Abstract
Traditionally, Ilex paraguariensis leaves are consumed in tea form or as typical drinks like mate and terere, while the fruits are discarded processing and has no commercial value. The aim of this work to evaluate phytochemical properties, total phenolic compounds, antioxidant and antimicrobial activity of extracts of Ilex paraguariensis fruits obtained from supercritical CO2 and compressed propane extraction. The extraction with compressed propane yielded 2.72 wt%, whereas with supercritical CO2 1.51 wt% was obtained. The compound extracted in larger amount by the two extraction solvents was caffeine, 163.28 and 54.17 mg/g by supercritical CO2 and pressurized propane, respectively. The antioxidant activity was more pronounced for the supercritical CO2 extract, with no difference found in terms of minimum inhibitory concentration for Staphylococcus aureus for the two extracts and better results observed for Escherichia coli when using supercritical CO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Yerba (or erva) mate (Ilex paraguariensis) is a tree shrub belonging to Aquifoliaceae family, with natural growing in the states of Mato Grosso do Sul, São Paulo, Paraná, Santa Catarina and Rio Grande do Sul, reaching also part of Paraguay and Argentina (Groppo 2015). The Ilex paraguariensis has been extensively studied due to a variety of pharmacological antioxidant properties (Bravo et al. 2007; Boaventura et al. 2013a), bactericidal ability (Burris et al. 2015), stimulant (Athayde et al. 2000), diuretic, antidiabetic (Boaventura et al. 2013b), usually attributed to the presence of phenolic compounds and alkaloids.
To get a sense of the Ilex paraguariensis market, only in South of Brazil it can be found more than 40 mate processing industries. To supply these industries, about 180 thousand medium and small rural properties dedicated almost exclusively to cultivate this raw material (Jacques et al. 2007a). Considering the fact that all of those industries direct their efforts to produce the same product (mate leaves for teas) the processing is still done in a very rudimentary form. Due to recent availability of this raw material from other countries, the strong competition established has required company investments towards improved agronomic techniques and also in the development of new processes to produce higher-value products (Jacques et al. 2007b).
The Ilex paraguariensis produces ripe fruits between January and March, presenting a purplish red color, being eaten by native birds, which spread out the plant in the region (Peixoto et al. 2000). The bitter taste of the fruit and its strong color indicates the presence of phenolic compounds (Miró et al. 1998). According to Canhoto (2010) phenolic compounds are secondary metabolites produced by plants, which can generate competitive advantages as antimicrobial action. It is well known that the chemical structure of phenolic compounds is directly connected to their antioxidant activity, which stabilizes free radicals, and can inhibit for example LDL (low-density lipoprotein) cholesterol oxidation, named the bad cholesterol (Vaccari et al. 2009). It should also be noted that while an adult mate plant produces around 150 kg of leaves, up to 20 kg of fruits could be obtained, which may comprise a non-negligible amount of an interesting, non-waste, raw material.
Because of the economic and safety concerns related to the toxicity of conventional (organic solvent) extraction methods, the use of pressurized and supercritical fluids has emerged as a plausible alternative. Extraction of vegetable matrices with supercritical fluids is probably the most classical and well established application of supercritical fluid technology. Carbon dioxide is the most commonly used supercritical solvent in the extraction of flavor and fragrance compounds due to its well-known properties (Reverchon 1997; Reverchon and de Marco 2006; Melo et al. 2014). Nevertheless, propane and n-butane can also be a good choice because their critical pressures are relatively low compared to that of carbon dioxide and present a dielectric constant of around 1.7, which is quite similar to carbon dioxide, 1.6 (Weast et al. 1988). Of course, for safety reasons, the flammability of propane will require adoption of additional security policies.
As liquid propane exhibits low compressibility and very low solubility in water, it behaves like a piston fluid that increases the system pressure changing favorably the extraction of non-polar components. Furthermore, propane is plenty available, cheaper, can be used in much lower pressures compared to carbon dioxide and can also be easily separated from the final product by system depressurization. Besides the mild temperature and pressure operating conditions, the use of short-chain hydrocarbons, like propane and n-butane, allows reduction of extraction time. However, literature is somewhat scarce on the vegetable matrices extraction using pressurized propane (Illés et al. 1997, 2000; Hamdan et al. 2008; Daood et al. 2002; Corso et al. 2010; Freitas et al. 2008; Silva et al. 2015a, b; Santos et al. 2015; Zanqui et al. 2015a, b; Jesus et al. 2013; Pederssetti et al. 2011; Nimet et al. 2001; Sparks et al. 2006).
Carelli et al. (2011) extracted Ilex paraguariensis leaves using supercritical CO2. The extract obtained demonstrated potential for natural antibiotics from this plant due to the antimicrobial activity exhibited against microorganisms such as Staphylococcus aureus and Pseudomonas aeruginosa. Though literature is somewhat vast concerning supercritical-CO2 extraction of mate leaves (Jacques et al. 2007a, b), to the best of our knowledge no report was found regarding compressed fluid extraction of mate plant fruits, which means that possible application of such waste biomass has been completely neglected.
In this context, the aim of this study was to perform the phytochemical characterization, to determine the amount of total phenolic compounds, and the antioxidant and antimicrobial activity (Escherichia coli (gram negative) and Staphylococcus aureus (gram positive) microorganisms) of extracts of Ilex paraguariensis fruits obtained from supercritical CO2 and compressed propane extraction.
Materials and methods
Plant material
Ilex paraguariensis ripened fruits were collected in January, −26.87961 latitude, longitude −52.9538 in the Pinhalzinho city, western state of Santa Catarina-Brazil, and Ilex paraguariensis A. St. Hil. was identified as belonging to the Aquifoliaceae family for Marciela Batistela in Biology Laboratory of Unochapecó where one voucher specimen was deposited under the number 3169. Fruit samples were washed and then dried in oven with forced air circulation at 40 °C overnight followed by grinding, packing and stored under nitrogen atmosphere prior to extraction experiments.
Pressurized fluid extraction
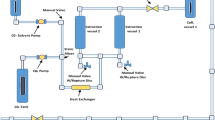
The experimental apparatus consists basically of a solvent reservoir, two thermostatic baths, a syringe pump (ISCO 260D), a 130 cm3 jacketed extraction vessel, an absolute pressure transducer (Smar, LD301) equipped with a portable programmer (Smar, HT 201) with a precision of 0.12 bar, a collector vessel with a glass tube, and a cold trap. Amounts of around 30 g of dried of finely comminuted fruits (average particle size of 0.14 mm) were charged into the extraction vessel. The solvent was pumped at a constant flow rate of 2 mL/min into the bed, which was supported by two 300 mesh wire disks at both ends and was kept in contact with the herbaceous matrix for at least 30 min to allow system stabilization. The extract was then collected by opening the micrometering valve and extraction continued until no significant mass was extracted, as measured on a precision scale balance (Shimadzu, Model AY220 with 0.0001 g accuracy).
For propane (White Martins, 99.5% purity, Florianópolis, Brazil) an experimental 22 full design was executed based on a previous work (Freitas et al. 2008) in the temperature range of 35–55 °C and from 40 to 60 bar. Solvent density was estimated using the HBT (P–V-T) correlation for compressed liquids (Reid et al. 1987). It should be noted that in the temperature and pressure ranges investigated in this work, propane behaves like a compressed liquid and thus exhibits small density variations, around 0.46 g cm3.
For carbon dioxide (White Martins, 99.9% purity, Florianópolis, Brazil), an experimental 22 design was also followed in the temperature range of 35–55 °C and from 150 to 250 bar, such extraction conditions based on previous works (Jacques et al. 2007a, b). Carbon dioxide densities were estimated using Angus correlation (Angus et al. 1985). In both cases, experiments were accomplished isothermally at constant pressure. A whole experimental run lasted in general 6 h, including all steps involved: sample weighing, temperature stabilization (baths, extractor), extraction, and depressurization. Based on triplicate experiments carried out for all the experimental conditions of both experimental designs, the overall average standard deviation of the yields noticed was about 0.15 wt%.
The output of compressed fluids was computed based on the volume decay of the syringe pump reservoir, with a resulting accuracy of ±0.01 g in pressurized solvents delivery. With known values of pressure and temperature in the syringe pump reservoir, solvent densities were calculated and accordingly the mass of solvents charged into the extraction vessel. For more information on the methods used in the extraction process, the reader is addressed to the works of Jacques et al. (2007a, b) and Rodrigues et al. (2004).
The extracts obtained from the use of compressed solvents extraction, CO2 and propane, were collected at the end of the extraction vessel through a glass flask immersed in a cold trap. The solvent was easily evaporated to constant weight, which was a simple task due to the high volatility of the solvents employed. Then, the extracts were stored in freezer (~−18 °C) until the chemical and biological analyses. Extracts were then ready to follow the specific analyses mentioned.
Determination of total phenolic content
Total phenolics content was determined by the Folin–Ciocaulteu method based on the technique described by Shahidi and Naczk (2002) with modifications. Triplicate solutions using both extracts were prepared at a concentration of 0.5 mg/mL in methanol analytical grade and protected from light. Afterwards, water was added and subsequently the Folin–Ciocalteu reagent (Vetec, Rio de Janeiro, Brazil) and after 3 min saturated Na2CO3 was added. After 1 h absorbance was read at 765 nm in a spectrophotometer. Quantification was performed based on a standard curve of gallic acid and the total phenolic was expressed in mg of gallic acid equivalents (GAE)/100 g of dry extract.
Gas chromatography/mass spectrometry
The extracts (from CO2 and propane) were analyzed through GC/MS (Shimadzu QP5050A), using a capillary column DB5 (30 m × 0.25 mm, 25 µm). Column temperature was programmed 70 °C/3 min, 4 °C/min to 260 °C, 2.5 °C/min to 300 °C/25 min. Helium was the carrier gas and the injection port and detector temperatures were 290 and 300 °C, respectively. The components were identified by matching their mass spectra with those of Wiley library database and by comparison of retention times with standards. In all samples it was added an internal standard (biphenyl 100 ppm) and the content of each compound was calculated by the ratio between the peak area of the compound by the peak area of internal standard (Boligon et al. 2013). Caffeine, theobromine, phytol, palmitic acid, squalene, vitamin E, β-sitosterol and stigmasterol were quantified through the injection of authentic standards (Sigma-Aldrich Co). Three replicate were performed for each sample.
Antioxidant activity by DPPH (2,2-difenil-1-pricril-hidrazil) method
The antiradical powers of the different concentrations of the Ilex paraguariensis extracts (0.01, 0.02, 0.04, 0.08, 0.16 and 0.32 mg/mL) and 50 ml of each dilution was mixed with 1.95 ml of methanolic DPPH solution standard (0.24 mg/mL—Sigma-Aldrich, Steinheim, Germany) were determined by measuring the decrease in the DPPH absorbance at 517 nm after 24 h in the dark compared to a blank (Brand-Williams et al. 1995). This analysis was carried out in triplicate (n = 3), and the results are expressed as the means of % inhibition of the DPPH radical. The concentration of Ilex paraguariensis extracts that could scavenge 50% of the DPPH radical (IC50) was calculated via regression analysis using the GraphPad Prism Program version 6.0.
Microbiological analysis
Microbiological analyses were performed into sterile 96 well microplates-bottomed “U” in triplicate. A volume of 200 μL of the sample was prepared in a concentration of 2000 μg/mL, using 10% DMSO as a diluent and it was filtered (Millipore 0.45 μm) and inoculated with two fold serial dilutions (1000, 500, 250, 125, 62.5, 31.25, 15.6 and 7.8 μg/mL). The microbial inoculum at a concentration of 0.5 McFarland standard, which corresponds to 108 CFU/mL (Colony Forming Units), was subsequently diluted 10 times in sterile 0.9% saline, and from this solution a volume of 5 μL was added to all wells. The wells of the column 7 have been reserved for the negative control of the inhibitory activity of DMSO diluent. The wells in column 8 received only Mueller–Hinton (Merck®) broth and microbial inoculum, providing positive control of bacterial viability and column 9 wells received only Mueller–Hinton broth for verification plate sterility (Ostrosky et al. 2008). The microplates were incubated in bacteriological incubator at 35 °C for 18 h. Then, it was added to each well 20 μL of an aqueous solution of TTC (triphenyltetrazolium chloride, 0.5% w/v) and the microplates were again incubated for a further 3 h. The presence of a red color in the wells was interpreted as negative test of the inhibitory effect/anti-microbial activity, while the absence of red color as positive proof of inhibitory action. The minimum inhibitory concentration (MIC) was determined according to the methods described by Liu and Yang (2012) and was defined as the lowest concentration, in mg/mL able to inhibit microbial growth, tested in this work against Escherichia coli (gram negative) and Staphylococcus aureus (gram positive) microorganisms, acquired from NEWAPROV.
Results and discussion
The first step was to evaluate the effect of temperature and pressure on the extraction yield of mate fruits and results are shown in Table 1. As can be seen from this table, extraction with pressurized propane provided higher yields compared to supercritical CO2 for almost all conditions, with the best results from the two solvents being 2.72 and 1.51 wt%, for propane and CO2, respectively. The extractions with propane required the application of lower pressure and in all cases shorter extraction times, typically 90–120 min for propane compared to 3–4 h for CO2.
Thus, in the temperature range investigated, compressed propane presented a hydrostatic behavior, working as mechanical press or piston fluid that increases the system pressure, changing favorably the extraction of non-polar components. Such characteristic may be advantageous due to the possibility of saving energy and reducing operational pressure expenditures with consequent lower capital investments. Thus, it seems that working at relatively lower pressures and mild temperatures may be advantageous over supercritical carbon dioxide use, since lower investment (capital expenditure—Capex) and operational costs (operational expenditure—Opex) are involved when using compressed propane.
According to Lanza et al. (2005) the oils are constituted by mostly triglycerides, which have high solubility in propane. Pederssetti et al. (2011) performed canola seed extraction with CO2 from 40 to 60 °C and pressures from 20 to 25 MPa, while propane was employed from 30 to 60 °C and pressures of 8 to 12 MPa. They concluded that compressed propane required shorter times and lower pressures relative to carbon dioxide. Nimet et al. (2001) studied sunflower seed extraction with CO2 at temperatures of 40 to 60 °C and pressures from 19 to 25 MPa and with propane at temperatures from 30 to 60 °C and pressures of 8 to 12 MPa and observed that for all conditions investigated propane allowed higher extraction yields of sunflower oil.
The extracts of Ilex paraguariensis fruits showed different concentrations of phenolic compounds for the solvents investigated, but with no significant difference, as can be seen in Table 2.
The antioxidant activity of different Ilex paraguariensis fruit extracts was measured by the DPPH method and Fig. 1 shows the antioxidant activity in DPPH radical capture percentage concentration of 0.32 mg/mL extract. Table 2 also shows the IC50 of the extracts, which is the concentration capable of inhibiting 50% of DPPH, therefore the lower the better is the antioxidant activity IC50. Compared to propane, the extract obtained with supercritical CO2 showed a slightly higher amount of total phenolic compounds (9.25 mg GAE/100 g sample), but with no significant difference by Tukey test (p < 0.05). Ghafoor et al. (2012) obtained 2.52 mg GAE/mL of extract obtained from grape seeds with supercritical CO2 at 46 °C and 167 bar. Pessoa et al. (2015) found total phenolic compounds between 14.6 and 17.0 mg GAE/g for the extract obtained from pequi with pressurized propane at 60 °C and 15 MPa. Mesomo et al. (2012) obtained better results in terms of phenolic compounds extraction from ginger using propane at 50 °C and 10 MPa than with CO2 under 40 °C and 16.5 MPa.
Percentage of antioxidant activity by DPPH radical capture for the Ilex paraguariensis fruit extracts from supercritical CO2 and pressurized propane obtained at the central point of the experimental designs. Columns with the same letter do not differ by T test (p < 0.05). Values represent the mean ±standard deviation
The antioxidant activity conducted by the capture of DPPH radical was more efficient for the extract obtained with carbon dioxide, 17.77%, while that coming from propane showed 11.27% radical inhibition. The IC 50 was lower for the extracts obtained with CO2, 13.46 mg/mL, while the extracts obtained by propane required 20.14 mg/mL. Such result might be related to the higher amount of phenolic compounds found in the CO2 extracts and also due to the presence of vitamin E in this solvent, not found in the pressurized propane extracts.
Caffeine was the compound found in greater amount for the solvents, with 163.28 mg/g sample for CO2 and 54.17 mg/g for pressurized propane. Peres et al. (2013) found caffeine concentrations up to 268 mg/mL in mate samples, but the compounds found in higher concentrations were caffeoylquinic acids with concentrations up to 289 mg/mL. Table 3 shows the concentration of the eight compounds quantitatively identified by GC/MS, with caffeine as the major compound. The well-known much more pronounced hydrophilic characteristic of supercritical carbon dioxide compared with propane, and consequently its extracting solvent power for polar compounds compared with propane is clearly demonstrated by the chemical profile of the extracts obtained, shown in Table 3. From this table it can be seen that supercritical carbon dioxide extracts much more caffeine and theobromine than propane, compounds for which the antioxidant activity is closely related. This is the reason why supercritical carbon dioxide extract show a better antioxidant activity compared with propane, which in turns provides greater extraction yield but loaded with (apolar) compounds (perhaps waxes, not characterized in this work) that do not contribute effectively to the antioxidant activity.
The minimum inhibitory concentration for the two extracts was tested on two microorganisms, as presented in Table 4. Duarte et al. (2007) and Wang et al. (2008) classified the extracts as strong inhibitors for MIC values below 500 μg/mL, moderate inhibitors for MIC between 600 and 1500 μg/mL and weak inhibitors for MIC above 1600 μg/mL. Thus, the two extracts obtained in this work may be considered weak inhibitors for Staphylococcus aureus with the minimum inhibitory concentration found of 2000 μg/mL.
For Escherichia coli culture, the extract obtained with CO2 required a concentration of 1000 μg/mL and was considered a moderate inhibitor, while 2000 μg/mL was necessary for the extract obtained with propane pressurized, and thus was a weak inhibitor.
Oliveira et al. (2013) using grape extract obtained with supercritical CO2, noted a moderate inhibitory effect against Staphylococcus aureus and Escherichia coli. Czaikoski et al. (2015) using Eupatorium intermedium flower extracts obtained with supercritical carbon dioxide and pressurized propane, noticed the efficacy against Staphylococcus aureus, whereas Escherichia coli was completely resistant to the extracts.
Conclusion
Extraction yields of mate fruits in the temperature and pressure range investigated were, in general, higher for compressed propane compared to supercritical carbon dioxide. Antioxidant and antimicrobial activity for both compressed extraction solvents tested were detected with slight advantage for the CO2 extract. Microbiological test of both extracts showed the same weak minimum inhibitory concentration for Staphylococcus aureus, while for Escherichia coli the extract obtained from the application of supercritical CO2 was classified as moderate inhibitor, and that obtained with propane was considered weak inhibitor. Regarding chemical profile, the supercritical CO2 extract presented a higher concentration of caffeine, theobromine, palmitic acid and vitamin E, whereas for propane higher concentrations of phytol, squalene, β-sitosterol and Stigmasterol were found. Most importantly, this work demonstrates the potential use of an agro-industrial waste as a raw material, completely neglected, for producing value-added compounds.
References
Angus S, Armstrong B, Reuck K (1985) International thermodynamic tables of the fluid state: chlorine (tentative tables). Butterworths, London
Athayde ML, Coelho GC, Schenkel EP (2000) Caffeine and theobromine in epicuticular wax of Ilex paraguariensis A St-Hil. Phytochemistry 55:853–857
Boaventura BCB, Murakami ANN, Prudêncio ES, Maraschin M, Murakami FS, Amante ER, Amboni RDMC (2013a) Enhancement of bioactive compounds content and antioxidant activity of aqueous extract of mate (Ilex paraguariensis A. St. Hil.) through freeze concentration technology. Food Res Int 53:686–692
Boaventura BCB, Di Pietro PF, Kleina GA, Stefanutoa A, Morais EC, Andrade F, Wazlawika E, Silva EL (2013b) Antioxidant potential of mate tea (Ilex paraguariensis) in type 2 diabetic mellitus and pre-diabetic individuals. J Funct Foods 5:1057–1064
Boligon AA, Feltrin AC, Athayde ML (2013) Antioxidant and antimicrobial properties of Guzuma ulmifolia essential oil. Am J Essent Oils Nat Prod 1:23–27
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Bravo L, Goya L, Lecumberri E (2007) LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res Int 40:393–405
Burris KP, Higginbotham KL, Stewart CN Jr (2015) Aqueous extracts of yerba mate as bactericidal agents against methicillin-resistant Staphylococcus aureus in a microbiological medium and ground beef mixtures. Food Contr 50:748–753
Canhoto MJ (2010) Biotecnologia vegetal da clonagem de plantas à transformação Genética. Imprensa da Universidade de Coimbra, Coimbra, p 199 (in Portuguese)
Carelli G, Macedo SMD, Valduga AT, Corazza ML, Oliveira JV, Franceschi E, Vidal R, Jaskulski MR (2011) Avaliação preliminar da atividade antimicrobiana do extrato de erva-mate (Ilex paraguariensis A. St.-Hil.) obtido por extração com CO2 supercrítico. Rev Bras de Plant Méd 13:110–115 (in Portuguese)
Corso MP, Fagundes-Klen MR, Silva EA, Cardozo Filho L, Santos JN, Freitas LS, Dariva C (2010) Extraction of sesame seed (Sesamun indicum L.) oil using compressed propane and supercritical carbon dioxide. J. Supercrit Fluids 52:56–61
Czaikoski K, Mesomo MC, Scheer AP, Santa ORD, Queiroga CL, Corazza ML (2015) Kinetics, composition and biological activity of Eupatorium intermedium flower extracts obtained from SC CO2 and compressed propane. J Supercrit Fluids 97:145–153
Daood HG, Illés V, Gnayfeed MH, Mészáros B, Horváth G, Biacs PA (2002) Extraction of pungent spice paprika by supercritical carbon dioxide and subcritical propane. J Supercrit Fluids 23:143–152
Duarte MCT, Leme EE, Delarmelina C, Soares AA, Figueira GM, Sartoratto A (2007) Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J Ethnopharmacol 111:197–201
Freitas LS, Oliveira JV, Dariva C, Jacques RA, Caramão EB (2008) Extraction of grape seed oil using compressed carbon dioxide and propane: extraction yields and characterization of free glycerol compounds. J Agric Food Chem 56:2558–2564
Ghafoor K, Fahad Y, AL-Juhaimi FY, Choi H (2012) Supercritical fluid extraction of phenolic compounds and antioxidants from grape (Vitis labrusca B.) seeds. Plant Foods Hum Nutr 67:407–414
Groppo M (2015) Aquifoliaceae in Lista de Espécies da Flora do Brasil, Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB4904. Accessed 20 May 2015 (in Portuguese)
Hamdan S, Daood HG, Markus MT, Illés V (2008) Extraction of cardamom oil by supercritical carbon dioxide and sub-critical propane. J Supercrit Fluids 44:25–30
Illés V, Szalai O, Then M, Daood H, Perneczki S (1997) Extraction of hiprose fruit by supercritical CO2 and propane. J Supercrit Fluids 10:209–218
Illés V, Daood HG, Perneczki S, Szokonya L, Then M (2000) Extraction of coriander seed oil by CO2 and propane at super- and subcritical conditions. J Supercrit Fluids 17:177–186
Jacques RA, Krause LC, Freitas LS, Dariva C, Oliveira JV, Caramão EB (2007a) Influence of drying methods and agronomic variables on the chemical composition of mate tea leaves (Ilex paraguariensis A. St.-Hil) obtained from high-pressure CO2 extraction. J Agric Food Chem 55:10081–10085
Jacques RA, Santos JG, Dariva C, Oliveira JV, Caramão EB (2007b) GC/MS characterization of mate tea leaves extracts obtained from, high-pressure CO2 extraction. J Supercrit Fluids 40:354–359
Jesus AA, Almeida LC, Silva EA, Filho LC, Egues SMS, Franceschi E, Fortuny M, Santos AF, Araujo J, Sousa EMBD, Dariva C (2013) Extraction of palm oil using propane, ethanol and its mixtures as compressed solvent. J Supercrit Fluids 81:245–253
Lanza M, Ndiaye PM, Tavares FW, Oliveira D, Dariva C, Oliveira JV (2005) Phase behavior of castor oil in compressed propane and n-butane. J Supercrit Fluids 34:215–221
Liu TT, Yang TS (2012) Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food system. Int J Food Microbiol 156:68–75
Melo MMR, Silvestre AJD, Silva CM (2014) Supercritical fluid extraction of vegetable matrices: applications, trends and future perspectives of a convincing green technology. J Supercrit Fluids 92:115–176
Mesomo MC, Scheer AP, Perez E, Ndiaye PM, Corazza ML (2012) Ginger (Zingiber officinale R.) extracts obtained using supercritical CO2 and compressed propane: kinetics and antioxidant activity evaluation. J Supercrit Fluids 71:102–109
Miró PC, Ferreira A, Aquila ME (1998) Alelopatia de frutos de erva-mate (Ilex paraguariensis) no desenvolvimento do milho. Pesqui Agropecu Bras Bras 33:1261–1270 (in Portuguese)
Nimet G, Silva EA, Palú F, Dariva C, Freitas LS, Neto AM, Cardozo-Filho L (2001) Extraction of sunflower (Heliantus annuus L.) oil with supercritical CO2 and subcritical propane: experimental and modeling. Chem Eng J 168:262–268
Oliveira DA, Salvador AA, Smânia A Jr, Smânia EFA, Maraschin M, Ferreira SRS (2013) Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J Biotechnol 164:423–432
Ostrosky EA, Mizumoto MK, Lima ME, Keneko TM, Nishikawa SO, Freitas BR (2008) Métodos para avaliação de atividade antimicrobianan e determinação da concentração mínima inibitória (CMI) de plantas medicinais. Rev Bras Farmacogn 18:301–307
Pederssetti MM, Palú F, Silva EA, Rohling JH, Cardoso-Filho L, Dariva C (2011) Extraction of canola seed (Brassica napus) oil using compressed propane and supercritical carbon dioxide. J Food Eng 102:189–196
Peixoto AM, Toledo FF, Reichardt K, Filho MJ, Souza JSI (2000) Enciclopédia agrícola brasileira: E–H, 3rd edn. Editora da Universidade de São Paulo, São Paulo (in Portuguese)
Peres RG, Tonin FG, Tavares MFM, Rodriguez-Amaya DB (2013) HPLC-DAD-ESI/MS identification and quantification of phenolic compounds in ilex paraguariensis beverages and on-line evaluation of individual antioxidant activity. Molecules 18:3859–3871
Pessoa AS, Podestá R, Block JM, Franceschi E, Dariva C, Lanza M (2015) Extraction of pequi (Caryocar coriaceum) pulp oil using subcritical propane: determination of process yield and fatty acid profile. J Supercrit Fluids 101:95–103
Reid RC, Prausnitz JM, Poling BE (1987) The properties of gases and liquids, 4th edn. McGraw-Hill, New York
Reverchon E (1997) Supercritical fluid extraction and fractionation of essential oils and related products. J Supercrit Fluids 10:1–37
Reverchon E, De Marco I (2006) Supercritical fluid extraction and fractionation of natural matter. J Supercrit Fluids 38:146–166
Rodrigues MRA, Krause LC, Caramão EB, Santos JG, Dariva C, Oliveira JV (2004) Chemical composition and extraction yield of the extract of Origanum vulgare obtained from sub-and supercritical CO2. J Agric Food Chem 52:3042–3047
Santos KA, Bariccatti RA, Cardozo-Filho L, Schneider R, Palú F, Silva C, Silva EA (2015) Extraction of crambe seed oil using subcritical propane: kinetics, characterization and modeling. J Supercrit Fluids 104:54–61
Shahidi F, Naczk M (2002) Food phenolics: sources, chemistry, effects and applications. CRC, Boca Raton, London
Silva CM, Zanquia AB, Gohara AK, Souza AHP, Cardozo-Filho L, Visentainer JV, Chiavelli LUR, Bittencourt PRS, Silva EA, Matsushita M (2015a) Compressed n-propane extraction of lipids and bioactive compounds from Perilla (Perilla frutescens). J Supercrit Fluids 102:1–8
Silva CM, Zanqui AB, Souza AHP, Gohara AK, Chaves MA, Gomes STM, Cardozo-Filho L, Souza NE, Matsushita M (2015b) Chemometric study of perilla fatty acids from subcritical n-propane extracted oil. J Braz Chem Soc 26:14–21
Sparks D, Hernandez R, Zappi M, Blackwell D, Fleming T (2006) Extraction of rice bran oil using supercritical carbon dioxide and propane. JAOCS 83:885–891
Vaccari NFS, Soccol MCH, Ide GM (2009) Compostos fenólicos em vinhos e seus efeitos antioxidantes na prevenção de doenças. Rev de Ciênc Agrovet 8:71–83 (in Portuguese)
Wang Y-S, He H-P, Yang J-H, Di Y-T, Hao X-J (2008) New monoterpenoid coumarins from Clausena anisum-olens. Molecules 13:931–937
Weast RC, Astle MJ, Beyer WH (1988) CRC handbook of chemistry and physics. CRC Press, Boca Raton
Zanqui AB, Morais DR, Silva CM, Santos JM, Gomes STM, Visentainer JV, Eberlin MN, Cardozo-Filho L, Matsushita M (2015a) Subcritical extraction of flaxseed oil with n-propane: composition and purity. Food Chem 188:452–458
Zanqui AB, Morais DR, Silva CM, Santos JM, Chiavelli LUR, Bittencourt PRS, Eberlin MN, Visentainer JV, Cardozo-Filho L, Matsushita M (2015b) Subcritical extraction of salvia hispanica L. oil with n-propane: composition, purity and oxidation stability as compared to the oils obtained by conventional solvent extraction methods. J Braz Chem Soc 26:282–289
Acknowledgements
The authors thank CNPq, FAPERGS, CAPES for the financial support and scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, C.E.F., Scapinello, J., Bohn, A. et al. Phytochemical profile, antioxidant and antimicrobial activity of extracts obtained from erva-mate (Ilex paraguariensis) fruit using compressed propane and supercritical CO2 . J Food Sci Technol 54, 98–104 (2017). https://doi.org/10.1007/s13197-016-2440-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2440-4