Abstract
Citrus plants strongly depend on mycorrhizal symbiosis because of less or no root hairs, but few reports have studied if their root traits and physiological status could be altered by different arbuscular mycorrhizal fungi (AMF). In a pot experiment we evaluated the effects of three AMF species, Glomus mosseae, G. versiforme and Paraglomus occultum on the root traits and physiological variables of the trifoliate orange (Poncirus trifoliata L. Raf.) seedlings. Root mycorrhizal colonization was 58–76% after 180 days of inoculation. AMF association significantly increased plant height, stem diameter, leaf number per plant, shoot and root biomass. Mycorrhizal seedlings also had higher total root length, total root projected area, total root surface area and total root volume but thinner root diameter. Among the three AMFs, greater positive effects on aboveground growth generally ranked as G. mosseae > P. occultum > G. versiforme, whilst on root traits as G. mosseae ≈ P. occultum > G. versiforme. Compared to the non-mycorrhizal seedlings, contents of chlorophyll, leaf glucose and sucrose, root soluble protein were significantly increased in the mycorrhizal seedlings. In contrast, root glucose and sucrose, leaf soluble protein, and activity of peroxidase (POD) in both leaves and roots were significantly decreased in the mycorrhizal seedlings. It suggested that the improvement of root traits could be dependent on AMF species and be related to the AMF-induced alteration of carbohydrates and POD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant root is the major underground organ to take up water and nutrients and synthesize some organic compounds, and its functions are affected by biotic and abiotic factors, particularly the activities of soil microorganisms (Osmont et al. 2007; Hodge et al. 2009; Yao et al. 2009; Wu et al. 2010). Arbuscular mycorrhiza (AM), a symbiosis between plant roots and soil AM fungi (AMF), improves the supply of water and nutrients to the host plant and thus the growth of host plants (Smith and Read 2008). As a result, an improvement of root traits by AMF can enhance uptake of water and nutrients in drier soil and then the performance of host plants (Wu et al. 2009, 2010; Miransari 2010).
Recent studies have also shown that mycorrhization affects root longevity, morphology, and structure of host plants (Schellenbaum et al. 1991; Hodge et al. 2009). For example, Schellenbaum et al. (1991) reported that the branches of Vitis vinifera roots were increased after colonized by Glomus fasciculatum. Mycorrhizal colonization promoted the formation of lateral roots of high order, induced more fine roots and less coarse roots (Yao et al. 2009). Root architectural alteration in AMF-colonized strawberry (Fragaria xananassa) or citrus (Citrus tangerine) could increase root functioning to explore more water and nutrients under salt or Phytophthora fragariae stressed conditions (Norman et al. 1996; Wu et al. 2010). Meanwhile, effects of AM colonization on root phosphorus (P) uptake were stronger in juvenile than in adult plants (Padilla and Encina 2005). However, AM colonization negatively correlated with fine-root diameter in both fertile and infertile soils (Zangaro et al. 2007) and did not have significant effects on pea (Pisum sativum) root biomass production under ambient or elevated CO2 concentrations (Gavito et al. 2001). Moreover, it is unclear whether different AMF species vary in their effect on root morphology of the host plants. As a result, further studies on the roles of AMF in root morphology and root functioning are needed.
Mycorrhizal inoculation not only affected root morphology but also physiological status in host plants. The presence of AMF under drought stress could significantly increase the activity of leaf, but not root peroxidase (POD) (Wu et al. 2006), an enzyme relates to plant stress tolerance, the lignification process in root xylem and percentage of rooting (Lepeduš et al. 2004; Metaxas et al. 2004; Jebara et al. 2005). In addition, leaf chlorophyll and soluble protein were higher, but leaf soluble sugar was lower in five AMF inoculated tree seedlings (Cassia siamea, Delonix regia, Erythrina variegata, Samanea saman, and Sterculia foetida) grown under nursery conditions (Manoharan et al. 2008). However, it is unclear if some physiological variables will be also altered to response the mycorrhizal-induced alteration of root traits.
Trifoliate orange (Poncirus trifoliata L. Raf.), a close relative to Citrus, is greatly demanded as the main rootstock for citrus plantation in China. Generally, trifoliate orange has less root hairs and is strongly dependent on AMF in fields. The aims of the present study were to evaluate (1) alterations of root traits after inoculated with three AM fungal species from two genera, and (2) corresponding responses of some physiological variables (carbohydrate, chlorophyll, POD, and soluble protein) to these AMF-induced alterations of root traits.
Materials and methods
Plant growth and mycorrhizal inocula
Seeds of trifoliate orange were surface sterilized for 5 min in 70% ethanol and germinated on moistened filter papers under dark at 25°C. Four seven-day-old seedlings were transplanted to one plastic pot (18 × 12 × 13 cm) containing 3.1 kg of autoclaved growth media (xanthi-udic ferralsols/vermiculite/sphagnum, 5/2/1, v/v/v). Plants were grown under 600–850 μmol/m2/s, 26/18°C (day/night) and 65–95% relative humidity in a non-environmentally controlled plastic greenhouse at the Yangtze University from March 20 to September 27, 2009. The position of the pots was changed every week for eliminating the environmental error.
This experiment was a randomized complete block design with four AM [G. mosseae (BGC XZ02), G. versiforme (BGC NM04B), Paraglomus occultum (BGC BJ04B) or non-AMF control (same amount of autoclaved inocula)] treatments (4 replicates for each treatment). Each AM treatment was regarded as a block. Fifteen g fresh AM inocula were supplied to the growth media (5 cm depth) before transplant. The inocula were commercially supplied by the Beijing Academy of Agriculture and Forestry Sciences and consisted of spores, hyphae, root fragments and cultured sands.
Plant harvest and variable analysis
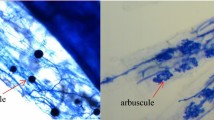
Shoots and roots were harvested, and height, stem diameter, and leaf numbers were measured after 180 days of growth. Root system was scanned with an Epson Expression/STD 4800 Scanner and analyzed with the WinRHIZO software (Regent Instruments Inc., Quebec, Canada). After scanning, the fresh root systems were divided into two parts: one for the determination of POD and soluble protein (see below) and AM mycorrhization (entry points, vesicles and arbuscules) (Phillips and Hayman 1970; Wu et al. 2008); another was oven-dried (75°C, 48 h) and ground (0.5 mm) for glucose and sucrose analysis. Leaves were also ground (0.5 mm) for glucose and sucrose analysis.
Frozen leaf and root samples (0.2 g) were homogenized in 8 ml of 0.1 mol phosphate buffer (pH 7.8, containing 0.1 mmol EDTA, 1 mmol ASC, 1 mmol DTT and 2% PVP) and centrifuged at 4,200g for 10 min, and the resulting supernatant was used for analysis of POD activity and soluble protein. Soluble protein was determined using bovine serum albumin as the standard (Bradford 1976), and POD activity was determined according to the method described by Amako et al. (1994). POD activity was expressed in A470/min/mg protein. Determination of leaf chlorophyll was followed by Lichtenthaler (1987). Extracts for determinations of glucose and sucrose were prepared from 50 mg of samples homogenized with 4 ml 80% alcohol (12.5:1, w/v) at 80°C for 40 min and centrifuged at 2,500g for 5 min. The supernatant was used for glucose and sucrose analysis. Glucose and sucrose contents of leaves and roots were determined with the methods of Zhang and Zai (2004).
Statistical analysis
Data (means ± SE, n = 4) were analyzed by one-way variance (ANOVA) with the SAS 8.1 software and least significant differences (LSD) were compared at P < 0.05.
Results and discussion
Mycorrhizal formation
Neither mycorrhizal colonization nor structures were observed in the non-AMF control seedlings. Among the three mycorrhizal inoculations, root colonization (58–76%) in the 6-month-old trifoliate orange seedlings was significantly highest with G. mosseae, greater with G. versiforme and least with P. occultum (Table 1). In contrast, numbers of arbuscules, vesicles and entry points were similar among the three mycorrhizal inoculations, expect a significant lower vesicle number with the P. occultum treatment. These results suggested that mycorrhizal formation and development in trifoliate orange seedlings might be AMF species dependent.
Plant growth and root morphology
In general, plant height, stem diameter, leaf number and biomass production were significantly highest in both the G. mosseae and P. occultum treatment, greater in the G. versiforme treatment and least in the non-AMF treatment (Table 2). Compared to the non-AMF treatment, plant height, stem diameter, leaf number, shoot, root or total dry weight was significantly respectively increased by 25.6, 22.3, 30.1, 48.0, 77.8 or 55.9% with the inoculation of G. mosseae, 26.8, 15.7, 19.6, 32.0, 44.4 or 35.3% with P. occultum and 11.1, 9.0, 13.3, 12.0, 33.3, or 17.6% with G. versiforme. These results suggested the enhancement of plant growth might also be AMF species dependent.
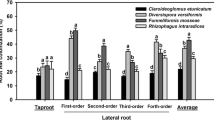
Such enhancement trends in the root morphological traits were also generally true among the four AMF treatments (Table 2). Compared to the non-AMF seedlings, a range of 20.9–42.3% (root total length), 15.9–39.7% (total root projected area), 15.3–39.6% (total surface area), and 14.3–46.4% (total root volume) were respectively higher in the G. mosseae-, G. versiforme- and P. occultum-colonized seedlings. These alterations of root morphological traits were greatest when inoculated with P. occultum, greater with G. mosseae and least with G. versiforme. Indeed, growth and development of plant roots are strongly affected by mycorrhizal fungi (Hodge et al. 2009) and root traits, such as total length, tap length, diameter, branching, volume, surface area and number were altered by AMF colonization (Schellenbaum et al. 1991; Hooker et al. 1992; Atkinson et al. 2003; Gutjahr et al. 2009; Yao et al. 2009; Orfanoudakis et al. 2010). Our results in these root traits of trifoliate orange (Table 2) are consistent with those in grapevine, beach plum and red tangerine (Augín et al. 2004; Zai et al. 2007; Wu et al. 2010) and might indicate that the magnitude of root morphological alteration could be dependent on AMF species. Alteration of root traits may derive from the enhanced root nutrient uptake by AMF and hence contribute to plant growth. In addition, compared to the non-AMF treatment, G. mosseae and G. versiforme inoculations significantly decreased root diameter in trifoliate orange seedlings by 6.0 and 4.3%, respectively (Table 2). This is in agreement that AM root colonization negatively correlated with the fine-root diameter of twelve native woody species in both fertile and infertile soils of southern Brazil (Zangaro et al. 2007).
Physiological variables
Compared to the non-AMF seedlings, the leaf chlorophyll contents of the G. mosseae-, G. versiforme- and P. occultum-colonized seedlings increased by 50.3, 60.0, and 71.0%, respectively (Table 3). Our results in trifoliate orange are consistent with these in Karna Khatta (Citrus karna) and Troyer Citrange (Poncirus trifoliata × C. sinensis) (Murkute et al. 2006). Significantly higher leaf glucose (18.3–28.9%) and sucrose (16.4–19.1%), but lower root glucose (19.6–39.0%) and sucrose (12.3–31.2%) contents, were observed in the AMF inoculated seedlings than in the un-inoculated ones (Table 3). The significantly higher leaf sucrose and glucose contents in the AMF seedlings might imply that plant photosynthesis was enhanced with the increase of leaf chlorophyll (Table 3). In contrast, root sucrose and glucose were decreased in all AM trifoliate orange seedlings (Table 3), which is reasonable since ~20% of the photosynthetically fix carbon are generally consumed by AMF for their belowground functions (Smith and Read 2008). In addition, better root morphological structures in AMF seedlings should consume more root carbohydrates to sustain its growth and development (Rolland et al. 2006; Ingram and Malamy 2010). Therefore, we suggested that the AMF-induced alternation of plant carbohydrates might be directly related to root and mycorrhizal development.
Compared to the non-mycorrhizal control, 17.3, 13.4, and 16.1% of leaf soluble protein were decreased, but 186.5, 401.7, and 28.2% of root soluble protein were increased respectively with the inoculations of G. mosseae, G. versiforme and P. occultum (Table 3), implying that the magnitude of AMF enhancement in protein synthesis of trifoliate orange seedlings were also species different. These results are consist with that AMF inoculation with G. mosseae and G. versiforme stimulated soluble protein contents in various host plants, including Cassia siamea, Delonix regia, Echinacea purpurea, Erythrina variegata, Prunus cerasifera, Samanea saman, Sterculia foetida and Zea mays (Berta et al. 1995; Boucher et al. 1999; Manoharan et al. 2008; Araim et al. 2009). However, a decrease of soluble protein was observed in micropropagated Prunus cerasifera roots when inoculated with an ericoid mycorrhizal Hymenoscyphus ericae (Berta et al. 1995). This inconsistency is likely to attribute to different mycorrhizal fungal and/or plant species used.
Compared with the non-AMF seedlings, POD activity of leaf or root in the G. mosseae-, G. versiforme- and P. occultum-infected seedlings was decreased by 35.7, 20.1% and 54.5 or 85.4%, 84.8 and 51.0%, respectively (Table 3). Such a decrease in POD activity was AMF species dependent as P. occultum-colonized or G. mosseae-colonized seedlings showed the lowest leaf or root POD activity, respectively. In contrast, higher activity of POD in mycorrhizal plants was also observed under salt or drought stress (Ghorbanli et al. 2004; Wu et al. 2006). These inconsistent results might result from either different plant species (soybean, Ghorbanli et al. 2004) or different experimental conditions (Wu et al. 2006). High POD also could be related with low percentage of rooting in the case of Arbutus unedo cuttings (Metaxas et al. 2004), but the decrease of POD activity was accompanied by a decrease of lignin with an increase in NAA (α-naphthylacetic acid) in soybean roots (Chen et al. 2002), which induces root elongation and decreases root diameter in Annona cherimola (Padilla and Encina 2005; Zangaro et al. 2007). As a result, the alteration of POD activity under AMF inoculation might contribute to root morphological alternation with diverse mechanisms.
Conclusion
Our results showed that the tested root traits of trifoliate orange seedlings were significantly generally improved by all three AM fungal species especially G. mosseae and P. occultum, and such improvements might be related to the AMF-induced alterations of carbohydrates and POD activity. Further studies will be required to examine whether these alterations could be consistent with other plants.
References
Amako K, Chen GX, Asade K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Araim G, Saleem A, Arnason JT, Charest C (2009) Root colonization by an arbuscular mycorrhizal (AM) fungus increases growth and secondary metabolism of purple coneflower, Echinacea purpurea (L.) Moench. J Agric Food Chem 57:2255–2258
Atkinson D, Black KE, Forbes PJ, Hooker JE, Baddeley JA, Watson CA (2003) The influence of arbuscular mycorrhizal colonization and environment on root development in soil. Eur J Soil Sci 54:751–757
Augín O, Mansilla JP, Vilariño A, Sainz M (2004) Effects of mycorrhizal inoculation on root morphology and nursery production of three grapevine rootstocks. Am J Enol Vitic 55:108–111
Berta G, Trotta A, Fusconi A, Hooker JE, Munro M, Atkinson D, Giovannetti M, Morini S, Fortuna P, Tisserant B (1995) Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiol 15:281–293
Boucher A, Dalpé Y, Charest C (1999) Effect of arbuscular mycorrhizal colonization of four species of Glomus on physiological responses of maize. J Plant Nutri 22:783–797
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen LM, Cheng JT, Chen EL, Yiu TJ, Liu ZH (2002) Naphthaleneacetic acid suppresses peroxidase activity during the induction of adventitious roots in soybean hypocotyls. J Plant Physiol 159:1349–1354
Gavito ME, Curtis PS, Jakobsen I (2001) Neither mycorrhizal inoculation nor atmospheric CO2 concentration has strong effects on pea root production and root loss. New Phytol 149:283–290
Ghorbanli M, Ebrahimzadeh H, Sharifi M (2004) Effects of NaCl and mycorrhizal fungi on antioxidative enzymes in soybean. Biol Plant 48:575–581
Gutjahr C, Casieri L, Paszkowski U (2009) Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol 182:829–837
Hodge A, Berta G, Doussan C, Merchan F, Crespi M (2009) Plant root growth, architecture and function. Plant Soil 321:153–187
Hooker JE, Munro M, Atkinson D (1992) Vesicular-arbuscular mycorrhizal fungi induced alteration in poplar root system morphology. Plant Soil 145:207–214
Ingram PA, Malamy JE (2010) Root system architecture. In: Kader JC, Delseny M (eds) Advances in botanical research, vol 55. Elsevier, New York, pp 75–117
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162:929–936
Lepeduš H, Cesar V, Krsnik-Rasol M (2004) Guaiacol peroxidases in carrot (Daucus carota L.) root. Food Technol Biotechnol 42:33–36
Lichtenthaler K (1987) Chlorophyll and carotinoids: pigments of photosynthetic brommembranes. Method Enzymol 148:351–382
Manoharan PT, Pandi M, Shanmugaiah V, Gomathinayagam S, Balasubramanian N (2008) Effect of vesicular arbuscular mycorrhizal fungus on the physiological and biochemical changes of five different tree seedlings grown under nursery conditions. Afr J Biotechnol 7:3431–3436
Metaxas D, Syros T, Yupsanis T, Economous AS (2004) Peroxidases during adventitious rooting in cuttings of Arbutus unedo and Taxus baccata as affected by plant genotype and growth regulator treatment. Plant Growth Regul 44:257–266
Miransari M (2010) Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol 12:563–569
Murkute AA, Sharma S, Singh SK (2006) Studies on salt stress tolerance of citrus rootstock genotypes with arbuscular mycorrhizal fungi. Hort Sci 33:70–76
Norman JR, Atkinson D, Hooker JE (1996) Arbuscular mycorrhizal fungal-induced alteration to root architecture in strawberry and induced resistance to the root pathogen Phytophthora fragariae. Plant Soil 185:191–198
Orfanoudakis M, Wheeler CT, Hooker JE (2010) Both the arbuscular mycorrhizal fungus Gigaspora rosea and Frankia increase root system branching and reduce root hair frequency in Alnus glutinosa. Mycorrhiza 20:117–126
Osmont KS, Sibout R, Hardtke CS (2007) Hidden branches: developments in root system architecture. Annu Rev Plant Biol 58:93–113
Padilla IMG, Encina CL (2005) Changes in root morphology accompanying mycorrhizal alleviation of phosphorus deficiency in micropropagated Annona cherimola Mill. Plant Sci Hortic 106:360–369
Phillips JM, Hayman DS (1970) Improved producers for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann Rev Plant Physiol 57:675–709
Schellenbaum L, Berta G, Ravolanirina F, Tisserant B, Gianinazzi S, Fitter AH (1991) Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.). Ann Bot 68:135–141
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. 3rd. Academic Press, San Diego
Wu QS, Xia RX, Zou YN (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 163:1101–1110
Wu QS, Xia RX, Zou YN (2008) Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur J Soil Biol 44:122–128
Wu QS, Levy Y, Zou YN (2009) Arbuscular mycorrhizae and water relations in citrus. In: Tennant P, Benkeblia N (eds) Citrus II. Tree and forestry science and biotechnology 3:105–112
Wu QS, Zou YN, He XH (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304
Yao Q, Wang LR, Zhu HH, Chen JZ (2009) Effect of arbusuclar mycorrhizal fungal inoculation on root system architecture of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings. Sci Hortic 121:458–461
Zai XM, Qin P, Wan SW, Zhao FG, Wang G, Yan DL, Zhou J (2007) Effects of arbuscular mycorrhizal fungi on the rooting and growth of beach plum (Prunus maritima) cuttings. J Hortic Sci Biotech 82:863–866
Zangaro W, Nishidate FR, Vandresen J, Andrade G, Nogueira MA (2007) Root mycorrhizal colonization and plant responsiveness are related to root plasticity, soil fertility and successional status of native woody species in southern Brazil. J Trop Ecol 23:53–62
Zhang ZL, Zai LJ (2004) Expermental instructment of plant physiology (in Chinese), 3rd edn. Higher Education Press, Beijing
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.: 30800747), the Key Project of Chinese Ministry of Education (No.: 211107), and the Science-Technology Research Project for Excellent Middle-aged and Young Talents of Hubei Provincial Department of Education, China (No.: Q20111301).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, QS., Zou, YN., He, XH. et al. Arbuscular mycorrhizal fungi can alter some root characters and physiological status in trifoliate orange (Poncirus trifoliata L. Raf.) seedlings. Plant Growth Regul 65, 273–278 (2011). https://doi.org/10.1007/s10725-011-9598-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9598-6