Abstract
Despite the effective microbial inactivation of high pressure processing (HPP), too intensive pressurization can cause undesirable quality changes in fish muscle. Combination treatments can be applied to retain sensory attributes after HPP for ready-to-eat fishery products. This present study thus investigated the impacts of different initial sample temperatures (− 18, 2, 20, and 55 °C) on raw grass carp slices under 300, 400, and 500 MPa, followed by microbiological, sensorial, chemical, and structural analyses. Increasing the pressure level at 20 °C led to whitening, hardening, and decreases in water holding capacity of grass carp. For example, 400 MPa induced a L* increase from 59.6 to 80.7, loss in muscle transparency, 5.1% of weight loss, and a hardness increase from 390.2 to 540.5 g. Correspondingly, fiber shrinkage and the compaction of tissue structure were observed in pressurized fish muscle. When grass carp were pressurized at a low initial temperature (2 °C), the appearance remained semi-transparent under 300 MPa, and the changes in physicochemical quality were similar to the treatments at 20 °C. HPP treatments at a subzero (− 18 °C) or mild-heated (55 °C) initial temperature caused greater quality changes when compared to other temperature conditions, e.g., L* > 70 under 300 MPa, and hardness > 700 g under 400 MPa. The myofibrillar protein contents dropped more significantly as the initial temperature increased. Overall, a better understanding of the impacts of pressurization temperatures would thus help to monitor fish quality and develop appropriate means for novel ready-to-eat fish-based products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amongst the most widely marketed food products, aquatic foods have yielded up to 178 million tons of global production in 2020 [1]. In many countries and regions, people have the habit of consuming raw aquatic foods, e.g., crabs, clams, hake, and salmon for Portugal [2], or raw/marinated fish fillets, shrimps, crabs, shellfish, squids in south China. However, safety issues often arise because aquatic foods are highly susceptible to microbial activity. High pressure processing (HPP) is a highly effective method for bacterial inactivation and minimum quality alteration [3].
HPP has been applied to prolong the shelf life of fish [4] and crustaceans [5]. In case of shellfish, pressurized products have been indicated to have satisfactory sensory preferences and overall attributes similar to raw products [6, 7]. However, too intense pressure treatments can induce undesirable quality alteration of some certain aquatic species: for example, raw fish meat is highly sensitive to pressure, and the appearance turns opaque under 200–300 MPa [8]. Evident changes in color, hardness, and water holding capacity (WHC) of fish muscle have been found correspondingly [9,10,11]. Factors influencing the quality changes after HPP treatments involve aquatic food species, pressure intensity, pressurization duration, temperature, and so on [12].
Increasing or decreasing pressurizing temperature has been proven to enhance microbial inactivation after HPP treatments [13]. For example, lowering the pressurizing temperature from 15 to 1.5 °C was found to cause an increased reduction of Vibrio parahemolyticus by 2.3 log CFU/g [14], and the Vibrio vulnificus counts were decreased by 3–4 log CFU/g at 40–50 °C under 150 MPa for 4 min [15]. In addition, low temperatures (5 °C or subzero conditions) were able to reduce the changes in surface color and hardness of beef and pork after 200–800 MPa [16,17,18]. HPP conducted at mild heat (40 °C) has been investigated to cause less cook loss and strong WHC in beef under 200–400 MPa at 40 °C when compared to 20 °C [19]. Appropriate settings of treating pressure and temperature can thus have the potential to improve the quality of food products. Grass carp is amongst the most common ingredients for traditional Cantonese-style sashimi (raw ready-to-eat fish) products. Despite the remarkable microbial inactivation effects of HPP, there are still challenges regarding preserving the raw attributes during the processing of fish meat due to the sensitivity to pressure [20]. The investigation on temperature–pressure combination techniques would be expected to be helpful in providing indications for a novel ready-to-eat fish product in practice. However, the impacts of temperatures and pressure on fish quality still require to be clarified.
Therefore, this present study focused mainly on the quality changes in grass carp products after HPP treatments, by evaluating the microbial and physicochemical properties in the cases of different high pressure intensities and initial sample temperatures. Besides, techniques such as sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Raman spectroscopy were mainly used to explain the mechanisms of HPP-induced quality changes by measuring the protein denaturation degree [20, 21]. The present study thus aimed at the alteration in muscle microstructure by using an optical microscope and a scanning electron microscope.
Materials and methods
Raw fish material
Fresh grass carps (Ctenopharyngodon idellus) were obtained from a local supermarket (Freshhema Supermarket, Guangzhou, China), where the fish were able to be kept alive in a temporary cultivation tank. The grass carps (ca. 6 kg for each) were slaughtered and gutted at the supermarket, and then directly transported on ice to the laboratory within 30 min for further preparation. After washing the fish’s surface, inedible parts such as fish heads, fins, and tails were cut from the fish body using a standard kitchen chopping knife. The fish were manually filleted, carefully removing the major bones and obtaining the muscle. To ensure uniformity in quality, the central dorsal parts of the muscle were further manually processed into slices as samples (ca. 1 cm thick, ca. 10 g). For each grass carp fillet, nine slices were obtained. All samples were weighed and individually vacuum-packed in PE bags for further HPP treatments.
High pressure processing (HPP) treatments
In this study, four groups of grass carp fillets—frozen status (FS), low temperature (LT), room temperature (RT), and mild heat (MH)—were treated with HPP at different initial sample temperatures (− 18 °C for FS, 2 °C for LT, 20 °C for RT, and 55 °C for MH). To ensure that the initial temperatures reached the desired values, the samples were prepared one day before the HPP treatments (Section Raw fish material) and temporarily stored in a standard kitchen fridge/freezer (Midea, China) for 24 h. For the FS group, freezing was done at − 18 °C for 24 h prior to the HPP treatments, and the freezing rate was approximately 2 °C/h. For the other groups, the samples were stored at 2 °C for 24 h.
HPP treatments were performed under 300, 400, and 500 MPa for 10 min, by using a hydrostatic press (UUPF/5L/800 MPa, KeFa High Pressure Technology Co., Ltd, China) with a pressure chamber of 5 L and a maximum pressure of 500 MPa. The pressurizing rate was settled at 300 MPa/min, and a rapid decompression was achieved within 10 s. The whole HPP experiments were repeated three times as three repetition batches, and nine slices obtained from the same fillet were treated under each HPP condition in each batch.
For the RT group, the samples were directly pressurized at room temperature (20 °C), using water in the pressure chamber as the pressure transmitting medium. For the FS and LT groups, nine slices were first transferred from the fridge to a 500-mL insulation PTFE barrel (filled with ice water at 0 °C) within 5 min and brought to the HPP device (Fig. 1). Considering that heat transfer could occur between the PTFE barrel and HPP chamber, the core temperature of samples was measured by using a thermocouple data logger (Center 309, Center Technology Corporation, China) before and after HPP treatments. For the MH group, the heat insulation barrel containing nine slices and warm water at 55 °C was put into the HPP chamber. The temperature changes before and after pressurization were also recorded. Table 1 shows the temperatures of the fish slices before and after HPP treatments. In total, 324 slices (9 × 3 pressure levels × 4 temperatures × 3 repetition batches) were pressurized, and in the meantime, 27 (9 × 3 repetition batches) unpressurized samples (0.1 MPa) and 27 (9 × 3 repetition batches) cooked samples (after boiling in hot water at 100 °C for 1 min) were also prepared as the experimental controls. Out of the nine samples from each batch under each treatment condition, six slices were randomly selected for photographing and physicochemical assessments including weighing, color, texture and water holding capacity (WHC) analyses, one temporarily frozen at − 18 °C for no longer than 2 days for microbiological analysis, one temporarily frozen at − 18 °C for no longer than 7 days for protein content measurements, one temporarily frozen at − 18 °C for no longer than 14 days for optical microscopic observation and scanning electron microscope.
High pressure processing device, consisting of the following parts: 1-plug, 2-pressure chamber, 3-pressure transmitting media (water in this present study), 4-PTFE barrel for temperature insulation, 5-temperature insulation media (ice water or warm water in this present study), 6-experimental material (grass carp fillet in this present study), 7-computer/monitor, 8-data logger, 9-adapter, 10-pressure sensor, 11-pressure release valve, 12-pressure multiplier, and 13-water tank
Microbiological analysis
For microbiological analysis, grass carp slices (10.0 ± 0.1 g) were aseptically weighed into physiological peptone saline solution (PPS; 0.85% m/v NaCl + 0.1% m/v neutralized bacteriological peptone, Qianhui Ltd., Guangzhou, China) by ten times. After homogenization, appropriate decimal dilution series were made to determine the total plate counts on Plate Count Agar (Qianhui Ltd., Guangzhou, China) by using pour plating methods. The agar plates were cultivated at 22 °C for 3 days and the colonies visible on the plates were enumerated. The total plate counts for each HPP treatment condition were averaged over three repetition batches.
Physicochemical analysis
First, from each repetition batch, a portable chromameter (CR-400, Konica Minolta Holdings Inc., Japan) was used to determine the color indexes with the CIELAB color space mode, including L* (lightness), a* (redness), b* (yellowness), and ∆E (total color difference). For each slice, one spot on the surface with uniform color was measured through a transparent film. In total, the color indexes for each repetition batch were averaged over five spots, and the indexes for each HPP treatment were averaged over three batches.
Second, after the non-destructive colorimetric analysis, the slices were evaluated by texture and WHC measurements. Three cubes (1 × 1 × 1 cm) were cut from obtained from the central part of each grass carp slice, one measured by Texture Profile Analysis (TPA) tests using a Texture Analyzer (TA-XT2, Stable Micro Systems, UK), one by cook loss analysis, and one by centrifuge loss analysis. For texture assessments, the muscle cubes were placed at the center of the base plate. Measurements were carried out at room temperature using a 36-mm compression probe (P36/R), with the parameters set as follows: pre-test speed 5.0 mm/s, test speed 1.0 mm/s, post-test speed 5.0 mm/s, compression ratio 50%, trigger force 5 g, rest period between two compression cycles 5 s. The texture indexes, including hardness (g), springiness (%), and chewiness (g), were calculated automatically in the Exponent software (6.1.23.0, Stable Micro Systems, UK) on the basis of the force–time test figures. The WHC of the grass carp was determined by measuring the weight loss of the muscle cubes after cooking and centrifuging. For cook loss, the cubes were cooked in boiling water (100 °C) for 1 min, and for centrifuge loss, the cubes were centrifuged at 4000 rpm for 15 min. The values of cook loss and centrifuge loss were calculated according to the equation below (Eq. 1)
where m1 and m2 refer to the respective weights of the muscle cubes before and after cooking/centrifuging. In total, the texture and WHC indexes for each repetition batch were averaged over six measurements, and the indexes for each HPP treatment were averaged over three batches.
The protein composition in grass carp was determined using the method described elsewhere [22]. After removing the skin, the grass carp slices were ground, and 1.0 ± 0.1 g of the mince was added into 25 mL of phosphate buffer A (15.6 mmol/L Na2HPO4, 3.5 mmol/L KH2PO4, pH 7.5). After placing at 0–4 °C for 2–4 h, the homogenate was centrifuged at 4000 rpm for 15 min, and the supernatant was considered sarcoplasmic protein extract. The precipitate was subsequently mixed with 25 mL of phosphate buffer B (0.5 mol/L KCl, 15.6 mmol/L Na2HPO4, 3.5 mmol/L KH2PO4, pH 7.5). The mixture was placed at 0–4 °C for 2–4 h again, followed by centrifuging under the same condition. The supernatant was considered as myofibrillar protein extracts. The contents of sarcoplasmic and myofibrillar protein were determined by Bradford’s method using a spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The concentrations of myofibrillar and sarcoplasmic protein for each HPP condition were averaged over three repetition batches.
Microstructure and image analysis
One grass carp slice was randomly selected from each HPP condition and frozen at − 60 °C for over 12 h. A vacuum freeze drier (Alphal-4Lplus, Christ, Germany) was used to remove the internal moisture of the slices and preserve the fibrillar structure. For optical microscopic observation, the dehydrated slices were immersed in molten paraffin (50–60 °C) under vacuum, enabling paraffin to penetrate between the interfibrous spacing. The solidified samples were then cut into Section (10 µm thick) perpendicularly to the fiber direction and stained using 10% Eosin Y solution. The histological images of tissue sections were captured under a 40× magnification in ProgRes® CapturePro 2.8.8 software using an optical microscope (CX21, Olympus, Japan). For SEM analysis, muscle cubes (5 × 5 × 2 cm) were cut from the dehydrated slices using a scalpel and stuck to the conducting resin for gilding (the cut surface of the tissue facing up). The microstructure was subsequently captured under a 100× magnification using a scanning electron microscope (EVO 18 SEM, Zeiss, Germany).
Statistical analysis
One-way analysis of variance (AVONA) was used for describing differences between mean values among three sample replicates by Duncan’s new multiple range test (p < 0.05). Plotting of figures was performed by Origin v.2015.
Results and discussion
Microbial inactivation
Figure 2 presents the decreases in total bacterial loads in grass carp slices after HPP treatments at different initial temperatures. In raw fish samples, over 5 log CFU/g of TPC were detected, and the introduction of high pressure lowered the total bacterial load significantly (p < 0.05). HPP treatments under 300 MPa ensured TPC below 5 × 104 CFU/g. When pressurized under 500 MPa, the TPC of fillet samples were ca. 2 log CFU/g. Similarly, a 1–2 log cycle reduction of total bacterial counts was found in salmon carpaccio under 250 MPa [23]. Furthermore, various previous studies focused on the microbial aspects and have guaranteed the safety of pressurized aquatic products: for example, the levels of Salmonella and Listeria monocytogenes in salmon were lowered by 1–1.5 log CFU/g under 250 MPa or 400 MPa for 3 min [24]. When pressurizing milkfish under 300 MPa for 5 min, the remaining numbers of coliform and E. coli were decreased to 40 and < 3 MPN/g, and treatments above 400 MPa ensured that the coliform numbers were lower than 3 MPN/g [25].
Total plate counts of grass carp slices after high-pressure treatments (300, 400, and 500 MPa) for 10 min at different initial sample temperatures. For the group codes (FS, LT, RT, and MH), see Table 1. Unpressurized samples (0.1 MPa) were used as experimental controls. Data are presented as the average ± standard deviation among three repetition batches. Different letters with the same numbers indicate the significant difference between different pressure levels at the same initial temperature (p < 0.05)
Increasing or lowering temperatures slightly improved the effects on bacterial inactivation. By contract with the RT group, total colony counts of the FS and MH groups under 500 MPa were approximately 0.5 log lower. As reported previously, most microorganisms were tolerant to pressure in the range of 20–30 °C [12]. However, the inactivation levels of microbes in food matrices were elevated when HPP treatments were conducted at low or high temperatures [13]. For example, at 15, 5, and 1.5 °C, Vibrio parahemolyticus counts in oyster homogenate after 250 MPa-pressurization for 5 min were reduced by 4.3, 6.2 and 7.2 log CFU/g, respectively [14]. HPP treatments under 193 MPa for 10 min at − 20 °C lowered the total colony counts of salmon and cod by 1.5 log, with psychrotrophic bacteria, E. coli, Staphylococcus aureus undetected [26]. Compared to room temperature, mild heating at 40–50 °C combined with HPP (150 MPa for 4 min) can also reduce Vibrio vulnificus counts by 3–4 log [15]. These results potentially indicated that temperature and pressure had synergistic effects on food safety control. Considering that the initial contamination is amongst the crucial factors determining the progression of microbial spoilage, the implementation of HPP is able to prolong the shelf-life of fish-based products during chilled storage. Despite the notable promise in microbial activation, only slight differences were observed amongst various initial product temperatures. This can be likely because the previous research targeting microbial inactivation mostly used samples that were singly inoculated with relatively high colony numbers (approximately 107 CFU/g) [14, 15]. The corresponding reduction levels after HPP treatments were more evident when compared to naturally contaminated samples with lower initial microbial counts (Fig. 2) and more complex matrices. In addition, a challenge when applying HPP in solid products is that different food structures or properties (such as nutrients, density, aw) can lead to the differences in pressure transmission [27, 28]. Further investigations should focus on comparisons between naturally contaminated or manually inoculated fillet samples and homogenates, and the constitution and interaction within the pressurized microbiota. Overall, irrespective of the applied initial temperatures, HPP treatments under 300 MPa already ensure the total bacterial numbers below the quality and safety limit in a Chinese standard GB 10136 for ready-to-eat aquatic foods.
Changes in appearance and physicochemical quality
Figure 3 presents the color changes in grass carp slices after HPP treatments at different initial temperatures. The pictures of the grass carp appearance are shown in Fig. 6. Similarly, 300 MPa was able to induce whitening when pressurization was conducted at room temperature, and slices under 400 MPa already turned entirely white and opaque (Fig. 6). Colorimetric results show that HPP treatments under 400 MPa significantly increased the surface L* of grass carp from 59.6 to 80.7 (Fig. 3). Similarly, notable changes in color quality were also found in various fish species, including salmon (L*: from 41.9 to 62.4 under 300 MPa for 15 min), tuna (L*: from 39.8 to 58.8 under 300 MPa for 15 min) [29], and grass carp (L*: from 55.1 to 78.3 under 600 MPa for 15 min) [11], indicating the high sensitivity to pressure treatments of fish muscle. A more compact tissue microstructure as a result of high pressure can lead to surface whitening of the fish muscle [30]. More explanations regarding the protein and structural properties are presented in the subsequent sections. When performing HPP treatments under the FS and MH conditions, the color was significantly altered at the lowest pressure, returning L* values around 72–78 and ∆E around 20–25 under 300 MPa (similar to the samples pressurized under 400 MPa at room temperature). On the contrary, less color changes were found in the LT group when compared to FS and MH, and the increasing levels of L* were similar to the HPP treatments performed at room temperature (Fig. 3). In the case of pork, HPP treatments under 200–800 MPa at 5 °C caused less L* increase (from ca. 62 to ca. 72) than at room temperature (from ca. 62 to ca. 78) [16]. These results indicate that freezing and mild heating might lead to greater quality changes after high pressure treatments, while moderate cooling showed the potential in preserving raw attributes.
Changes in the color indexes (lightness L*, total color difference △E) of grass carp slices after high-pressure treatments (300, 400, and 500 MPa) for 10 min at different initial sample temperatures. For the group codes (FS, LT, RT, and MH), see Table 1. Unpressurized samples (0.1 MPa) and cooked samples (cooked at 100 °C for 1 min) were used as experimental controls. Data are presented as the average ± standard deviation among three repetition batches. Different letters with the same numbers indicate the significant difference between different pressure levels at the same initial temperature (p < 0.05), and an asterisk “*” denoted for “cooked samples” indicates a significant difference between cooked samples and all pressurized samples (p < 0.05)
Figure 4 presents the weight loss ratios and changes in grass carp texture after different HPP treatments. After pressurization, the weight loss in grass carp increased from 3.5 to 5.5% under higher pressure levels (from 300 to 500 MPa) at room temperature. Correspondingly, when grass carp was treated under 500 MPa at room temperature, the hardness rose from 390.2 to 719.7 g, and the chewiness from 99.3 to 249.5 g. The springiness of the grass carp slices was approximately 60% under 500 MPa, higher than the unpressurized samples. Similarly, notable changes in fish meat texture were also found in various fish species, including salmon (shear force: from ca. 1 N to ca. 4 N under 300 MPa for 15 min), tuna (shear force: from ca. 1.5 N to ca. 3 N under 300 MPa for 15 min) [29], and grass carp (hardness from 4824 to 7118 g and chewiness from 699 to 2622 g under 600 MPa for 15 min) [11]. The quality of albacore muscle changed significantly as pressure rose from 0.1 to 500 MPa, with L* increasing from 50 to 70 and hardness from 5 to 25 N [31]. The mass decrease (due to juice loss) and the hardness increase can be associated with myofibrillar protein denaturation and aggregation under high pressure [32]. When focusing on LT, RT, and MH groups, the increasing levels of weight loss, hardness, and chewiness were larger when initial temperatures were higher (Fig. 4). After cooking treatments, grass carp slices suffered from irreversible quality changes, such as complete whitening, loss of transparency, tissue damage (Fig. 6), significant decreases in L* (Fig. 3), higher weight loss, and lower hardness and chewiness (Fig. 4). This also hints that heat can aggregate the quality alterations under high pressure. Similarly, the hardness value of barramundi fish increased significantly from 23.2 N to 25.7 N under 200 MPa and decreased to 19.7 N under 300 MPa at 4–5 °C [33]. The hardness and chewiness values of pork were 12.7 kg and 2.2 kg, respectively, when pressurized under 215 MPa at 15.5 °C, both smaller than the treatments at 29.4 °C [34]. Dry-cured ham pressurized under 600 MPa at 7 °C had smaller lightness and shear force than 20 °C and 35 °C [18]. When compared to 20 °C, pressurization under 200–400 MPa at 40 °C caused less cook loss but more whiteness change in beef meat [19]. As HPP treatments were carried out below 0 °C (at frozen status), the weight loss ratios were much higher (Fig. 4). This phenomenon was similarly found when pressurizing pre-frozen beef carpaccio at − 30 °C and can be likely due to fiber structure damage by irregular ice crystals [35]. This could be expected to have an aggregated quality deterioration when the temperature rose from − 18 to 3 °C, and the samples had been thawed (Table 1). On the contrary, some beef studies have reported minor impacts of pre-freezing techniques (ca. − 30–− 35 °C) on meat color and texture after HPP treatments under 300–400 MPa [17, 35, 36], which could be attributed to no thawing during HPP duration (sample temperature below − 30 °C) [37].
Weight loss ratios and changes in texture indexes (hardness, springiness, chewiness) of grass carp slices after high-pressure treatments (300, 400, and 500 MPa) for 10 min at different initial sample temperatures. For the group codes (FS, LT, RT, and MH), see Table 1. Unpressurized samples (0.1 MPa) and cooked samples (cooked at 100 °C for 1 min) were used as experimental controls. Data are presented as the average ± standard deviation among three repetition batches. Different letters with the same numbers indicate the significant difference between different pressure levels at the same initial temperature (p < 0.05), and an asterisk * denoted for “cooked samples” indicates a significant difference between cooked samples and all pressurized samples (p < 0.05)
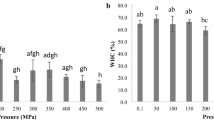
Figure 5 presents the water holding capacity changes in grass carp slices after HPP treatments at different initial temperatures. The cook loss and centrifuge loss of slices after HPP treatments ranged from 12.1 to 16.9% and from 23.3 to 26.4% (Fig. 5). WHC changes were also found in various aquatic foods following two potential tendencies—(1) a continuous decrease as a function of pressure that could result from irreversible protein denaturation and fiber compression [3, 10]; or (2) a slight WHC increase (related to the release of low molecular protein, water infiltration into protein core and increased protein hydration) followed by a reduction above a critical pressure threshold [38]. In the latter case, a peak for WHC can be observed under a certain pressure level. For example, the WHC peaks for skipjack tuna [9] and sea bass [10] were under around 150 MPa and 250 MPa. WHC of gilthead sea bream [39], cod [40], and grass carp (Fig. 5) decreased continuously under higher pressures. For example, the WHC of salmon and tuna were detected at approximately 70 g/100 g water before HPP treatments and dropped to 55 g/100 g water under 300 MPa (measured using a centrifuging loss based method) [29]. Slight differences were found between LT, RT, and MH groups when grass carp slices were pressurized under 300–500 MPa (Fig. 5). While slices from the FS group had the highest WHC changes amongst all the examined temperature scenarios, with the centrifuge loss reaching 28.2% under 500 MPa and cook loss reaching 18.4% under 300 MPa. These changing patterns were consistent with the high weight loss and hardness when slices were treated with an initial subzero temperature (Fig. 4), possibly owing to fiber damage and the resulting weakened water holding capacity [35].
Changes in water holding capacity (centrifuge loss ratios, cook loss ratios) of grass carp slices after high-pressure treatments (300, 400, and 500 MPa) for 10 min at different initial sample temperatures. For the group codes (FS, LT, RT, and MH), see Table 1. Unpressurized samples (0.1 MPa) and cooked samples (cooked at 100 °C for 1 min) were used as experimental controls. Data are presented as the average ± standard deviation among three repetition batches. Different letters with the same numbers indicate the significant difference between different pressure levels at the same initial temperature (p < 0.05), and an asterisk “*”denoted for “cooked samples” indicates a significant difference between cooked samples and all pressurized samples (p < 0.05)
Fiber structure
Figure 6 presents the changes in the fiber structure of grass carp slices after HPP treatments at different initial temperatures. In unpressurized samples, fibers were arranged in orderliness with no evident interconnection. Much spacing existing between fibers allowed for water-dwelling, which could be linked to the low hardness and strong water holding capacity of raw grass carp slices (Figs. 4 and 5). Under 300 MPa, the semitransparency of fish slices corresponded with the loose microstructure. When the treated pressure exceeded 400 MPa, fibrillar compression was observed at room temperature (group RT, Fig. 6). The consequent fiber shrinkage and structural compaction could be associated with the hardening of the fish texture (Fig. 4), as smaller diameters and thicker arrangement of muscle fibers have been reported to correspond with higher hardness values [41]. Similarly, myofiber shrinkage was observed in the 600-MPa treated sea bass fillets in accordance with the increase of hardness [42].
Changes in appearances and microstructure of grass carp slices after high-pressure treatments (300, 400, and 500 MPa) for 10 min at different initial sample temperatures, by using an optical microscope (a, 40×) and a scanning electron microscope (b, 100×). For the group codes (FS, LT, RT, and MH), see Table 1. Unpressurized samples (0.1 MPa) and cooked samples (cooked at 100 °C for 1 min) were used as experimental controls
According to a light scatter theory proposed by Hughes et al. [30], the changes in fiber structure can have an impact on the alteration of appearance and surface color. To be specific, when incident light travels inside the fiber structure, it mostly separates into three different directions, i.e., transmitted through the tissue, absorbed by the tissue, or reflected into human eyes [30]. As indicated in Fig. 6, a loose fiber structure was observed in the unpressurized fillet samples. In this case, more incident light could be transmitted through interfibrous spacing, and less could be perceived by human eyes [30], leading to a semi-transparent appearance (Fig. 6). 400 MPa caused the shrinkage of muscle fibers, and the reduction of interfibrous spacing (Fig. 6). This compaction of microstructure could potentially obstruct light from transmitting and cause an increased proportion of light reflection [30]. Therefore, a greater perceived whiteness and opacity were detected in the pressurized samples (Fig. 3).
When it regards different temperatures, grass carp slices from the LT group still had much interfibrous spacing and less fiber shrinkage under 300 MPa than the FS and MH groups. Correspondingly, the appearance of grass carp slices from the LT group remained semi-transparent and “raw-like” under 300 MPa (Fig. 6). However, when grass carp samples were pressurized under frozen or mild heating conditions, HPP led to a more compact tissue microstructure compared to the LT and RT groups (Fig. 6). Incident light was easily reflected instead of traveling through inter-filament spacing (Fig. 6), resulting in an increase in L* under 300 MPa (Fig. 3). When the used pressure was above 400 MPa, no significant differences were found between four groups; all of the slices showed opaque and completely white (Fig. 6), which was consistent with high levels of L* at around 80 (Fig. 3). After traditional thermal treatments, the heat caused damage in fish tissue and induced a significant whitening (Fig. 6), which would be considered unfavorable when considering the development of raw ready-to-eat fish products. Overall, these phenomena could indicate that pre-freezing and mild heat had negative influences on maintaining the raw attributes of fish muscle. While on the other hand, no significant differences in tissue structure were found between the LT and RT groups. As previously stated, lowering the pressurizing temperature from room temperature to 5 °C or 15.5 °C has been documented to induce a smaller increase in the whiteness, hardness and shear force of pork [16, 34]. The differences in quality changes between HPP pork and grass carp could be mainly related to the fact that the strength of pork myofibrillar protein was stronger than fish [21]. Another reason would be related to technical aspects: a more effective heat insulation system could be developed to ensure a low and stable temperature during the pressurization duration, as minor impacts of pre-freezing techniques (ca. – 30–− 35 °C) have been reported on meat color and texture under 300–400 MPa [37].
Protein property
Figure 7 presents the changes in myofibrillar and sarcoplasmic protein contents of grass carp slices after HPP treatments at different initial temperatures. The levels of both sarcoplasmic and myofibrillar protein decreased as pressure rose. Under all conditions, the reduction of myofibrillar protein was more evident than that of sarcoplasmic protein, which is in accordance with the SDS-PAGE found in sea bass protein extracts [42]. The temperatures had little influence on the contents of sarcoplasmic protein, whereas the extent of myofibrillar protein decreased significantly as temperature increased. Under 500 MPa, the FS group had the highest myofibrillar protein contents at 4.4 g/100 g while the MH group had the lowest at 0.57 g/100 g, likely suggesting the heat sensitivity and low pressure-stability of myofibrillar protein. The quality changes under high pressure could also be related to the alteration of protein properties [43]. The rheological and textural characteristics of raw fish muscle have been considered to be governed more by the myofibrillar and connective tissue proteins, rather than sarcoplasmic proteins [32]. Correspondingly, the decrease of myofibrillar proteins was more significant than sarcoplasmic proteins in the pressurized grass carp (Fig. 7). Soluble proteins in albacore were reduced notably to ca. 20% of the original contents [31]. De Oliveira et al. have reported that the irreversible degradation of myofibrillar protein (myosin under 100–200 MPa, actin under 200 MPa) led to an increase in hardness, gumminess, and adhesiveness, whereas sarcoplasmic protein remained stable up to 400 MPa [3]. In particular, these changes could be related to the unfolding of actin and sarcoplasmic proteins and the connection of new hydrogen bonds [33]. However, limited differences were found between the solubility of sarcoplasmic protein extracted from pork meat pressurized under 5 or 20 °C [44]. Under these two temperature scenarios, most band densities intensified under 200 MPa, which could be related to an increased concentration of these proteins, especially tropomyosin and troponin-T, indicating an increased fraction of solubilization, while the myosin-HC band decreased [44].
Changes in the myofibrillar protein and sarcoplasmic protein contents of grass carp slices after high-pressure treatments (300, 400, and 500 MPa) for 10 min at different initial sample temperatures. For the group codes (FS, LT, RT, and MH), see Table 1. Unpressurized samples (0.1 MPa) and cooked samples (cooked at 100 °C for 1 min) were used as experimental controls. Data are presented as the average ± standard deviation among three repetition batches. Different letters with the same numbers indicate the significant difference between different pressure levels at the same initial temperature (p < 0.05)
Further insights into the protein conformational structures would be characterized by using spectrometry-based techniques such as Raman spectrometry [38] or Fourier-transform infrared spectroscopy (FTIR) [45]. For example, the primary structure referring to the amino acid sequence is highly tolerant to high pressure because the disruption energy of covalent bonds is far in excess of pressurization energy [38]. Amide I band (α-helix 1655 cm−1, β-sheet 1670 cm−1, β-turn 1685 cm−1, and random coil 1665 cm−1) have been used to indicate the alteration of secondary structures [38, 46]. The tertiary and quaternary structures of protein can be reflected by amino acid residue exposure status: e.g., the amplitude ratio at 850 cm−1 and 830 cm−1 provides information on the microenvironment around tyrosyl residues [46, 47]. Several relevant studies have discovered a decrease of ordered conformational structures (α-helix, β-sheet) and an increase of disordered structures (β-turn, random coil) under high pressure in the case of myofibrillar protein of chicken [38] and shrimp [47]. The reduction of α-helix and β-sheet can weaken hydrogen bond interaction [3, 43] and water holding capacity [3]. The I850/I830 ratio increased under 200 MPa in previous investigations on silver carp protein extracts [46] and hake sarcoplasmic protein [48]. The release of hydrophobic residue groups could be associated with protein unfolding and structural destabilization caused by high pressure [38]. Preliminary Raman scanning trials on minced grass carp indicated a decrease of α-helix proportions from 36.1 to 30.7% and an increase of random coil from 23.5 to 27.7% when pressure rose to 500 MPa[20], which could be associated with high centrifuge/cook loss of grass carp muscle under 300–500 MPa (Fig. 5). However, it should be noted that the complexity and variability of food matrices pose a challenge to analyze the protein properties within the whole food samples using Raman spectroscopy [49]. The protein conformational information indicating the impacts of pressure and temperature could be further investigated using protein extracts isolated from unpressurized and pressurized grass carp samples under different initial temperatures.
Conclusion
High pressure processing has shown remarkable bacterial inactivation and the maintenance of raw attributes in contrast to heat treatments. However, the fish muscle was extremely sensitive to pressure (300–500 MPa), accompanied by surface whitening (L* and opacity increase), hardening, weight loss, water holding capacity decrease, etc. Grass carp quality was affected by different initial temperatures when performing HPP treatments. Decreasing and increasing the temperature caused a slightly higher reduction in microbial population. Under 300 MPa, the grass carp slices treated at 2 °C appeared semi-transparent and encountered less quality changes than the samples treated under frozen and mild heating status. Further investigations from the microstructural aspect indicate that the increase of whiteness and hardness could be associated with the compaction of microstructure. The contents of myofibrillar protein were also found to have a greater decrease when the grass carp slices were pressured at higher temperature.
Data availability
Data presented in this work can be obtained from the corresponding author upon request.
References
FAO (2022), https://www.fao.org/3/cc0461en/cc0461en.pdf.
C. Cardoso, H. Lourenço, S. Costa, S. Gonçalves, M. Leonor Nunes, J Food Prod Mark. 22, 421–435 (2016). https://doi.org/10.1080/10454446.2014.949982
F.A. de Oliveira, O.C. Neto, L.M.R. dos Santos, E.H.R. Ferreira, A. Rosenthal, Trends Food Sci. 66, 1–19 (2017). https://doi.org/10.1016/j.tifs.2017.04.014
L. Otero, M. Pérez-Mateos, F. Holgado, G. Márquez-Ruiz, M. López-Caballero, Innov. Food Sci. Emerg. Technol. 51, 41–50 (2019). https://doi.org/10.1016/j.ifset.2018.05.003
S. Humaid, D. Nayyar, J. Bolton, B. Perkins, D.I. Skonberg, High Press. Res. 40, 444–463 (2020). https://doi.org/10.1080/08957959.2020.1774753
B.H. Hughes, N.J. Greenberg, T.C. Yang, D.I. Skonberg, J. Food Sci. 80, C40–C48 (2015). https://doi.org/10.1111/1750-3841.12717
D. Kingsley, D. Kuhn, G. Flick, J. Oh, L. Lawson, G. Meade, C. Giesecke, Am. J. Food Technol. 9, 209–216 (2014)
R. Mengden, A. Röhner, N. Sudhaus, G. Klein, Innov. Food Sci. Emerg. Technol. 32, 9–15 (2015). https://doi.org/10.1016/j.ifset.2015.10.002
W. Jiranuntakul, N. Nakwiang, P. Berends, T. Kasemsuwan, T. Saetung, S. Devahastin, J. Food Sci. 83, 2324–2336 (2018). https://doi.org/10.1111/1750-3841.14318
B. Teixeira, L. Fidalgo, R. Mendes, G. Costa, C. Cordeiro, A. Marques, J.A. Saraiva, M.L. Nunes, Innov. Food Sci. Emerg. Technol. 22, 31–39 (2014). https://doi.org/10.1016/j.ifset.2013.12.005
P. Yu, C. Yan, F. Yang, Y. Xu, Q. Jiang, W. Xia, J. Aquat. Food Prod. Technol. 27, 1093–1105 (2018). https://doi.org/10.1080/10498850.2018.1534916
B.Q. Truong, R. Buckow, C.E. Stathopoulos, M.H. Nguyen, Food Eng. Rev. 7, 109–129 (2015). https://doi.org/10.1007/s12393-014-9084-9
R. Sehrawat, B.P. Kaur, P.K. Nema, S. Tewari, L. Kumar, Food Sci. Biotechnol. 30, 19–35 (2021). https://doi.org/10.1007/s10068-020-00831-6
S. Phuvasate, Y.C. Su, Int. J. Food Microbiol. 196, 11–15 (2015). https://doi.org/10.1016/j.ijfoodmicro.2014.11.018
A.G. Kural, H. Chen, Int. J. Food Microbiol. 122, 180–187 (2008). https://doi.org/10.1016/j.ijfoodmicro.2007.11.074
K.H. Bak, G. Lindahl, A.H. Karlsson, V. Orlien, Meat Sci. 92(4), 374–381 (2012). https://doi.org/10.1016/j.meatsci.2012.02.002
S. Bulut, Food Bioprocess Technol. 7, 3033–3044 (2014). https://doi.org/10.1007/s11947-014-1339-1
E. Coll-Brasas, J. Arnau, P. Gou, J. Lorenzo, J. García-Pérez, E. Fulladosa, Meat Sci. 152, 127–133 (2019). https://doi.org/10.1016/j.meatsci.2019.02.014
R. McArdle, B. Marcos, J. Kerry, A. Mullen, Meat Sci. 86, 629–634 (2010). https://doi.org/10.1016/j.meatsci.2010.05.001
L. Chen, B. Li, Z. Ruan, J. Qian, High Press. Res. (2023). https://doi.org/10.1080/08957959.2023.2198647
H. Wang, Z. Yang, H. Yang, J. Xue, Y. Li, S. Wang, M. Zhang, LWT 153, 112458 (2022). https://doi.org/10.1016/j.lwt.2021.112458
C. Niamnuy, S. Devahastin, S. Soponronnarit, Food Chem. 108, 165–175 (2008). https://doi.org/10.1016/j.foodchem.2007.10.058
J. Gómez-Estaca, M.E. López-Caballero, M.Á. Martínez-Bartolomé, A.M.L. de Lacey, M.C. Gómez-Guillen, M.P. Montero, Int. J. Food Microbiol. 283, 28–36 (2018). https://doi.org/10.1016/j.ijfoodmicro.2018.06.015
I.S. Boziaris, F.F. Parlapani, C.A.M. DeWitt, Innov. Food Sci. Emerg. Technol. 74, 102811 (2021). https://doi.org/10.1016/j.ifset.2021.102811
H.F. Kung, C.S. Lin, S.S. Liu, C.Y. Huang, K. Chiu, Y.C. Lee, Y.H. Tsai, Food Control 134, 108768 (2022). https://doi.org/10.1016/j.foodcont.2021.108768
E. Malinowska-Pańczyk, I. Kołodziejska, Food Technology and Biotechnology 51, 570–576 (2013)
N.K. Rastogi, K.S.M.S. Raghavarao, V.M. Balasubramaniam, K. Niranjan, D. Knorr, Crit. Rev. Food Sci. Nutr. 47(1), 69–112 (2007). https://doi.org/10.1080/10408390600626420
M. Campus, Food Eng. Rev. 2(4), 256–273 (2010). https://doi.org/10.1007/s12393-010-9028-y
J. Gómez-Estaca, M. López-Caballero, M. Gómez-Guillén, A.L. de Lacey, P. Montero, Innov. Food Sci. Emerg. Technol. 10, 148–154 (2009). https://doi.org/10.1016/j.ifset.2008.10.006
J. Hughes, S. Oiseth, P. Purslow, R. Warner, Meat Sci. 98, 520–532 (2014). https://doi.org/10.1016/j.meatsci.2014.05.022
L. Cartagena, E. Puértolas, I. Martinez de Maranon, Food Bioprocess Technol. 12, 2074–2084 (2019). https://doi.org/10.1007/s11947-019-02369-w
Y.M. Zhao, M. de Alba, D.W. Sun, B. Tiwari, Crit. Rev. Food Sci. Nutr. 59, 728–742 (2019). https://doi.org/10.1080/10408398.2018.1495613
B.Q. Truong, R. Buckow, M.H. Nguyen, C.E. Stathopoulos, J. Food Eng. 169, 72–78 (2016). https://doi.org/10.1016/j.jfoodeng.2015.08.020
C. Souza, D. Boler, D. Clark, L. Kutzler, S. Holmer, J. Summerfield, J. Cannon, N. Smit, F. McKeith, J. Killefer, J. Food Sci. 77, S54–S61 (2012). https://doi.org/10.1111/j.1750-3841.2011.02458.x
S.R. Vaudagna, C.B. Gonzalez, B. Guignon, C. Aparicio, L. Otero, P.D. Sanz, Meat Sci. 92, 575–581 (2012). https://doi.org/10.1016/j.meatsci.2012.06.002
C. Realini, M. Guàrdia, M. Garriga, M. Pérez-Juan, J. Arnau, Meat Sci. 88, 542–547 (2011). https://doi.org/10.1016/j.meatsci.2011.02.008
A.C. Lowder, C.A. Mireles Dewitt, J. Food Process. Preserv. 38, 1840–1848 (2014). https://doi.org/10.1111/jfpp.12155
Z. Zhang, Y. Yang, X. Tang, Y. Chen, Y. You, Food Chem. 188, 111–118 (2015). https://doi.org/10.1016/j.foodchem.2015.04.129
M. Campus, M.F. Addis, R. Cappuccinelli, M.C. Porcu, L. Pretti, V. Tedde, N. Secchi, G. Stara, T. Roggio, J. Food Eng. 96, 192–198 (2010). https://doi.org/10.1016/j.jfoodeng.2009.07.013
K. Angsupanich, M. Edde, D. Ledward, J. Agric. Food Chem. 47, 92–99 (1999). https://doi.org/10.1021/jf980587p
K. Honikel, C. Kim, R. Hamm, P. Roncales, Meat Sci. 16, 267–282 (1986)
T. Tsironi, L. Anjos, P.I. Pinto, G. Dimopoulos, S. Santos, C. Santa, B. Manadas, A. Canario, P. Taoukis, D. Power, J. Food Eng. 262, 83–91 (2019). https://doi.org/10.1016/j.jfoodeng.2019.05.010
K. Bak, T. Bolumar, A. Karlsson, G. Lindahl, V. Orlien, Crit. Rev. Food Sci. Nutr. 59, 228–252 (2019). https://doi.org/10.1080/10408398.2017.1363712
A. Grossi, K. Olsen, T. Bolumar, Å. Rinnan, L.H. Øgendal, V. Orlien, Food Chem. 196, 1005–1015 (2016). https://doi.org/10.1016/j.foodchem.2015.10.062
M. Martínez, G. Velazquez, D. Cando, R. Núñez-Flores, A.J. Borderías, H. Moreno, Innov. Food Sci. Emerg. Technol. 41, 323–329 (2017). https://doi.org/10.1016/j.ifset.2017.04.010
C. Qiu, W. Xia, Q. Jiang, Eur. Food Res. Technol. 238, 753–761 (2014). https://doi.org/10.1007/s00217-014-2155-6
M. Lv, H. Zhang, K. Mei, W. Yang, Z. Wang, J. Aquat. Food Prod. Technol. 29, 220–228 (2020). https://doi.org/10.1080/10498850.2020.1718818
G. Villamonte, L. Pottier, M. de Lamballerie, Eur. Food Res. Technol. 242, 667–675 (2016). https://doi.org/10.1007/s00217-015-2574-z
H. Jayan, H. Pu, D.W. Sun, Crit. Rev. Food Sci. Nutr. 62(16), 4294–4308 (2022). https://doi.org/10.1080/10408398.2021.1945534
Acknowledgements
This work was supported by Hainan Province Scientific Research Institute’s Technical Innovation Project (Grant number: SQKY2022-0040), and Guangdong Province Key Area Research and Development Project (Grant number: 2019B020212002). L.C. was funded by China Scholarship Council (CSC) for the scholarship under the Grant CSC No.202006150027.
Author information
Authors and Affiliations
Contributions
LC: Conceptualization, Formal analysis, Investigation, Methodology, Writing—original draft; Writing—review & editing; BL: Conceptualization, Supervision, Writing—review & editing; ZR: Writing—review & editing; JQ: Resources.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no known competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Li, B., Ruan, Z. et al. Effects of temperature prior to high-pressure processing on the physicochemical and structural properties of raw grass carp. Food Measure 18, 538–549 (2024). https://doi.org/10.1007/s11694-023-02190-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02190-2