Abstract
Effect of high pressure in inducing textural and functional modification has been investigated in pink perch (Nemipterus japonicus) mince. Fish mince undergone pressurization at 200, 400 and 600 MPa for a holding period of 10 min and was compared against cooked mince (90 °C; 40 min). The treated mince at 400 and 600 MPa lost its natural viscosity and behaved like cooked mince through denaturation and formation of protein aggregates. Textural characterisation showed the retention of tenderness in 200 MPa treated samples, but become harder on application of higher pressures. Unlike heat gels, pressure induced gels were more smooth, white and elastic in nature. A decreased in reactive SH groups was observed in 400 and 600 MPa treated samples due to the formation of disulfide bonds. Hydrophobic concentration was higher in cooked and 600 MPa treatments whereas Ca2+−ATPase activity decreased after pressurization. On application of different pressures microbial reduction of 2–3 log cycles was achieved in the mince samples. Hence pressure treatments at lower ranges cannot alter the texture and functionality of protein and the mince can undergo processing as required. Besides extending shelf life, the treatments above 400 MPa can make irreversible effect on texture quality and protein functionality which is similar to that of cooking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish being a source of healthy protein diet has received huge demand worldwide due to its high nutritional potential, easy digestibility and less costly over other meat counterparts. Developing high valued products from the fish provides us with a good alternative through which these benefits can be further utilized. However, the perishable nature of fish always reflects on product quality and its market value. Besides satisfying the consumer needs for healthy and nutritious food, maintaining quality and ensuring safety of the product is also given paramount importance.
In recent years many possibilities have been introduced in advanced technologies both in thermal and non-thermal sectors like high pressure processing, pulsed light, pulse electric field, cold plasma, microwave heating, di electric, Ohmic heating etc. for food processing. The possibilities in non-thermal preservation technologies have simultaneously gained importance, in response to the world-wide interest for more fresh and natural food products. Among the non-thermal technologies, High pressure processing (HPP) has turned to be an explored and exploited technology with a huge commercial potential. HPP products find its place in the world food market with high quality and high value addition (Sarika and Bindu 2019).
High Pressure Processing (HPP) is considered as the most successfully adopted non thermal technology in the food sector, by which the food products are subjected to a very high ranges of pressures (100–1000 MPa), having a lethal effect on the vegetative microorganisms, (both spoilage and pathogenic) and enzymes together with/without thermal destruction. The principle behind the high-pressure inactivation of microorganism and modification in the food properties are based on the protein denaturation, enzyme inactivation (Barbosa-Canovas et al. 1997) and agglomeration of cellular proteins (Farr 1990) in addition to the changes happening in the permeability of cell membrane. The vegetative microbial cells inactivation mostly occurs in the pressure range of 200 to 600 MPa at ambient conditions (Georget et al. 2015; Rastogi et al. 2007) which is mostly used in the industrial scenarios. Also it is stated that the microbial stability of food, is the result of a combination of different factors, but is determined by pressure effects on microorganisms in a matrix and the possibility of those to recover after treatment (Bolumar et al. 2020). Since this technology finds its major application in the field of food preservation by extending the shelf life of food and improve the quality and safety of products, so considered as a post package decontamination technology (Bajovic et al. 2012) or cold sterilization method. Because of this wide range of application, the number of HPP units in industrial operation is growing exponentially (Jung and Tonello-Samson 2018).
The attracting benefit of HPP in seafood industry is, retaining the active bioactive compounds in addition to maintaining the natural freshness, taste and texture of the product. Hence this technology has also created wide scope in the health foods sector or high valued nutraceutical products. As this HP process doesn’t involve heat, it can eliminate the development of cooked off flavours and textural changes which in conventional cooking impedes its acceptance as fresh foods. Hence the advantage of minimal effect on flavour and nutritional attributes of final products can be employed as a new dimension in product development. High pressure processing offers texturization in meat making use of its effect on fish myofibrillar proteins (Venugopal 2006). Now this technology also finds application in seafood industry for the production of surimi, kamaboko or other minced products. So high pressure can be used for making textural and functional modification through the process of cold gelation and unlike heat induced one the gel characteristics are different and needs to be studied. Extensive research was done in shelf life extension of various perishable fish and fish products by high pressure applications (Turong et al. 2015; Bindu and Das 2018; Sarika and Bindu, 2019). However, modifications in mechanical and functional properties of fish mince will create a new market demand for cold pasteurized fish foods. This potential application of HP has been achieved by making changes in the protein structure either reversibly or irreversibly at different pressure ranges without breaking covalent bonds unlike in cooking process.
The term fish mince is actually used for mechanically recovered fish flesh. Mincing occurs naturally during separation rather than deliberately as if it were passed through a mincer. Even fish mince can be indeed considered as a product that retains most of its textural characteristics when compared with fish (Taylor et al. 2007). Mincing helps in accelerating the deconformation, aggregation and cross linking of the myofibrillar proteins, that helps in developing various value added mince based products. In order to make value added products from fish, the mince has to undergone thermal modifications like cooking, pasteurization for better shelf life and once the product reaches consumer, it has to be cooked or reheated before consumption. These consecutive heating processes resulted in the loss of natural taste and texture of the product. Also, the consumer desire towards healthier foods fortified with bioactive compounds has created demand for the cold pasteurization methods. Pressure application minimised the protein denaturation, increased the nutritional and sensorial qualities and maintain inherent properties which can be best lose in conventional methods of processing.
Pink perch (Nemipterus japonicus) was taken as a candidate species for the study. This fish is an important deep-water inhabitant of family Nemipteridae, constitutes about 4.32% of the marine catch of India, but was considered as a trash fish earlier and hardly consumed until 1990. However, after the introduction of surimi technology in India, the Pink perch became one of the most important fish species in the manufacture of surimi because of its abundance, low cost and white meat. Now this fish is of great commercial value and find a diversified market with high export potential. In the light of this background, the focus of the study was to analyze the textural and functional alterations occurring in pink perch mince after different pressure treatments and compared against conventional process of cooking.
The observations of the study will help the food processors or researchers to use this technique as an alternative to conventional heating methods for the development of many mince-based products including surimi based or restructured products with minimum quality loss and extended shelf life. The significance lies in the introduction of a non-thermal processing method for enhancing the textural and functional quality of fish mince for developing novel products, which can satisfy the need for high quality, nutritionally healthier, minimal processed and safe foods without any additives.
Material and methods
Pink perch (Nemipterus japonicus) of about 200–250 g weight was selected as candidate species for the study. Fishes caught from the deep sea trawlers were brought to laboratory from local fish market of Cochin under iced condition. The fishes were thoroughly washed and cleaned with chilled potable water, weighed then descaled, headed and gutted before processing. Fillets were made manually and the muscle was extracted using a de-boning machine (BADDER 694, Lubeck, Germany) equipped with a drum of hole 3 mm diameter in size. The extracted pink perch mince was taken and filled in sausage type tubular casing (polyamide casing material of 30 mm dia) for attaining uniform and convenient shape. These samples were packed under 95% vacuum using a vacuum sealer (VAC-STAR, Model No: 101051) in EVOH pouches before processing. No ingredients were added and all chemicals used for analysis were of analytical grade and obtained from Merck (India), Sigma Aldrich Co and Hi-media.
The experimental design consists of 5 sets of samples of which 1st set was kept as control designated as raw/un cooked mince and 2nd as cooked mince (treated at 90 °C for 40 min). The 3rd, 4th and 5th sets were pressurized at 200, 400 and 600 MPa pressures with a dwell time of 10 min, process temperature of 30 °C and ramp rate of 600 MPa/min respectively. Each process was repeated for 4–5 batches. For analysis average triplicate sample size (n = 3) were taken on random basis in order to reduce the experimental errors. The samples were analysed for physico-chemical and functional changes and compared against the raw and cooked samples.
High pressure processing equipment
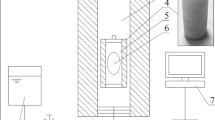
Application of pressure was done in HPP equipment, an iso-lab system (FPG 7100:9/2C, Stansted Fluid Power Ltd. Essex, UK) consisting a pressure chamber of cylindrical shape having dimension of 570 × 70 mm (length x diameter) and a holding capacity of 2L. The vessel is filled with pressure transmitting fluid consisting 30% propylene glycol in distilled water. A slight increase in temperature (2–4 °C) in the pressure transmitting fluid has been noticed with ramp rate, which was brought down to the set temperature by the cooling system of the machine (Ever Cool, Type EPIALT-7.5). Pressure holding time does not include pressure come-up or release time. An average come up time of 60–70 s for required pressure build up and a decompression time of 72 s was noticed. The process cycle is fully automated, following cycle initiation after loading the samples. Packed samples were subjected to a range of pressure like 200–600 MPa pressures at a constant temperature (30 °C) and holding time (10 min).
Conventional cooking
Conventional method of cooking fish mince was done in a water bath set at 90 °C. Mince samples filled in sausage type casing and packed in EVOH pouches were kept in water bath and cooked at a temperature of 90 °C for a period of 40 min. The heated samples were then immediately cooled down to 3–4 °C by keeping in ice and water for 15 min.
Physico-chemical analysis
Viscosity
Viscosity measurements were done in extracted mince using extraction buffer (EB) in the ratio 1:10 (mince: EB). The extract was then homogenized at 9000 rpm for 2 min and centrifuged at 9000 × g for 15 min at 4 °C (Binsi et al. 2007). The protein concentration in the supernatant was determined by Lowry method. Two related measures of fluid viscosity like kinematic and rotational viscosity was analysed for the samples.
The kinematic viscosity was determined for total proteins in extraction buffer by using Schott instruments with Micro Ostwald viscometer, Germany. This apparatus works by passing a specific quantity of extract through a capillary of defined width and length and measures its flow time in seconds. The kinematic viscosity is calculated using the instrument constant equation (v = K*t) where K = 0.01 mm2/s2and t is the flow time (s) and expressed in m2/s (SI Unit).
Rotational viscosity of total proteins extracted in extraction buffer was measured at 25 ± 1 °C by using a Brookfield digital viscometer (DV-E, USA) with a spindle LV1 and it is calculated by dividing shear stress by shear strain. When spindle begins to rotate at define speed, four torque values were recorded and the average viscosity was calculated. Triplicate measurements were taken for different shear (10–100 rpm) and the corresponding viscosity was recorded in centi poise (cP) or mPa.s units. Brookfield viscosity standard (10 cP) was used for calibration. Flow curve was plot against viscosity and shear rate and from the flow curve yield point or yield viscosity was calculated. Yield viscosity is the lowest shear stress value above which a material behaves like a fluid and below as solid. The measurements were taken by extrapolating the flow curve to the Y axis and corresponding values were calculated.
Texture profile analysis
Instrumental texture profile analysis (TPA was carried out in a texture analyser (TA Plus, Lloyd instruments-Ametek, Hampshire, UK) equipped with a 50 N load cell (Bourne 1978). Analysis was carried out after equilibrating the products at room temperature for 15 min and then placed the samples on the platform of TA machine and subjected to two bite test. TPA was done by using cylindrical plunger of 50 mm diameter with a test speed of 12 mm/min and a compression of 40%. The trigger was set at 1 N and force time curve was recorded for each test. From the recorded data, peak force, time difference and area of peaks were calculated and the texture parameters were computed using Nexygen software (Nexygen Plus, Lloyd Materials Testing).
Textural parameters were calculated as hardness (N) as the maximum peak force during the first compression cycle, cohesiveness as the ratio of the positive force areas during two compression cycle, springiness (mm) as the height that the sample recovers during the time elapses between the end of the first and the start of the second compression, gumminess (N) as the product of hardness and cohesiveness and chewiness (N.mm) as the product of gumminess and springiness.
Gel strength
Gel strength of the samples was determined using a steel ball probe having diameter 10 mm. A uniform sample dimension was made by cutting the mince at 4 × 2 cm width x height and before loading in the texture analyser (TA Plus, Lloyd instruments- Ametek, Hampshire, United Kingdom). Test speed was set at 50 mm/min and depression limit of 10 mm was used. The gel strength was expressed as the product of breaking force and breaking strain (g.cm).
Colour analysis
The color coordinates ie. L, a, b was measured using Hunter lab colorimeter (Hunter lab, Reston, VA, USA) based on the method described by Hunter and Harold (1987). The tri stimulus L* a* b* measurement mode was used as it relates to the human eye response to colour. The L* represents lightness ranges from 0 to 100 with 100 being absolute white and 0 for absolute black, + a ∗ denotes redness whereas − a ∗ denotes green and + b ∗ for yellowness and − b ∗ for blueness. Samples taken were calibrated to white and black calibration plate and the measurements were taken in triplicates from different spots and average value was taken.
Fourier transform- infrared spectroscopy (FTIR)
FTIR spectra of the samples in the range of 400 to 4000 cm−1 were determined using Thermo Nicolet Avatar 370 ESP infrared spectrophotometer at data acquisition rate of 4 cm-1 per point. Samples were made into tablets of 2 mg in 100 mg potassium bromide (KBr) approximately and spectra were obtained. All spectra obtained in terms of absorbance vs wave length were analysed after the background subtraction using OMNIC software (Thermo Fisher Scientific).
Determination of sulfhydryl (SH) groups
Protein preparation: Protein preparation was done based on the method developed by Jaczynski and Park (2004) and the concentration was adjusted to 1 mg/mL. Reactive (surface) and total SH groups were determined using the Ellman’s method (1959). The amount of total and reactive SH was measured at 412 nm with a molar extinction coefficient of 13,600 M–1 cm–1 using a spectrophotometer (Model DU640, Beckman Instruments).
Total SH (conc) or reactive SH is calculated as SH = A*D/ε where A = Absorbance, D = Dil. factor) and ε = molar attenuation coefficient.
Determination of surface hydrophobicity
Protein surface hydrophobicity was measured with bromophenol blue method developed by Chelh et al. (2006) based on the interaction of protein with hydrophobic chromophore bromophenol blue (BPB) and separated by centrifugation and the absorbance was measured at 595 nm against a blank of phosphate buffer. The bounded BPB was estimated by the formula:
Calcium-activated adenosine triphosphatase (Ca2+−ATPase) enzyme activity
Ca2+−activated ATPase enzyme activity was determined based on Noguchi and Matsumoto 1970 method modified by Binsi et al. (2007) for fish-based samples and expressed as µg P/mg protein/min at 27 °C. The inorganic phosphorus released was estimated by spectrophotometric reading taken at 660 nm. The determination of micromoles of phosphate liberated was done using a standard curve (Tausky and Shorr 1952).
Microbiological analysis
The enumeration of aerobic bacteria was done by estimating the aerobic plate count (APC) using pour plate technique (AOAC 2016). The 3 M petri films (FDA 2001) was used for adding the estimation of APC and the distribution of suspension was done with a plastic spreader device. Enumeration was done after incubation at 35 ± 2 °C for 48 ± 2 h.
Statistical analysis
SPSS 20 (IBM-statistical package for social science software, version 20, Chicago IL. USA) was used as software for the statistical data analysis. Experiments were done and the average of triplicates (n = 3) was taken as mean values with standard error (SE). Data analysis was performed using oneway Analysis of Variance (ANOVA) with post-hoc multiple comparison analysis using Duncan’s Multiple Range Test (DMRT). The level of significance was set up at p ≤ 0.05.
Results and discussion
Viscosity
Viscosity profile was done to analyse the resilience property of protein which are important for the quality evaluation of fish mince for its utilization in forming convenient type mince-based products. The measurements were done for both kinematic and rotational viscosities. Absolute viscosity often refers to the fluids internal resistance to flow on application of a force and the ratio between absolute viscosity to density is known as kinematic viscosity. A significant difference in kinematic viscosity was noticed (Fig. 1a) after high pressure processing. For viscosity measurements the protein concentration was adjusted to 1 mg/ml after extraction in all samples. Mince in raw state had a kinematic viscosity of 3.26 ± 0.44 × 10–6 m2/s which showed a reduction, after cooking and high pressure applications. The reduction was less observed at 200 MPa (2.22 ± 0.02 × 10–6 m2/s) due to less conformational changes occurred at that pressure (Zare 2004; Ramirez-Suarez and Morrissey 2006). So that after heating, the mince might have lost its natural viscosity due to heat induced protein denaturation. Similarly, pressurization at 400 MPa and above, the loss of natural viscosity might have happened and so, the samples treated at 400 and 600 MPa behaved like cooked ones. In turn the solubility pattern was also comparable with the changes in viscosity, profile when studied. The variation in the reduced viscosity value at any given concentration of protein solution is influenced by various factors such as nature of protein and other composite substances (Karthikeyan et al. 2006). The drastic reduction in viscosity is stated as that under certain conditions like heating or pressurization fish proteins are more susceptible in forming aggregates that reduces the protein solubility and viscosity (Jimenez-Colmenero and Borderias 1983). The viscosity mainly associated with myofibrillar proteins and reduction in viscosity measurements indicates association–dissociation/aggregation phenomenon of proteins especially in cooked, 400 and 600 MPa treated samples. Ojagh et al. (2011) observed a similar reduction in viscosity of Atlantic salmon muscle, subjected to high pressure processing (300 MPa for 5 °C and 40 °C) and compared against cooked muscle.
Rotational viscosity refers to the fluid viscosity at a given shear rate and it is calculated by dividing shear stress (from torque) by shear strain (angular velocity). Flow curves were plotted for each treatment samples and the yield viscosity were calculated. The yield point measurements were calculated by extrapolating the flow curve to the Y axis. From the curve it is observed that similar to cooked mince, the treated mince (Fig. 1b) at 400 and 600 MPa exhibited a yield viscosity and this yield viscosity is due to random aggregation of protein, which showed a thinning behaviour on further shearing. So, the natural viscosity of the mince was lost in high pressure treated samples above 400 MPa as in cooked ones. The changes observed in mince below 400 MPa treatments were smaller when compared to untreated/raw mince which might due to unique nature and size of protein aggregates formed during cooking and different high-pressure conditions (Ojagh et al. 2011).
Texture profile analysis
Table 1 shows the changes in textural parameters of fish mince after pressure treatment and heat application. The mince after cooking showed a hardness of 10.47 ± 0.67 N but in pressure treatments it was observed in the range of 5.49 ± 0.10 N to 13.31 ± 1.13 N. The lower hardness value observed in 200 MPa, indicates the retention of tenderness in mince but increased with an increase of pressures. The pressure can induce protein denaturation, aggregation and gelation similar to that happened in the heat gelation. But the nature of the bonds formed during heating and pressurization was different that can cause reversible and irreversible effect on structural modification. At lower pressure ranges, non covalent interactions were usually formed which are sometime reversible (Sarika et al. 2019). HP above 400 MPa can cause irreversible protein denaturation due to more unwinding of myofibrillar protein and the formation of new hydrogen bonded networks, which eventually increased the hardness of the product.
Retention of tenderness after HPP treatment (200 MPa) is due to the breakdown of lysosome and subsequent release of proteolytic enzymes to the medium (Hugas et al. 2002). Also the connective tissue protein like collagen is stabilized by hydrogen bonds, which are least affected by low pressures, but pressure effects are mostly based on myofibrillar fractions (Gekko and Koga 1983). Hardness of mince was also contributed by the formation of S–S bonds at higher pressures, which is similar to the di sulphide interactions during cooking. Hence at low pressures, tenderness was retained in mince whereas higher pressure can impart hardness or toughness to the fish mince. Since the 200 MPa treated mince didn’t show detrimental changes in textural and functional qualities, it can be reprocessed for making products without losing the functionality.
A similar cohesiveness and springiness nature was observed in pressure treated and heat-treated samples. The changes were not significant between the 200–400 MPa treatments but cohesive and elastic property of the 600 MPa treated mince was similar to cooked gels. This might be due to the ordered aggregation of protein and formation networks at high pressures which resulted in characteristics more near to gelation. Gumminess and chewiness had followed a similar trend with hardness values. The springiness and chewiness are used to determine the muscle protein gel textural characteristics. Suzuki et al. (2006) reported that above 200 MPa the cohesion between mince protein was increased in reformed meat or fish type products. Similar observation by Angsupanich and Ledward (1998) that the characteristic pressure-treated texture of cod fish meat is different from that seen in both raw or cooked ones, being harder, chewier and gummier than the cooked product which appear to be the different response of the myofibrillar proteins to heat and pressure.
Gel strength
Like thermal gelation, high pressure can also induce gelation in fish mince. Changes in the gel strength properties of fish mince after different treatments are shown in Fig. 2. Pressure processed gels at 400 MPa (300.29 ± 18.12 g.cm) showed gel strength similar to that of cooked gels (293.34 ± 7.97 g.cm) and 600 MPa gels were showing the highest value. Gel strength of 134.32 ± 1.83 g.cm was observed in 200 MPa, which are weak gels but were glossier and less opaque in appearance than hard cooked gels. Gelation of muscle proteins results from the transformation of an amorphous viscous solution to a three-dimensional elastic network (Karthikeyan et al. 2006). Results shows that pressure induced solubilization might have happened in the mince without the prior formation of a sol state by additives or salt or formed with mechanical action which resulted in an ordered aggregation of myofibrillar protein, similar to that happened in heat gelation. High pressure can depolymerise actomyosin and actin as well as enhance solubilization of myofibrillar proteins; (Cheftel and Culioli 1997). The effects of HPP upon gelation properties are different and depends upon the protein, treatment conditions such as the applied pressure, duration, and temperature under pressure etc. (Jimenez Colmenero et al. 1998; Jimenez Colmenero 2002). The mechanism of protein denaturation differs depending on the pressure/temperature combinations as well as state of meat; whether raw or heated (Sun and Holley, 2011; Jimenez Colmenero 2002).
Proper gel strength was observed in pressure gels at 400 MPa and above, even though the nature of the pressure and heat induced gels was different. The findings were supported by the observations of Messen et al. (1997) where pressure upto 300 MPa myosin tail–tail interactions were not involved, resulting in the formation of weaker gels than heat induced. In cooking the difference is constituted by the extensive unfolding of proteins, which resulted in more stable networks with higher interactions. Similar observation by Ashie and Lanier (1999) was noticed during high pressure effect (100–300 MPa) on the gelation of Alaskan pollack surimi and turkey breast muscle and higher values were observed after 400 and 600 MPa pressurization with more similar values in cooked and 400 MPa. Higher pressures can induce more protein unfolding and stronger interactions, making the gels stronger and the gels formed were smoother and more elastic especially in marine fish species and organoleptically superior than those produced by heat (Sarika and Bindu 2019; Bindu et al. (2013).
Colour attributes
High pressure provokes drastic changes in colour attributes of fish mince. The lightness values indicated by L* increased with increases in pressure especially in cooked and 600 MPa treated samples. The colour attributes of raw mince were observed as L* 53.18 ± 0.73, a* 1.77 ± 0.19 and b*15.41 ± 0.41 value, but after heating the values changed as L* 69.08 ± 0.19, a*0.13 ± 0.01 and b* 16.07 ± 0.20. The changes in colour attributes especially L* and b* were not significant at 200 MPa pressurization (L* 55.07 ± 0.19, a*3.35 ± 0.06 and b* 15.19 ± 0.33), but an increase was noticed in a* value. The increase in a* might correspond to the release of myoglobin into the muscle. However, application of pressure above 200 MPa showed an increase in colour attribute as L * 69.03 ± 0.12, a*0.85 ± 0.17 and b* 12.76 ± 0.12 and L* 69.36 ± 1.34, a* 0.79 ± 0.14 and b*12.25 ± 0.21 in 400 MPa and 600 MPa respectively. The changes in the values were observed to be similar as in cooked mince. The increase in L* value and decrease in a* value corresponds to the loss of transparency, which is due to the denaturation of globin protein and the release of heme, making the mince whiter as observed in cod and mackeral subjected to high pressure (Oshima et al. 1993). Truong et al. (2016) found high pressure above 200 MPa resulted in increased whiteness and loss of translucency in barramundi fish muscle. The raw mince colour was retained below 150 or 200 MPa, and with 250 MPa or higher, muscle become opaque like milky white as if it is cooked. An increase in pressure causes decrease in a* and increase in b* values, which corresponds to the loss of natural colour of the mince. These changes in the colour attributes of fish meat at higher pressure ranges will impair the consumer’s perspective of retaining raw colour and texture after high pressure processing and affect its commercialization. However, these changes are not so relevant in fish mince, because it is further processed for making various mince-based products.
Total and reactive sulfhydryl (-SH) groups
The sulfhydryl groups on the protein surface are considered to be the most important functional groups in proteins (Cao, et al., 2012). The quantification of SH groups can be used to analyse the conformational changes in protein.
The total and reactive (surface) or free SH groups in pink perch mince changed on application of pressure and heat (Fig. 3). The total and reactive SH in control raw mince was found to be 17.29 ± 0.48 µmoles/g and 9.90 ± 0.28 µmoles/g respectively. On application of pressure, total SH groups increased to 24.21 ± 0.52 µmoles/g, and reactive or free SH groups decreased to 5.25 ± 1.11 µmoles/g, at 200 MPa and gradually decreased with increase in pressure. Cooked mince had the lowest levels of SH groups with 8.40 ± 0.99 µmoles/g (total) and 0.55 ± 1.07 µmoles/g (reactive) respectively, due to heat-induced denaturation and subsequent formation of disulfide bonds. Increase in total SH groups after HPP is due to the conformational changes that happened in the protein which promotes myosin unfolding causing more exposure of buried sulfhydryl groups (Chen et al. 2014). Sulfur-containing amino acids, especially methionine, are among the most hydrophobic amino acids which always found in the interior of protein. High pressure can make available of these amino acids for interactions. Similar to cooking the formation of S–S bonds were noticed more at higher pressures of 400 and 600 MPa, leading to the decrease in reactive and total SH groups. The energy generated during HP treatment is not enough to disrupt S–S bonds (213.1 kJ/mol), as a very high pressure of 10,000 MPa can only provide 8.37 kJ/mol of energy (Wang et al. 2019). The increase in reactive SH groups at 200 MPa was also likely because of reduction of SS bonds to SH groups and lower protein degradation. Wang et al. (2019) also observed positive relationship in low salt golden threadfin bream myosin between R-SH content and high pressure indicating greater myosin unfolding when the applied pressure increased (0–600 MPa).
Surface hydrophobicity
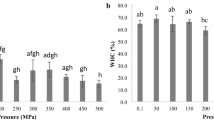
The surface hydrophobicity is related to the amount of hydrophobic amino acid residues distributed on the protein surface which significantly influences the aggregation of myosins (Nakai and Li-Chan 1988). The surface hydrophobicity of the protein was measured using bromophenol blue method developed by Chelh et al. (2006) and the changes observed with pressure and heat, shown in Fig. 4. Were statistically significant (ANOVA p < 0.05). This method was used as a non-invasive approach account for the total hydrophobic residues based on the interaction of protein with hydrophobic chromophore bromophenol blue (BPB) which was separated by centrifugation and measured spectrophotometrically at 595 nm. Mince in raw state (control) had a hydrophobic concentration of 836.84 ± 15.27 µg BPB/g protein increased to 1090.77 ± 10.01 µg BPB/g protein after cooking and increased to 888.78 ± 35.11, 981.12 ± 15.27 and 1021.52 ± 10.27 µg BPB/g protein on application of 200, 400 and 600 MPa pressures respectively. Increase in surface hydrophobicity is due to the increase in the exposure of hydrophobic core, which in turn depends on the extent of pressure applied. The sulfhydryl and hydrophobic groups were mainly present at the interface of α-helical regions located in the myosin interior (Guo et al. 2017) and transfer of these residues to the surface suggests disruption of the myosin α-helices by external pressure. So, the denaturation and degradation of protein most likely resulted in exposure of hydrophobic core, which are normally buried in the interior of protein structure. When pressure application increased from 200 to 600 MPa, the concentration of hydrophobic cores increased similar to that in cooking. So, application of pressure and heat resulted in more exposure of these hydrophobic cores, and as a result the hydrophobicity was found to be more in cooked and 600 MPa treated samples. The results indicated that absorption difference spectroscopy of BPB provides a valuable supplement to fluorescence for determining the presence of hydrophobic sites on the surface of proteins as well as a method for measuring binding constants (Bertsch, 2003).
Ca2 + -ATPase activity
The determination of actomyosin Ca2+ ATPase activity extracted from the fish mince can be considered as an index of protein quality of the fish species. The raw mince had an activity of 0.25 ± 0.002 µg Pi/mg protein/min, which decreased to 0.2 ± 0.004 µg Pi/mg protein/ min on cooking. But at 200, 400 and 600 MPa the activity reduced from 0.23 ± 0.01, 0.22 ± 0.002 to 0.21 ± 0.001 µg Pi/mg protein/ min respectively. Even though the reduction in values observed was small, it was statistically significant (ANOVA, P < 0.05) during high pressure application. The gradual reduction in enzyme activity was found at increasing pressure application, indicating that myosin underwent denaturation at high pressures. Quali and Valin (1981) stated that activity loss in Ca2 + -ATPase enzyme was due to proteolysis of myosin molecules. Also observations from Ko et al. (2006) supported that actomyosin extraction decreased after pressure application in tilapia meat. Ko (1996) observed that actomyosin extraction in milk fish was not affected when pressurized below 100 MPa, but the activity reduced to 20% of fresh meat by 200 MPa due to protein denaturation. Since extractable actomyosin is thought to be from undenatured protein, reduction in Ca2+−ATPase activity at higher pressures accounts for greater protein denaturation and reduction in extractable actomyosin concentration. The result also indicates that application of high pressures in the applied range did not cause inactivation of Ca2+−ATPase activity; instead, the activity got reduced in pink perch mince when compared to raw mince.
FTIR spectroscopy
The changes in FTIR spectra of pressurized samples in respect to control are shown in Fig. 5. Which helps to examine the changes in the secondary structure of protein due to heat and pressure treatment. The protein characteristics set of absorption regions when exposed to infrared spectrum were identified as Amide A, B and Amide I-VII and arise from the amide bonds of the protein. Among them, vibrational bands of the protein backbone (Amide I and Amide II bands) are sensitive to secondary structure of a protein.
The Amide I band of the raw mince was found 1656.91 cm−1. However, upon heat cooking and pressure treatment there is a shift in the Amide I band. The amide I band for the cooked and pressure treated sausages are 1606.65 cm−1 (cooked), 1657.26 cm−1 (200 MPa) and 1657.28 cm−1(400 MPa). The exact absorption peak of the Amide I depends on the hydrogen bonding and conformation of the protein structure (Uriarte-Montoya et al. 2011). The shifting of the Amide I band due to application of different treatments can cause pressure induced changes in the chemical bonds of the protein. High pressure can cause more unfolding of the protein structure and creates non covalent interactions. Pressure-induced frequency-shifts between the involved atoms have been reported in FTIR characteristics of protein (Heremans et al. 1996; Taniguchi and Takeda (1996); Liu et al., 2021). This is due to O–H hydrogen bond strengthening ‘at the expense’ of the C=O bond. Further study on the resolution of Amide I band will help in clearly identifying the nature of secondary structure such as α-helix, β-sheet or random coil of the protein.
Microbial quality
The commercial application of high pressures in the food sector focused on shelf life extension of the products through microbial inactivation. Table 2 shows the changes associated with different processing conditions in the aerobic count. Uncooked mince had an initial plate count of 4.92 ± 0.01 log10 CFU/g. After 200 MPa pressure application, the total aerobic count in the mince was observed to be 3.63 ± 0.03 log10CFU/g, showing a reduction of 1 log cycle whereas in 400 and 600 MPa pressure application, a log reduction of 2.26 ± 0.01 and 1.53 ± 0.02 log10CFU/g was achieved. Also 2-log reduction was achieved after cooking (2.28 ± 0.02 log10CFU/g). Statistically results showed a significant reduction from one to three log cycles when pressures increased from 200 to 600 MPa (ANOVA p < 0.05). Lau and Turek (2007) reported that pressures above 200 MPa and above normal temperature, can inactivate most of the vegetative and pathogenic microorganisms and the inactivation depends on peak pressures. Principle of microbial inactivation is that high pressure can induce protein denaturation resulting in disruption of cellular bound enzymes. Also pressure can cause cell membrane damage that affect the cellular fluid transport mechanism and microbial enzyme denaturation, resulting in inactivation of microorganisms (Torres and Velazquez (2005). Protein denaturation and subsequent inactivation of microorganisms were found to be more in high pressure treated minces especially above 400 MPa, which can be well utilized for ready to eat food products.
Conclusion
On subjecting pink perch mince to high pressures of 200, 400 and 600 MP for a holding time of 10 min, texture characteristics were not affected upto 200 MPa but at higher pressures properties changed. Gel strength increased with increase in pressures. High pressure application at 400 and 600 MPa made considerable changes in total and reactive SH groups and surface hydrophobicity measurements. Hence the loss of protein functionality was not observed at 200 MPa pressure, but at higher pressure ranges the effect on fish mince was similar as cooking. Changes in protein conformation were minimum at 200 MPa. Microbiologically 2–3 log reduction was achieved when pressure increased from 200 to 600 MPa. So, application of pressure at lower ranges can retain the functionality of the protein, but above 400 MPa, pressure can be used to modify the textural and functional properties. Hence pink perch mince processed at higher pressure ranges can be used for extending shelf life. In contrast, treated mince at lower pressures can be further utilized for fabrication of many value-added fish products like mince based or surimi based, restructured and imitation products with enhanced functionality.
References
Angsupanich K, Ledward DA (1998) High pressure treatment effects on cod (Gadus morhua) muscle. J Food Chem 63(1):39–50
AOAC 2016. Official Methods of Analysis of AOAC International. 990.12. Aerobic plate count in foods (17.2.07), Chapter 17 pp 12.
Ashie INA, Lanier TC (1999) High pressure effects on gelation of surimi and turkey breast muscle enhanced by microbial transglutaminase. J Food Sci 64:704–708
Bajovic B, Bolumar T, Heinz V (2012) Quality considerations with high pressure processing of fresh and value-added meat products. Meat Sci 92:280–289
Barbosa-Canovas GV, Pothakamury UR, Palou E, Swanson BG (1997) Nonthermal preservation of foods. Marcel Dekker, New York
Bindu J, & Das S 2018. High-pressure applications for preservation of fish and fishery products. *Research Methodology in Food Sciences: Integrated Theory and Practice*, 341.
Bindu J, Ginson J, Kamalakanth CK, Asha KK, Gopal TKS (2013) Physico-chemical changes in high pressure treated Indian white prawn (Fenneropenaeus indicus) during chill storage. Innov Food Sci Emerg Technol 17:37–42
Binsi PK, Shamasundar BA, Dileep AO (2007) Physico-chemical and functional properties of proteins from green mussel (Perna viridis) during ice storage. J Sci Food Agric 87(2):245–254
Bolumar T, Orlien V, Sikes A, Aganovic K, Bak K, Guyon C, Stübler A-S, Lamballerie M, Hertel C, Brüggemann D (2020) High-pressure processing of meat: molecular impacts and industrial applications. Comprehensive Rev Food Sci Food Safety. https://doi.org/10.1111/1541-4337.12670
Bourne MC (1978) Texture profile analysis. Food Technol 32:62–72
Cao Y, Xia T, Zhou G, Xu X (2012) The mechanism of high pressure-induced gels of rabbit myosin. Innov Food Sci Emerg Technol 16(39):41–46
Cheftel JC, Culioli J (1997) Effects of high pressure on meat: a review. Meat Sci 46:211–234
Chelh I, Gatellier P, Sante-Lhoutellier V (2006) Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci 74(4):681–683
Chen X, Chen CG, Zhou YZ, Li P, Ma F, Nishiumi T (2014) Effects of high pressure processing on the thermal gelling properties of chicken breast myosin containing κ-carrageenan. Food Hydrocolloids 40:262–272
Farr D (1990) High pressure technology in the food industry. Trends Food Sci Technol 1:14–16
Gekko K, Koga S (1983) The effect of pressure on thermal stability and in vitro 16 fibril formation collagen. Agric Biol Chem 47:1027–1033
Georget E, Sevenich R, Reineke K, Mathys A, Heinz V, Callanan M, Knorr D (2015) Inactivation of microorganisms by high isostatic pressure processing in complex matrices: a review. Innov Food Sci Emerg Technol 27:1–14
Guo M, Liu S, Ismail M, Farid MM, Ji H, Mao W et al (2017) Changes in the myosin secondary structure and shrimp surimi gel strength induced by dense phase carbon dioxide. Food Chem 227:219–226
Heremans K, Goossens K, Smeller L (1996) Pressure-tuning spectroscopy of proteins Fourier transform infrared studies in the diamond anvil cell. In: Markley JL, Northtrop DB, Royer CA (eds) High-pressure effects in molecular biophysics and enzymology. Oxford University Press, New York
Hugas M, Garriga M, Monfort JM (2002) New mild technologies in meat processing: high pressure as a model technology. Meat Science. 62:3
Hunter RS, Harold RW (1987) The measure of appearance. Willey Interscience, New York
Jaczynski J, Park JW (2004) Physicochemical changes in Alaska pollock surimi and surimi gel as affected by electron beam. JFS: Food Chem Toxicol 69(1):53–57
Jiménez Colmenero F (2002) Muscle protein gelation by combined use of high pressure/temperature. Trends Food Sci Technol 13:22–30
Jiménez Colmenero F, Fernández P, Carballo J, Fernández-Martín F (1998) High-pressure-cooked low-fat pork and chicken batters as affected by salt level and cooking temperature. J Food Sci 63:656–659
Jung S, Tonello-Samson C (2018) High hydrostatic pressure food processing: potential and limitations. In: Proctor A (ed) Alternatives to conventional food processing, 2nd edn. The Royal Society of Chemistry, London, pp 251–315
Karthikeyan M, Dileep AO, Shamasundar BA (2006) Effect of water washing on the functional and rheological properties of proteins from threadfin bream (Nemipterus japonicus) meat. Int J Food Sci Technol 41(9):1002–1010
Ko WC (1996) Effect of high pressure on gelation of meat paste and inactivation of actomyosin Ca-ATPase prepared from Milkfish. Fisheries Sci 62:101–104
Ko W-C, Jao C-L, Hwang J-S, Hsu KC (2006) Effect of high-pressure treatment on processing quality of tilapia meat fillets. J Food Eng 77(4):1007–1011
Lau MH, Turek EJ (2007) Determination of quality difference in low-acid foods sterilized by high pressure versus retorting. In: Doona CJ, Feeherry FE (eds) High pressure processing of foods. Blackwell Publishing
Liu H, Xu Y, Zu S, Wu X, Shi A, Zhang J, Wang Q, He N (2021) Effects of high hydrostatic pressure on the conformational structure and gel properties of myofibrillar protein and meat quality: a review. Foods 10:1872. https://doi.org/10.3390/foods10081872
Messens W, Van Camp J, Huyghebaert A (1997) The use of high pressure to modify the functionality of food proteins. Trend Food Sci Technol 8:107–112
Nakai S, Li-Chan E (1988) Hydrophobic interactions in food systems. CRC Press
Noguchi S, Matsumoto JJ (1970) Studies on the control of the denaturation of the fish muscle proteins during frozen storage. I. Preventive effect of sodium glutamate. Bull SosSci Fish 36:1078–1087
Ohshima T, Ushio H, Koizumi C (1993) High pressure processing of fish and fish -products. Trends Food Sci Technol 4(11):370–375
Ojagh SM, Nunez-Flores R, Lopez-Caballero ME, Montero MP, Gomez-Guillen MC (2011) Lessening of high-pressure-induced changes in Atlantic salmon muscle by the combined use of a fish gelatin–lignin film. Food Chem 125(2):595–606
Quali A, Valin C (1981) Effect of muscle lysosomal enzymes and calcium activated natural proteinase on myofibrillar ATPase activity: relationship with aging changes. Meat Sci 5:233–245
Ramirez-Suarez JC, Morrissey MT (2006) Effect of high pressure processing (HPP) on shelf life of albacore tuna (Thunnus alalunga) minced muscle. Innov Food Sci Emerg 7(1–2):19–27
Rastogi NK, Raghavarao KSMS, Balasubramaniam VM, Niranjan K, Knorr D (2007) Opportunities and challenges in high pressure processing of foods. Critical Reviews in Food Science and Nutrition 47(1):69–112. https://doi.org/10.1080/10408390600626420
Sarika Kunnath and Bindu Jaganath (2019) Non thermal processing of sea foods. In: Chauhan OP (ed) Non thermal processing of foods. CRC Press, pp 373–394
Sun XD, Holley RA (2011) Factors influencing gel formation by myofibrillar proteins in muscle foods. Comprehensive Rev Food Sci Food Safety 10:33–51
Suzuki A, Kim K, Tanji H, Nishiumi H, Ikeuchi Y (2006) Application of high hydrostatic pressure to meat and meat processing. In: Nollet LML, Toldra F (eds) Advanced technologies for meat processing. CRC Press, pp 194–217
Taniguchi Y, Takeda N (1996) High-pressure FTIR studies of the secondary structure of proteins. In: Markley JL, Northtrop DB, Royer CA (eds) High-pressure effects in molecular biophysics and enzymology. Oxford University Press, pp 87–95
Tausky HH, Shorr E (1952) A microcolorimetric method for the determination of inorganic phosphorus. J BiolChem 202:675–685
Taylor KDA, Himonides A, Alasalvar C (2007) 5- Increased processed flesh yield by recovery from marine by-products. In: Fereidoon S (ed) Woodhead publishing series in food science, technology and nutrition, maximizing the value of marine by products. Woodhead publishing
Torres JA, Velazquez G (2005) Commercial opportunities and research challenges in the high pressure processing of foods. J Food Eng 67:95–112
Truong BQ, Buckow R, Stathopoulos CE, Nguyen MH (2015) Advances in high-pressure processing of fish muscles. Food Eng Rev 7(2):109–129
Truong BQ, Buckow R, Nguyen MH, Stathopoulos CE (2016) High pressure processing of barramundi (Lates calcarifer) muscle before freezing: the effects on selected physicochemical properties during frozen storage. J Food Eng 169:72–78
Uriarte-Montoya MH, Santacruz-Ortega H, Cinco-Moroyoqui FJ, Rouzaud-Sandez O, Plascencia-Jatomea M, Ezquerra-Brauer JM (2011) Giant squid skin gelatin: chemical composition and biophysical characterization. Food Res Int 44:3243–3249
Venugopal V (2006) Mince and mince-based products Seafood Processing adding value through quick freezing retortable packaging cook chilling. CRC Press
Wang J, Lia Z, Zhenga B, Zhanga Y, Guo Z (2019) Effect of ultra-high pressure on the structure and gelling properties of low salt golden threadfin bream (Nemipterus virgatus) myosin. LWT Food Sci Technol 100:381–390
Zare Z 2004. High pressure processing of fresh tuna fish and its effects on Shelf Life. MSc Thesis. McGill University, Montreal, Quebec, Canada, pp. 45–76
Acknowledgements
Authors are grateful to the Indian Council of Agricultural Research for the technical and financial support. Authors also express gratitude to STIC (Sophisticated Test and Instrumentation Centre), CUSAT, Cochin for availing their instrumentation facility.
Funding
Authors express gratitute towards Indian Council of Agricultural Research for the funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kunnath, S., Jaganath, B., Panda, S.K. et al. Modifying textural and functional characteristics of fish (Nemipterus japonicus) mince using high pressure technology. J Food Sci Technol 59, 4122–4133 (2022). https://doi.org/10.1007/s13197-022-05466-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-022-05466-5