MS-A (2008) Análise da composição fitoquímica do extrato etanólico das folhas da Annona squamosa (ATA). Rev Bras Farm 89:180–184
Cavalcante UMT, Maia LC, Melo AMM, Santos VF (2002) Influência da densidade de fungos micorrízicos arbusculares na produção de mudas de maracujazeiro– amarelo. Pesq Agrop Brasileira 37:643–649
Ceccarelli N, Curadi M, Martelloni L, Sbrana C, Picciarelli P, Giovannetti M (2010) Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 335:311–323
Coelho IR, Cavalcante UMT, Campos MAS, Silva FSB (2012) Uso de fungos micorrízicos arbusculares (FMA) na promoção do crescimento de mudas de pinheira (Annona squamosa L., Annonaceae). Acta Bot Bras 26:933–937
Degáspari CH, Waszczynskyj N (2004) Propriedades antioxidantes de compostos fenólicos. Visao Acad 5:33–40
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–355
Empresa Brasileira de Pesquisa Agropecuária – EMBRAPA (2011) Laboratório de análises de solo e de planta. Petrolina, EMBRAPA semiárido
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Hernández-Ortega HA, Alarcón A, Ferrera-Cerrato R, Zavaleta-Mancera HA, López-Delgado HA, Mendoza-López MR (2012) Arbuscular mycorrhizal fungi on growth, nutrient status, and total antioxidant activity of Melilotus albus during phytoremediation of a diesel-contaminated substrate. J Environ Manag 95:S319–S324
Karagiannidis N, Thomidis T, Panou-Filotheou T (2012) Effects of Glomus lamellosum on growth, essential oil productionand nutrients uptake in selected medicinal plants. J Agric Sci 4:137–144
Krishna H, Singh SK, Sharma RR, Khawale RN, Grover M, Patel VB (2005) Biochemical changes in micropropagated grape (Vitis vinifera L.) plantlets due to arbuscular mycorrhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci Hortic 106:554–567
Lee J, Scagel CF (2009) Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem 115:650–656
Lohse S, Schliemann W, Ammer C, Kopka J, Strack D, Fester T (2005) Organization and metabolism of plastids and mitochondria in arbuscular mycorrhizl roots of Medicago truncatula. Plant Physiol 139:329–340
Mandal S, Evelin H, Giri B, Singh VP, Kapoor R (2013) Arbuscular mycorrhiza enhances the production of stevioside and rebaudioside - A in Stevia rebaudiana via nutritional and non-nutritional mechanism. Appl Soil Ecol 72:187–194
Manoharan PT, Shanmugaiah V, Balasubramanian N, Gomathinayagam S, Sharma MP, Muthuchelian K (2010) Influence of AM fungi on the growth and physiological status of Erythrina variegata Linn. grown under different water stress conditions. Eur J Soil Biol 46:151–156
Monteiro JM, Albuquerque UP, Lins Neto EM, Araújo EL, Albuquerque MM, Amorim ELC (2006) The effects of seasonal climate changes in the Caatinga on tannin levels in Myracrodruon urudeuva (Engl.) Fr. All, and Anadenanthera colubrina (Vell.) Brenan. Braz J Pharmacogn 16:338–344
Nell M, Vötsch M, Vierheilig H, Steinkellner S, Zitterl-Eglseer K, Franz C, Novak J (2009) Effect of phosphorus uptake on growth and secondary metabolites of garden sage (Salvia officinalis L.). J Sci Food Agric 89:1090–1096
Oliveira MS, Campos MAS, Albuquerque UP, Silva FSB (2013) Arbuscular mycorrhizal fungi (AMF) affects biomolecules content in Myracrodruon urundeuva seedlings. Ind Crop Prod 50:244–247
Pedone-Bonfim MVL, Lins MA, Coelho IR, Santana AS, Silva FSB, Maia LC (2013) Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrine (Vell.) Brenan) seedlings. J Sci Food Agric 92:1479–1484
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Rahmaty R, Khara J (2011) Effects of vesicular arbuscular mycorrhizal Glomus intraradices on photosynthetic pigments, antioxidant enzymes, lipid peroxidation, and chromium accumulation in maize plants treated with chromium. Turk J Biol 35:51–58
Rasouli-Sadaghiani M, Hassani A, Barin M, Danesh YR, Sefidkon F (2010) Effects of arbuscular mycorrhizal (AM) fungi on growth, essential oil production and nutrients uptake in basil. J Med Plant Res 4:2222–2228
Ratti N, Verma HN, Gautam SP (2010) Effect of Glomus species on physiology and biochemistry of Catharantus roseus. Indian J Microbiol 50:355–360
Rodrigues VEG, Carvalho DA (2001) Levantamento etnobotanico de plantas medicinais no domínio cerrado na região do alto Rio Grande – Minas Gerais. Cienc Agrotecnol 25:2–123
Rondon-Neto RB, Santos JS, Silva MA, Koppe VC (2010) Potencialidades de uso de espécies arbustivas e arbóreas em diferentes fisionomias de cerrado, em Lucas do Rio Verde/MT. Rev Biol Ciênc Terra 10:113–126
Rufino MSM, Alves RE, Brito ES, Morais SM, Sampaio CG, Pérez-Jiménez J, Saura-Calixto FD (2007) Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. Comunicado Técnico 127. Fortaleza, Embrapa Agroindústria Tropical
Samarão SS, Rodrigues LA, Martins AM, Manhães TN, Alvim LAM (2011) Desempenho de mudas de gravioleira inoculadas com fungos micorrízicos arbusculares em solo não-esterilizado, com diferentes doses de fósforo. Acta Sci Agron 33:81–88
Santos SCM, Mello JCP (2003) Taninos. In: Simões CMO, Schenkel EP, Gosmann G, Pallazzo DE, Mello JC, Mentz LA, Petrovick PR (eds) Farmacognosia: da planta ao medicamento, 5th edn. UFRGS, Porto Alegre, pp 615–656
Santos DR, Costa MCS, Miranda JRP, Santos RV (2008) Micorriza e rizóbio no crescimento e nutrição em N e P de mudas de angico-vermelho. Rev Caatinga 21:76–82
Silva FSB (2006) Fase assimbiótica, produção, infectividade e efetividade de fungos micorrízicos arbusculares (FMA) em substratos com adubos orgânicos. Tese de doutorado, Universidade Federal de Pernambuco
Silva LF, Silva ML, Cordeiro AS (2012) Análise do mercado mundial de madeireiras tropicais. Rev Polit Agric 21:48–54
Siqueira-Filho JA, Santos APB, Nascimento MFS, Espírito Santo FS (2009) Guia de Campo de Árvores da Caatinga. Editora e gráfica Franciscana Ltda, Petrolina
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 3rd edn. Academic Press, London
Sugai MAA, Collier LS, Saggin-Júnior OJ (2011) Inoculação micorrízica no crescimento de mudas de angico em solo de cerrado. Bragantia 70:416–423
Thamizhiniyan P, Panneerselvam M, Lenin M (2009) Studies on the growth and biochemical activity of Coleus aromaticus Benth. as influenced by AM fungi and Azospirillum. Recent Res Sci Technol 1:259–263
Toussaint J-P (2007) Investigating physiological changes in the aerial parts of AM plants: what do we where should we be heading? Mycorrhiza 17:349–353
Toussaint J-P, Kraml M, Nell SE, Smith FA, Steinkellner S, Schmiderer C, Vierheilig H, Novak J (2008) Effect of Glomus mosseae on concentration of rosmarinic and caffeic acids and essential oil compounds in basil inoculated with Fusarium oxysporum f. sp. Basilica. Plant Pathol 57:1109–1116
Ubessi-Macarini C, Negrelle RRB, Souza MC (2011) Produtos florestais não-madeiráveis e respectivo potencial de exploração sustentável, associados à remanescente florestal ripário do alto rio Paraná, Brasil. Acta Sci Biol Sci 33:451–462
Vermerris W, Nicholson R (2006) Phenolic compound biochemistry, p. 267
Vivot E, Munoz JD, Cruanes MC, Cruanes MJ, Tapia A, Hirschmann GS, Martínez E, Di Sapio O, Gattuso M, Zacchino S (2001) Inhibitory activity of xanthine-oxidase and superoxide scavenger properties of Inga verna subsp. affinis. Its morphological and micrographic characteristics. J Ethnopharmacol 76:65–71
Wu Q-S, Zou Y-N, He X-H, Luo P (2011) Arbuscular mycorrhizal fungi can alter some root characters and physiological status in Trifoliate orange (Poncirus trifoliate L. Raf.) seedlings. Plant Growth Regul 65:1–3
Zuanazi JAS, Montanha JA (2003) Flavonóides. In: Simões CMO. In: Schenkel EP, Gosmann G, Pallazzo DE, Mello JC, Mentz LA, Petrovick PR (eds) Farmacognosia: da planta ao medicamento, 3rd edn. UFRGS, Porto Alegre, cap.23
Zubek S, Mielcarek S, Turnau K (2012) Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 22:149–156
Acknowledgments
To the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (processo n° 473779/2011-0) for its financial support and to the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for granting a Master’s scholarship to the first author and to the student Hicaro Ribeiro Soares dos Santos for his help in the installation, removal and evaluation of the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lima, C.S., Campos, M.A.d.S. & da Silva, F.S.B. Mycorrhizal Fungi (AMF) increase the content of biomolecules in leaves of Inga vera Willd. seedlings. Symbiosis 65, 117–123 (2015). https://doi.org/10.1007/s13199-015-0325-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-015-0325-3