Abstract
The microbial diversity of Verbascum lychnitis community from industrial areas was investigated. The plants harbor a variety of endophytic fungi most of which belong to the Ascomycetes. The isolated fungal endophytes were identified according to ITS1-5.8S-ITS2 rDNA sequence similarity and were found to belong to 22 genera and 12 orders. The most frequently isolated genera were Diaporthe spp., Alternaria spp. and Pichia spp. An unidentified species from the Xylaria genus was isolated from V. lychnitis, which is a novel finding since most Xylaria species were reported to be solely wood-inhabiting fungi. The composition of fungal endophytes from the tailings site showed higher diversity, particularly in leaf tissues, than in non-tailings sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In natural and agricultural environments, plants are host to a multitude of microorganisms forming unique, integrated plant - microbial communities. A large variety of microorganisms colonizes the inner and outer surfaces of plant tissues. One of these groups are plant endophytes. The term “endophyte” was first used in XIX century to describe any microorganism colonizing internal plant tissues (cited from Petrini 1991). Since then the term has been evolving (Bayman 2007). Currently, one of widely accepted definitions describes endophytes as bacterial or fungal microorganisms that colonize symptomlessly the living, internal tissues of their host, even though they may, after an incubation or latency period, cause disease (Petrini 1991).
It has been hypothesized that the interaction between fungal endophytes and host plants is characterized by a well-established equilibrium between fungal virulence and plant defense, in which any shift in balance results in the development of plant disease. Nevertheless, endophytic fungi are thought to interact mutualistically with their host plant. The presence of the fungal endophyte may improve water and nutrient uptake and confer protection against both biotic and abiotic environmental stresses (Schulz and Boyle 2005) including drought (Ravel et al. 1997; Cheplick et al. 2000,) and high temperature (Redman et al. 2002), insect pests and herbivores (Siegel and Bush 1996; Schardl and Phillips 1997), low pH (Lewis 2004), high salinity (Waller et al. 2005) and metal toxicity (Monnet et al. 2001). The importance of endophytes is also emphasized by their ability to produce phytohormones, vitamins and other plant growth-promoting substances (Sirrenberg et al. 2007; Dai et al. 2008).
Species from the Verbascum genus, commonly known as ‘mulleins’, are the largest group of the Scrophulariaceae family, encompassing approximately 2500 species (Kahraman et al. 2011). Verbascum plants are widespread from the temperate to the subtropical zones and have modest soil requirements (Zielińska-Pisklak et al. 2013). They occur spontaneously and grow well at the slope of the tailings near Kraków (Poland), where growing conditions are extremely difficult (Turnau et al. 2010; Turnau et al. 2012).
Due to their medicinal properties, mulleins have been used in folk medicine since ancient times (Sezik et al. 2001; Leporatti and Ivancheva 2003; Turker and Gurel 2005; Sher 2011). Verbascum species are abundant in a number of bioactive substances such as saponins (Turker et al. 2004), iridoid and phenylethanoid glycosides (Akdemir et al. 2004; Akkol et al. 2007) and flavonoids (Akdemir et al. 2003) which confer tolerance to difficult environmental conditions of post-industrial wastes. Mulleins are also being utilized by the pharmaceutical industry. Verbascum species show potential for bioremediation of polluted soils accumulating toxic metals such as As, Pb, Zn (Turnau et al. 2010), Ba (Kowalska et al. 2012) Mo, Cu and Ag (Sagiroglu et al. 2006).
Due to a high concentration of toxic elements and the poor water holding capacity in the substratum, tailings are highly unfavorable for plant growth and the development of the necessary soil biota. The first 40–50 years of natural succession are characterized by the presence of pioneer species such as Fragaria vesca, Hippophae rhamnoides, Arrhenatherum elatius, Trifolium repens, Viola tricolor (Turnau et al. 2001) and grasses, particularly from the family Poaceae (Ryszka and Turnau 2007). Plants, besides their unquestionable impact on physical-chemical properties of the soil (Hinsinger 2013), are an important niche for beneficial microorganisms.
Verbascum species commonly occur at the tailings near Chrzanów, calcareous grasslands in Poland (Turnau et al. 2008) and in other post-industrial wastes in Europe (Góralska 2014). The species of Verbascum with the greatest ability to colonise degraded areas is V. thapsus. In the experiment designed by Turnau et al. (2008) V. thapsus with other plants from calcareous grassland were introduced onto the tailings site. According to the results V. thapsus was among a few plant species which survived, produced seeds and spread out on the tailings (Turnau et al. 2008). Survival under extreme conditions is also possible thanks to the ability of Verbascum seeds to survive up to 100 years (Turnau et al. 2012).
In our study we decided to focus on a species that spontaneously occur in large numbers on post-industrial sites - V. lychnitis. The plant cover in the area of sampling was restricted to grass species, with the exception of only a few dicots, including V. lychnitis. As mentioned previously, plants are a necessary niche for microorganisms engaged in detoxifying polluted soil. This relationship is mutual; for many plants the presence of fungal microorganisms is obligatory for survival (Redman et al. 2002). For others the presence of endophytic fungi is required for growth and reproduction (Clay 1984). With that being said, we can assume that the biodiversity of plants may correspond with the diversity of microorganisms present in the ecosystem. Thus, identyfing the diversity of plant associated endophytic fungi will be a step towards understanding the processes that allow plants to survive in extreme environments. In recent years plant-microbe consortia have been gaining interest for their potential use in phytoremediation. Pioneer plants are a potential reservoir for beneficial microorganisms, thus their identification can be an asset for phytoremediation.
The purpose of present study was to 1) investigate the diversity of microbial community of V. lychnitis growing at the slope of the tailings and non-tailings sites and 2) isolate and characterize the fungal endophytes from these plants.
2 Materials and methods
2.1 Research area
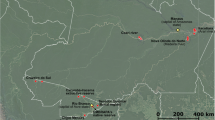
V. lychnitis plants were collected from the following locations: meadows located near Olkusz (N 50°18′26″ E 19°33′48″), wastelands located in the vicinity of Klucze (N 50°19′47″ E 19°33′32″) and three plots from the tailings of the ZG Trzebionka Mining Company located near Chrzanów (N 50°09′19″ E 19°25′10″). All of these sites are situated in an industrial region, 30–50 km west of Kraków, Poland where the toxic metal (Cd, Pb and Zn particularly) concentration in the soil is elevated (http://www.jura.eko.org.pl/doc/srodowisko/Gmina_Klucze.pdf, http://www.jura.eko.org.pl/doc/srodowisko/Gmina_Olkusz.pdf). In contrast to the Klucze and Olkusz sites the substratum from the tailings contained almost no organic matter and was P- and N- deficient. It was slightly alkaline (pH 7.4), and contained up to 108.15 μg g−1 of Cd, 2372.03 μg g−1 of Pb and 12067.0 μg g−1 of Zn, although the bioavailability of these elements is not particularly high (Orłowska et al. 2005a; Orłowska et al. 2005b).
2.2 Plant sampling
From the tailings site, Olkusz and Klucze, 16, 9 and 6 plants respectively were harvested. Juvenile (1 year) and mature (2 year old) plants were collected and left in distilled water overnight. The V. lychnitis samples from field sites were then separated into roots, shoots (stems, leaves) and seeds.
2.3 Endophyte isolation
Fungi isolation was performed twice from plants from each site. For isolation, plant tissues lacking visible injury were used. In order to eliminate epiphytic communities, the outer surface of plant tissues were initially surface sterilized.
Aerial parts were treated with 70 % ethanol (5 min), immersed in 1 % sodium hypochlorite solution (1 min) and incubated in 0.01 % Tween 20 solution (1 min). Samples were rinsed three times in sterile distilled water. Subsequently, plant tissues were dried on sterile filter paper, cut into 0.5 × 0.5 cm pieces and placed on potato dextrose agar (PDA) supplemented with oxytetracycline (50 mg/1) to prevent bacterial growth. Parafilm (Bemis, USA) - sealed petri dishes were incubated for 4–7 days in darkness at 25 °C. Plates were evaluated for hyphae of emerging fungi on a daily basis and transferred to fresh medium. In order to check the effectiveness of the surface sterilization procedure, aliquots of water from the last rinsing were smeared on a PDA plate (Santamaria and Bayman 2005 [modified]); Hallmann et al. 2006; Verma et al. 2007 [modified]; Compant et al. 2011).
The roots of Verbascum were rinsed in running tap water and surface sterilized with 99 % ethanol (1 min), incubated in 3 % hydrogen peroxide solution (5 min) and immersed in 75 % ethanol (5 min) (Compant et al. 2011 [modified]). Subsequently, roots were air-dried on sterile filter paper. The younger roots were cut to 1 cm segments and placed on PDA medium supplemented with oxytetracycline (50 mg/l).
Surface sterilization of the seeds was performed by immersion in 1 % sodium hypochlorite solution with 1 droplet (200 μl) of Tween 80 in 100 ml of solution (30 min). The samples were subsequently rinsed in sterile distilled water three times. The sterility of the seeds was verified by spreading aliquots of water from the last rinsing onto PDA medium. The endophytes were isolated from seeds by pressing in a sterile mortar after addition of 10 mM MgSO4 solution (Mastretta et al. 2009 [modified]). After incubation of the PDA plates supplemented with oxytetracycline (50 mg/l), the hyphae of growing fungi were transferred to fresh medium.
2.4 Endophyte identification
Isolated endophytic fungi were identified according to their macroscopic and microscopic characteristics and using molecular methods.
Genomic DNA was extracted from mycelium of fungi growing on PDA by cutting the mycelium from the youngest part of the agar culture (7–8 days old) and transferring to test tubes filled with two tungsten beads each. Subsequently, samples were homogenized in a tissue lyser (LT, Qiagen, Germany).
DNA was isolated with 2 % cetyltrimethyl ammonium bromide (CTAB) buffer according to the protocol (Gardes and Bruns 1993). Reaction mixtures (25 μl) contained 12.5 ul Dream Taq Green PCR Master Mix (Thermo Scientific) and primers (Genomed) ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) (Gardes and Bruns 1993) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al. 1990).
Amplification was carried out as follows: a preliminary denaturation step at 95 °C for 3 min was followed by 35 cycles of 30 s at 95 °C, 30 s at 55 °C, and 45 s at 72 °C, PCR products were separated by electrophoresis in 1.5 % agarose gel. For visualization gels were stained with Gel Red Nucleic Acid Stain (BIOTIUM).
PCR products were purified with 3 M sodium acetate solution and isopropanol (http://openwetware.org/wiki/Isopropanol_Precipitation_for_PCR_Purification), cycle sequenced with ABI BigDye Terminator ver.3.1 (Applied Biosystems, USA) and purified according to the manufacture’s protocol with 125 mM EDTA and 96 % ethanol. Sequences were analyzed in a sequenator 3130xl Genetic Analyzer (Applied Biosystems).
Finally, DNA sequences from each culture were edited using BioEdit (Hall 1999) and Chromas (www.technelysium.com.au) software. Using the BLASTn (Altschul et al.1997), sequences were compared to those published in the NCBI (www.ncbi.nlm.nih.gov) databases. A positive identification of species was confirmed if they shared ≥ 98 % ITS region sequence identity with the most similar (reference) sequence from NCBI databases. The obtained sequences within 2 % nucleotide difference were categorized into a single operational unit and assigned an identical name. The correctness of Latin names was adopted from www.indexfungorum.org. Sequences were submitted to GenBank and assigned accession numbers from KP174665 to KP174711
2.5 Biodiversity measurements
Shannon-Wiever index (H’) of biodiversity was applied to the fungal species community from the tailings and non-tailings sites.
3 Results
3.1 Isolation of endophytic fungi from V. lychnitis
In this study, a total of 263 fungal cultures were isolated from 31 V. lychnitis plants collected from the tailings site, Olkusz and Klucze (Table 1).
From fungal cultures, 47 isolates were identified based on ITS1-5.8S-ITS2 rDNA sequencing. 68 and 32 % of the identified taxa were isolated from juvenile and matured plants respectively.
3.2 Molecular identification of endophytic fungi
The isolated fungal endophytes belonged to 22 genera and 12 orders (Table 2). The most frequent genus was Diaporthe with 14.89 % of identified endophytes belonging to this genus, followed by Alternaria (12.76 %) and Pichia (8.51 %). Other genera were represented by one to three identified isolates. The Pleosporales occurred as the most prevalent order with 36.17 %, followed by Diaporthales and Hypocreales (14.89 % each). The identity percentage among obtained sequences compared with those available in GenBank ranged from 96 to 100 %.
3.3 Endophyte distribution in plant organs
Pyrenophora and Xylaria species were isolated from Verbascum leaves, while Alternaria and Chaetomium species were isolated from the shoots of the plants. Cochliobolus, Gibberella, Phoma, Pichia and Trichoderma species were isolated from all parts of Verbascum plants. Diaporthe spp. were isolated from leaves apart from one strain which inhabited plant roots. Diaporthe sp. was also the only genus which occurred in plants from all examined sites. Cadophora, Cochliobolus Pyrenophora and Xylaria species were isolated only from the tailings site.
3.4 Biodiversity and species richness
The Shannon-Weiner index was significantly higher (3.044) in plants from the tailings site than in plants from non-tailings sites (2.294).
The most abundant in fungal taxa were leaves (12 species) and roots (9 species) of plants growing on the tailings site (Table 2). In Klucze and Olkusz, the frequency of endophytes isolated from different parts of plants ranged from 1 to 4 species.
4 Discussion
The association of Verbascum with vesicular mycorrhizal fungi has been shown several times previously (Pendleton and Smith 1983; Harley and Harley 1987; Góralska 2014), however, the presence and identity of other, important classes of microbial organisms from mulleins have not so far been studied.
In this study the plants were found to harbor a variety of endophytic fungi most of which (97.87 %) belong to Ascomycetes. The most frequent genera were Diaporthe (Phomopsis), Alternaria and Pichia.
Fungi from the genus Phomopsis have been described as deleterious plant pathogens. Species such as P. viticola cause cane and leaf spots in grapevine, and its occurrence leads to significant yield loss (Scheper et al. 2000). P. amygdali infections result in twig canker and blight of almonds and peach (Tuset and Portilla 1989 cited from Udayanga et al. 2011). P. helianthi, reported by Heller and Gierth (2001), causes a stem canker of sunflower. Some species such as P. leptostromiformis which infects lupines (Lupinus sp.) may also be dangerous to animals causing lupinosis, a type of mycotoxicosis in sheep (Wood and Sivasithamparam 1989). However, species of Phomopsis are also commonly isolated as endophytes from leaves of different species of dicot plants, and in many cases these fungi dominate the endophyte assemblage of a host. Sun et al. (2011) showed that the Phomopsis sp. was one of the most dominant endophyte species in Acer truncatum. An unidentified fungus from this genus conferred tolerance against the plant pathogen Microcyclus ulei (Rocha et al. 2011). According to Suryanarayanan et al. (2002) Phomopsis sp., Colletotrichum sp. and Phyllosticta sp. contributes 49–70 % to a trees’ endophyte assemblage irrespective of the season and forest type, indicating that they are well adapted to survive as endophytes and elude different types of host defences.
Another highly represented fungus was Alternaria alternata, a common pathogen causing a variety of diseases of plants (Maiti et al. 2007; Abkhoo and Sabbagh 2013) including marketable fruits (Lee et al. 2013). It also belongs to a group of only eight species of this genus which have been identified as human pathogens (Farina et al. 2007). Fungi from this genus are also commonly isolated as endophytes from medicinal plants (Aly et al. 2008; Bhagat et al. 2012), Malus domestica (Camatti-Sartori et al. 2005), Coffea arabica (Fernandes et al. 2009), evergreen rhododendrons (Purmale et al. 2012) and Nicotiana tabacum (Spurr and Welty 1975). A. alternata has been routinely isolated as an endophyte of leaves from wheat (Larran et al. 2002). As in the case of Phomopsis sp., Alternaria sp. fungi are reported as common endophytes (Suryanarayanan et al. 2011). Since A. alternata is an opportunistic pathogen for humans, it should not be considered for phytoremediation.
The third most frequent endophyte species isolated from V. lychnitis was Pichia guilliermondi. Buzzini et al. (2003) isolated these ascomycetous yeasts from three rain forests, where Pichia sp. comprised 56 % of the total strains. This genus is reported as a biocontroller of pathogenic fungal growth in plants (Petersson and Schnürer 1995), and a source of antimicrobial metabolites (Zhao et al. 2010). However, Gai et al. (2009) showed that the supernatant from a culture of P. guilliermondii increased the in vitro growth of the pathogen, suggesting that the yeast could assist in the establishment of this pathogen in its host plant, therefore contributing to the development of disease symptoms.
The only isolated fungus belonging to Basidiomycetes was Rhizoctonia solani. This fungus has gained a reputation of a widespread and deleterious plant pathogen with unlimited host ranges (Parmeter 1970; Ohkura et al. 2009). However, it has also been reported as an orchid symbiont (Shimura et al. 2009) including orchids that occur on the industrial wastes examined in this study (Wojtczak 2013).
Seven of the endophyte genera/species found in the present study were represented by a single isolation. According to Sánchez Márquez et al. (2012) species that are isolated only once in an endophyte survey are considered rare in the host plant. The interactions between plants and rare species may represent unstable associations that possibly only occur when a given plant and fungal genus encounter one another.
Among grasses and perennial plants, the most common endophytic taxa belong to Ascomycota (Stone et al. 2004). The vast majority of endophytes isolated from V. lychnitis were ascomycetous. In V. lychnitis, Pleosporales was the most prevalent order followed by Diaporthales and Hypocreales. Sieber (2007) noticed that representatives of the orders Dothideales, Pleosporales, Mycosphaerellales and the Xylariales can be dominant in endophytic communities from angiosperms and gymnosperms. Stone et al. (2004) pointed out that Diaporthales and Hypocreales were among the most frequent isolates from foliage and woody perennials. The majority of the isolated species come from juvenile plants. Fungi isolation from young tissues is more efficient than from older tissues. The latter often contain many slow growing species from which isolation is difficult (Bacon and White 1994).
The H’ values for isolates from the tailings were higher in comparison to non-tailings sites, which indicates that the fungal community from plants growing on the tailings was more diverse, particularly in leaf tissues, in comparison with non-tailings sites. These data are inconsistent with published literature (Jurc et al. 1996; Lappalainen et al. 1999). In this study, higher H’ values for isolates from the tailings line reflect the diversity of fungal community in one particular species - V. lychnitis, but not the biodiversity of microorganisms at the site of sampling. In order to gain a comprehensive understanding of the effect of toxic metals on the diversity of microorganisms inhabiting the plants, a much more thorough study is necessary.
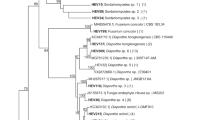
The most frequent species isolated from V. lychnitis leaves from the tailings site were Pyrenophora leucospermi (3 isolates), Alternaria alternata, Diaporthe sp. and Xylaria sp. (2 isolates of each). The most frequent species isolated from V. lychnitis leaves from non-tailings sites were Diaporthe sp. (3 isolates), Diaporthe neoartcii (1 isolate) and Alternaria alternata (2 isolates). P. leucospermi is a novel species isolated from plants belonging to the Proteaceae family also known as a pathogen causing severe blight in current-season leaves, stems, and flower heads (Crous et al. 2011). Alternaria sp., Diaporthe sp. and Xylaria sp. are all common endophytes but the isolation of Xylaria from V. lychnitis is particulary interesting, since most Xylaria species have been reported as wood-inhabiting fungi occurring commonly on decaying trees (Wang et al. 2014). The results may indicate that the most abundant endophytes have adapted to the long-term toxic metal stress conditions and the leaf tissue was their niche. Since A. alternata is an opportunistic pathogen for humans (Anaissie et al. 1989), only Diaporthe sp. and Xylaria sp. should be considered as potential candidates for phytoremediation. Endophytes from the Xylaria genus have not been reported in herbaceous species. In this study this fungi was found inhabiting the leaves of plants growing in the tailings site, thus we speculate that the presence of toxic metal in the substratum impacts the interaction between the plant and the fungi rendering it possible for the fungi to colonize V. lychnitis.
Some endophytic ascomycetous fungi are known to be very defensive against saprobic basidiomycetes. In the current study Phomopsis sp. and Xylaria sp. were one of the most frequent inhabitants in leaf tissue of plants from the tailings, which potentially indicate their defensive role against secondary colonizers. Similar results have been presented by Fukasawa et al. (2009), in which a Xylaria sp. in particular was highly combative against the secondary colonizer Phanerochaete filamentosa.
Another important factor possibly determining the fungal community is the life cycle of the fungi. Xylariaceous fungi are endophytes in trees; however, they remain dormant until triggered to grow and sporulate by natural leaf senescence, abscission or damage (Promputtha et al. 2010). Xylariaceous fungi have the advantage of occupying tree tissues earlier than other fungi that usually colonize plants from the outside via airborne or soil-borne spores or hyphae. This is probably the reason why small leaves and phyllome tissues are dominated by endophytic Xylariaceous species in their early stages of decomposition. It may be possible for endophytic fungi to become saprobes following the senescence of host tissue (Wong and Hyde 2001; Ghimire and Hyde 2004). According to Promputtha et al. (2010) saprobes may have evolved from endophytic fungi as a result of their ability to colonize leaves and produce specific enzymes during fungal succession. Indeed some endophytes may cause decay of the host tissue and then persist as saprobes after senescence (Ghimire and Hyde 2004; Oses et al. 2008).
5 Conclusions
The endophyte diversity of grass and tree species from different regions has been extensively studied and, to our knowledge, this is the first comprehensive report of the fungal endophyte diversity of V. lychnitis. This mullein species was earlier investigated for the presence of bioactive compounds (Alipieva et al. 2014) or in studies on succession (Rebele 2008). The most frequent genera isolated from V. lychnitis were typical endophytes inhabiting other monocotyledonous and dicotyledonous plants. The isolation of Xylaria sp. from the leaves of Verbascum, a biannual dicotyledon, is a novel finding. The composition of fungal endophytes at the tailings site showed higher diversity than at non-tailings sites.
References
Abkhoo J, Sabbagh SK (2013) Evidence of Alternaria alternata causing leaf spot of Aloe vera in Iran. J Phytopathol 162:516–518
Akdemir ZŞ, Tatli Bedir E, Khan IA (2003) Antioxidant Flavonoids from Verbascum salviifolium Boiss. FABAD J Pharm Sci 28:71–75
Akdemir ZŞ, Tatli Bedir E, Khan IA (2004) Acylatediridoid glycosides from Verbascum lasianthum. Turk J Chem 28:101–110
Akkol EK, Tatli II, Akdemir ZS (2007) Antinociceptive and anti-inflammatory effects of saponin and iridoid glycosides from Verbascum pterocalycinum var. mutense Hub.-Mor. Z Naturforsch C 62:813–820
Alipieva KI, Orhan IE, Cankaya IIT, Kostadinova EP, Georgiev MI (2014) Treasure from garden: chemical profiling, pharmacology and biotechnology of mulleins. Phytochem Rev 13:417–444
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Aly AH, Edrada-Ebel R, Indriani ID, Wray V, Muller WE, Totzke F, Zirrgiebel U, Schachtele C, Kubbutat MHG, Lin WH, Proksch P, Ebel R (2008) Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J Nat Prod 71:972–980
Anaissie EJ, Bodey GP, Rinaldi MG (1989) Emerging fungal pathogens. Eur J Clin Microbiol 8:323–330
Bacon CW, White JF Jr (1994) Stains, media, and procedures for analyzing endophytes. In: Bacon CW, White JF Jr (eds) Biotechnology of endophytic fungi of grasses. CRC Press Inc, Boca Raton, pp 47–56
Bayman P (2007) Fungal endophytes. In: Kubicek CP, Druzhinina IS (eds) The Mycota, vol 4, Environmental and Microbial Relationships, 2nd edn. Springer, Berlin, pp 213–27
Bhagat J, Kaur A, Sharma M, Saxena AK, Chadha BS (2012) Molecular and functional characterization of endophytic fungi from traditional medicinal plants. World J Microbiol Biotechnol 28:963–971
Buzzini P, Martini A, Cappelli F, Pagnoni UM, Davoli P (2003) A study on volatile organic compounds (VOCs) produced by tropical ascomycetous yeasts. A Van Leeuw J Microb 84:301–311
Camatti-Sartori V, da Silva-Ribeiro RT, Valdebenito-Sanhueza RM, Pagnocca FC, Echeverrigaray S, Azevedo JL (2005) Endophytic yeasts and filamentous fungi associated with southern Brazilian apple (Malus domestica) orchards subjected to conventional, integrated or organic cultivation. J Basic Microbiol 45:397–402
Cheplick GP, Perera A, Koulouris K (2000) Effect of drought on the growth of Lolium perenne genotypes with and without fungal endophytes. Funct Ecol 14:657–667
Clay K (1984) The effect of the fungus Atkinsonella hypoxylon (Clavicipitaceae) on the reproductive system and demography of the grass Danthonia spicata. New Phytol 98:165–175
Compant S, Mitter B, Colli-Mull JG, Gangl H, Sessitsch A (2011) Endophytes of grapevine flowers, berries, and seeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microbiol Ecol 62:188–197
Crous PW, Summerell BA, Swart L, Denman S, Taylor JE, Bezuidenhout CM, Groenewald JZ (2011) Fungal pathogens of Proteaceae. Persoonia: Mol Phylogeny Evol Fungi 27:20–45
Dai CC, Yu BY, Li X (2008) Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. Afr J Biotechnol 7:3505–3510
Farina C, Gotti E, Parma A, Naldi L, Goglio A (2007) Pheohyphomycotic soft tissue disease caused by Alternaria alternata in a kidney transplant patient: a case report and literature review. Transplant Proc 39:1655–1659
Fernandes MDRV, Pfenning LH, Costa-Neto CMD, Heinrich TA, Alencar SMD, Lima MAD, Ikegaki M (2009) Biological activities of the fermentation extract of the endophytic fungus Alternaria alternata isolated from Coffea arabica L. Braz J Pharm Sci 45:677–685
Fukasawa Y, Osono T, Takeda H (2009) Effects of attack of saprobic fungi on twig litter decomposition by endophytic fungi. Ecol Res 24:1067–1073
Gai CS, Lacava PT, Maccheroni W, Glienke C, Araújo WL, Miller TA, Azevedo JL (2009) Diversity of endophytic yeasts from sweet orange and their localization by scanning electron microscopy. J Basic Microbiol 49:441–451
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes -application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Ghimire SR, Hyde KD (2004) Fungal endophytes. In: Varma A, Abbott L, Werner D, Hampp R (eds) Plant surface microbiology. Springer, Berlin, pp 281–292
Góralska K (2014) Zastosowanie mikroorganizmów w rekultywacji hałdy cynkowej Z.G.”Trzebionka”. Dissertation, Jagiellonian University
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hallmann J, Berg G, Schulz B (2006) Isolation procedures for endophytic microorganisms. In: Schulz B, Boyle C, Sieber TN (eds) Microbial root endophytes. Springer, Berlin, pp 299–319
Harley JL, Harley EL (1987) A check-list of mycorrhiza in the British flora. New Phytol 105:1–102
Heller A, Gierth K (2001) Cytological observations of the infection process by Phomopsis helianthi (Munt.‐ Cvet) in leaves of sunflower. J Phytopathol 149:347–357
Hisinger P (2013) Plant induced changes in soil processes and properties. In: Gregory PJ, Nortcliff S (eds) Soil conditions and plant growth. Wiley, New York, pp 323–365
Jurc M, Jurc D, Gogala N, Simoncic P (1996) Air pollution and fungal endophytes in needles of Austrian pine. Phyton Ann Rei Botanicae 36:111–114
Kahraman C, Ekizoglu M, Kart D, Akdemir ZŞ, Tatli II (2011) Antimicrobial activity of some Verbascum species growing in Turkey. FABAD J Pharm Sci 36:11–15
Kowalska J, Stryjewska E, Bystrzejewska-Piotrowska G, Lewandowski K, Tobiasz M, Paldyna J, Golimowski J (2012) Studies of plants useful in the re-cultivation of heavy metals-contaminated wasteland - a new hyper accumulator of barium? Pol J Environ Stud 21:401–405
Lappalainen JH, Koricheva J, Helander ML, Haukioja E (1999) Densities of endophytic fungi and performance of leafminers (Lepidoptera: Eriocraniidae) on birch along a pollution gradient. Environ Pollut 104:99–105
Larran S, Perello A, Simon MR, Moreno V (2002) Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J Microbiol Biotechnol 18:683–686
Lee JH, Kim J, Kwak YS (2013) First report of black spot disease caused by Alternaria alternata on sweet persimmon fruits. Mycobiology 41:167–169
Leporatti ML, Ivancheva S (2003) Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J Ethnopharmacol 87:123–142
Lewis GC (2004) Effects of biotic and abiotic stress on the growth of three genotypes of Lolium perenne with and without infection by the fungal endophyte Neotyphodium lolii. Ann Appl Biol 144:53–63
Maiti CK, Sen S, Paul AK, Acharya K (2007) First report of leaf blight disease of Gloriosa superba L. caused by Alternaria alternata (Fr.) Keissler in India. J Gen Plant Pathol 73:377–378
Mastretta C, Taghavi S, van der Lelie D, Mengoni A, Galardi F, Gonnelli C, Barac T, Boulet J, Weyens N, Vangronsveld J (2009) Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int J Phytoremediation 11:251–267
Monnet F, Vaillant N, Hitmi A, Coudret A, Sallanon H (2001) Endophytic Neotyphodium lolii induced tolerance to Zn stress in Lolium perenne. Physiol Plant 113:557–563
Ohkura M, Abawi GS, Smart CD, Hodge KT (2009) Diversity and aggressiveness of Rhizoctonia solani and Rhizoctonia-like fungi on vegetables in New York. Plant Dis 93:615–624
Orłowska E, Jurkiewicz A, Anielska T, Godzik B, Turnau K (2005a) Influence of different arbuscularmycorrhiza fungal (AMF) strains on heavy metal uptake by Plantago lanceolata (Plantaginaceae). Pol Bot Stud 19:65–72
Orłowska E, Ryszka P, Jurkiewicz A, Turnau K (2005b) Effectiveness of arbuscular mycorrhizal fungal (AMF) strains in colonisation of plants involve in phytostabilisation of zinc wastes. Geoderma 129:92–98
Oses R, Valenzuela S, Freer J, Sanfuentes E, Rodriguez J (2008) Fungal endophytes in xylem of healthy Chilean trees and their possible role in early wood decay. Fungal Divers 33:77–86
Parmeter JR (1970) Rhizoctonia solani, biology and pathology. Univ of California Press
Pendleton RL, Smith BN (1983) Vesicular-arbuscular mycorrhizae of weedy and colonizer plant species at disturbed sites in Utah. Oecologia 59:296–301
Petersson S, Schnürer J (1995) Biocontrol of mold growth in high-moisture wheat stored under airtight conditions by Pichia anomala, Pichia guilliermondii, and Saccharomyces cerevisiae. Appl Environ Microbiol 61:1027–1032
Petrini O (1991) Fungal endophytes in tree leaves. In: Andrews JH, Hirano SS (eds) Microbial ecology of leaves. Springer, New York, pp 179–197
Promputtha I, Hyde KD, McKenzie EH, Peberdy JF, Lumyong S (2010) Can leaf degrading enzymes provide evidence that endophytic fungi becoming saprobes? Fungal Divers 41:89–99
Purmale L, Apine I, Nikolajeva V, Grantina L, Verkley G, Tomsone S (2012) Endophytic fungi in evergreen rhododendrons cultivated in vitro and in vivo. Environ Exp Bot 10:1–7
Ravel C, Courty C, Coudret A, Charmet G (1997) Beneficial effects of Neotyphodium lolii on the growth and the water status in perennial ryegrass cultivated under nitrogen deficiency or drought stress. Agronomie 17:173–181
Rebele F (2008) Vegetation development on deposit soils starting at different seasons. Plant Ecol 195:1–12
Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM (2002) Thermotolerance generated by plant/fungal symbiosis. Science 298:1581–1581
Rocha AC, Garcia D, Uetanabaro AP, Carneiro RT, Araújo IS, Mattos CR, Góes-Neto A (2011) Foliar endophytic fungi from Hevea brasiliensis and their antagonism on Microcyclus ulei. Fungal Divers 47:75–84
Ryszka P, Turnau K (2007) Arbuscular mycorrhiza of introduced and native grasses colonizing zinc wastes: implications for restoration practices. Plant Soil 298:219–229
Sagiroglu A, Sasmaz A, Sen O (2006) Hyperaccumulator plants of the Keban mining district and their possible impact on the environment. Pol J Environ Stud 15:317–325
Sánchez Márquez S, Bills GF, Herrero N, Zabalgogeazcoa Í (2012) Non-systemic fungal endophytes of grasses. Fungal Ecol 5:289–297
Santamarı’a J, Bayman P (2005) Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microbiol Ecol 50:1–8
Schardl CL, Phillips TD (1997) Protective grass endophytes: where are they from and where are they going? Plant Dis 81:430–438
Scheper RWA, Crane DC, Whisson DL, Scott ES (2000) The Diaporthe teleomorph of Phomopsis Taxon 1 on grapevine. Mycol Res 104:226–231
Schulz B, Boyle C (2005) The endophytic continuum. Mycol Res 109:661–686
Sezik E, Yeşilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T (2001) Traditional medicine in Turkey X. Folk medicine in central Anatolia. J Ethnopharmacol 75:95–115
Sher H (2011) Ethnoecological evaluation of some medicinal and aromatic plants of Kot Malakand Agency, Pakistan. Sci Res Essays 6:2164–2173
Shimura H, Sadamoto M, Matsuura M, Kawahara T, Naito S, Koda Y (2009) Characterization of mycorrhizal fungi isolated from the threatened Cypripedium macranthos in a northern island of Japan: two phylogenetically distinct fungi associated with the orchid. Mycorrhiza 19:525–534
Sieber TN (2007) Endophytic fungi in forest trees: are they mutualists? Fungal Biol Rev 21:75–89
Siegel MR, Bush LP (1996) Defensive chemicals in grass-fungal endophyte associations. In: Romeo JT, Saunders JA, Barbosa P (eds) Phytochemical diversity and redundancy in ecological interactions. Springer, US, pp 81–119
Sirrenberg A, Göbel C, Grond S, Czempinski N, Ratzinger A, Karlovsky P, Pawlowski K (2007) Piriformospora indica affects plant growth by auxin production. Physiol Plant 131:581–589
Spurr HW Jr, Welty RE (1975) Characterization of endophytic fungi in healthy leaves of Nicotiana spp. Phytopathology 65:417–422
Stone JK, Polishook JD, White JF (2004) Endophytic fungi. Biodiversity of Fungi. In: Foster MS, Bills GF (eds) Biodiversity of fungi: inventory and monitoring methods. Elsevier Academic Press, Burlington, pp 241–270
Sun X, Guo LD, Hyde KD (2011) Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers 47:85–95
Suryanarayanan TS, Murali TS, Venkatesan G (2002) Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Can J Bot 80:818–826
Suryanarayanan TS, Murali TS, Thirunavukkarasu N, Rajulu MG, Venkatesan G, Sukumar R (2011) Endophytic fungal communities in woody perennials of three tropical forest types of the Western Ghats, southern India. Biodivers Conserv 20:913–928
Turker AU, Gurel E (2005) Common mullein (Verbascum thapsus L.): recent advances in research. Phytother Res 19:733–739
Turker AU, Camper ND, Gürel E (2004) High-Performance Liquid Chromatographic determination of a saponin from Verbascum thapsus L. Biotechnol Biotechnol Equip 18:54–59
Turnau K, Ryszka P, Gianinazzi-Pearson V, van Tuinen D (2001) Identification of arbuscular mycorrhizal fungi in soils and roots of plants colonizing zinc wastes in southern Poland. Mycorrhiza 10:169–174
Turnau K, Anielska T, Ryszka P, Gawroński S, Ostachowicz B, Jurkiewicz A (2008) Establishment of arbuscular mycorrhizal plants originating from xerothermic grasslands on heavy metal rich industrial wastes–new solution for waste revegetation. Plant Soil 305:267–280
Turnau K, Ostachowicz B, Wojtczak G, Anielska T, Sobczyk Ł (2010) Metal uptake by xerothermic plants introduced into Zn-Pb industrial wastes. Plant Soil 337:299–311
Turnau K, Gawroński S, Ryszka P, Zook D (2012) Mycorrhizal-based phytostabilization of Zn–Pb tailings: lessons from the Trzebionka mining works (Southern Poland). In: Kothe E, Varma A (eds) Bio-geo interactions in metal-contaminated soils. Springer, Berlin, pp 327–348
Udayanga D, Liu X, McKenzie EH, Chukeatirote E, Bahkali HKD (2011) The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Divers 50:189–225
Verma VC, Gond SK, Kumar A, Kharwar RN, Strobel G (2007) The endophytic mycoflora of bark, leaf, and stem tissues of Azadirachtaindica A. Juss (Neem) from Varanasi (India). Microbiol Ecol 54:119–125
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Kogel KH (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci U S A 102:13386–13391
Wang XN, Huang WY, Du JC, Li CY, Liu JK (2014) Chemical constituents from the fruiting bodies of Xylaria euglossa Fr. and its chemotaxonomic study. Biochem Syst Ecol 54:157–159
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, London, pp 315–322
Wojtczak G (2013) Symbionty mykoryzowej storczyków z terenów skazonych metalami ciężkimi-badania molekularne. Dissertation, Jagiellonian University
Wong MK, Hyde KD (2001) Diversity of fungi on six species of Gramineae and one species of Cyperaceae in Hong Kong. Mycol Res 105:1485–1491
Wood PMR, Sivasithamparam K (1989) Diaporthe woodii (anamorph Phomopsis leptostromiformis)-A toxigenic fungus infecting cultivated lupins. Mycopathologia 105:79–86
Zhao J, Mou Y, Shan T, Li Y, Zhou L, Wang M, Wang J (2010) Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis. Molecules 15:7961–7970
Zielińska-Pisklak M, Szeleszczuk Ł, Wilczek K (2013) Dziewanna – starosłowiańska bogini wiosny. Lek w Polsce 3
Acknowledgments
This work was supported by The National Science Center (MAESTRO project) on the basis of DEC-2011/02/A/NZ9/00137 and a Grant from the Jagiellonian University (DS/MND/WBiN0Z/INoŚ/23/2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wężowicz, K., Rozpądek, P. & Turnau, K. The diversity of endophytic fungi in Verbascum lychnitis from industrial areas. Symbiosis 64, 139–147 (2014). https://doi.org/10.1007/s13199-015-0312-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-015-0312-8