Abstract
Supercritical fluid extraction (SFE) technique was applied and optimized for temperature, CO2 pressure and ethanol (modifier) concentration using orthogonal array design and response surface methodology for the extract yield, total phenols and antioxidants from grape (Vitis labrusca B.) seeds. Effects of extraction temperature and pressure were found to be significant for all these response variables in SFE process. Optimum SFE conditions (44 ~ 46 °C temperature and 153 ~ 161 bar CO2 pressure) along with ethanol (<7 %) as modifier, for the maximum predicted values of extract yield (12.09 %), total phenols (2.41 mg GAE/ml) and antioxidants (7.08 mg AAE/ml), were used to obtain extracts from grape seeds. The predicted values matched well with the experimental values (12.32 % extract yield, 2.45 mg GAE/ml total phenols and 7.08 mg AAE/ml antioxidants) obtained at optimum SFE conditions. The antiradical assay showed that SFE extracts of grape seeds can scavenge more than 85 % of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radicals. The grape seeds extracts were also analyzed for hydroxybenzoic acids which included gallic acid (1.21 ~ 3.84 μg/ml), protocatechuic acid (3.57 ~ 11.78 μg/ml) and p-hydroxybenzoic acid (206.72 ~ 688.18 μg/ml).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The trend for investigation of bioactive compounds, especially polyphenols, from natural sources (fruits, vegetables, cereals, herbs) has increased in recent years. These natural antioxidants have proved to be effective for inhibiting different human diseases due to their antiradical, antioxidant and anti-inflammatory properties [1]. Grape is a major fruit crop and in year 2010 it was cultivated on 7.1 million hectares around the globe with a total grape production of 67.1 million tones, approximately [2]. Most of the grape varieties, used for manufacturing different food products, contain seeds including Vitis labrusca B. which has 3–4 seeds per berry. During processing of grapes, high quantities of waste by-products remain, which can be a good and cheap source of high quality bioactive compounds having various health promoting properties. The grape pomace obtained, as by-product, constitutes around 20 % of the weight of processed grapes. Grape seeds constitute about 20 % of pomace and 60–70 % of total extractible phenolic compounds present in grapes [3]. These include several flavonoids with phenolic nature such as monomeric flavanols, dimeric, trimeric and polymeric procyanidins, and phenolic acids [4]. Phenolic compounds can be used in different therapeutic procedures with the purpose of free radical neutralization in biological systems [5]. The in vitro activity of grape extracts to inhibit the oxidation of human low-density lipoproteins correlates significantly to the presence of phenolics [6]. In addition, various anti-platelet aggregating effects and other potentially disease preventing cellular actions of phenolic compounds have been amply documented [7]. Extracts obtained from grape seeds have also been tested to possess anticancer and cancer chemopreventive properties during in vivo and in vitro studies [8].
The extraction of phenolic compounds from plant materials carries significant importance before their utilization and various studies have been carried out using different techniques [9]. These are usually extracted with methanol:water mixtures, which enables high gravimetric yields but fairly non-selective and complex extracts. Supercritical fluid extraction (SFE) using supercritical CO2 is considered one of the most suitable methods for producing natural antioxidants to be used in the food industry and pharmacological applications. The new and tougher regulatory requirements on the use of organic solvents have motivated need for active research activities on alternate ‘green’ technologies such as SFE [10]. Among different supercritical fluids, CO2 is often promoted as environment friendly, safe, non-toxic, non-carcinogenic, non-flammable, cheap and having modest critical conditions. Furthermore, it can be applied to a wide range of chemical and biochemical extraction processes. The selectivity of the supercritical CO2 can be adjusted by varying temperature and pressure to obtain fractions consisting of desirable compounds [11]. Above its critical point (31.1 °C, 7.38 MPa), where the distinction between a liquid and a gas disappears, the density of CO2 can vary by almost an order of magnitude with relatively small changes in temperature or pressure, so its solvating power can be regulated and controlled [12]. The extracts obtained by CO2-assisted SFE process are of outstanding quality and the yields are comparable with those obtained by organic solvent extraction methods [13]. In fact, SFE extracts are generally recognized as safe for use in food products, therefore, it may serve as a very promising technology in industry. CO2 is non-polar and may not be efficient alone for polar (e.g., phenolic) molecules, however, the addition of modifiers such as ethanol, methanol and water improves its polarity and solvent strength while retaining the sensitivity of solubility with respect to operating pressure and temperature of SFE [10]. SFE has been applied to extract oil from grape seeds and the effects of SFE process variables such as temperature, pressure, particle size of seeds, solvent flow rate and the direction of solvent flow, on the yield, quality and antioxidant potential of oil, have been investigated previously. Normally, SFE pressure of 150–300 bar and 30–60 °C temperature is used for the extraction of bioactives depending on different plant materials [10]. Relatively higher CO2 pressures (>200 bar) are applied to obtain oil from seeds [14]. In one such study the temperature was kept constant at 40 °C, however CO2 pressure varied between 280 and 550 bar to investigate the grape seed oil solubility and extraction kinetics [15]. In these reported applications of SFE from grape seeds, the quantities of extracted phenolics and antioxidants were not optimized. The levels of phenolics and antioxidants, observed in grape seeds oil, are lower than those in grape seeds (60–115 μg GAE/g and 0.14–1.166 μg/g of oil, respectively) [16]. Therefore, we planned SFE process for enhanced recovery of phenolic and antioxidant compounds from grape seeds. The recovery of oil free bioactive enriched extracts of grape seed by SFE is important for use in different food and pharmaceutical applications.
The objectives of our study were to optimize and study the effects of SFE process variables such as temperature, pressure and concentration of ethanol as modifier for the extraction of bioactive compounds from grape seeds by using an orthogonal array design and response surface methodology. We also aimed at studying the free radical scavenging activities and hydroxybenzoic acids contents of grape seeds extracts obtained using different experimental sets of SFE process.
Materials and Methods
Materials
Ripened grapes (Vitis labrusca B.), freshly harvested from a local farm in Kyungbuk province of Korea were excised from the stems and washed. Seeds were removed from grape berries and oven dried at 50 °C until the moisture level was constant (6.2 % w/w). Dried grape seeds were ground to a powdered form using an electrical grinder and passed through a 0.5 mm sieve. All the chemicals used were of analytical grade and they were purchased from Sigma Chemical Co. (St. Louis, MO) and Duksan Pure Chemical Co. (Ansan, Korea).
Methods
Supercritical Fluid Extraction
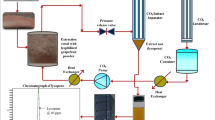
The system for supercritical fluid extraction (SFE) consisted of column thermostat (CO-1560, JASCO Corporation, Tokyo, Japan), solvent pumps (PU-1580, JASCO), UV/VIS detector (UV-1575, JASCO), back pressure regulator (880-81, JASCO), CO2 cylinder and a coolant circulator. 3 g powdered sample of grape seeds was kept in the extraction vessel and placed in the column thermostat set at specific temperature. Desired pressure was adjusted at the back pressure regulator and solvent pumps. The flow rates for CO2 and ethanol were fixed at 2 ml/min. Once the set temperature and pressure (at solvent pumps and back pressure regulator) were achieved after turning on the injection valve and the system was in equilibrium, the extraction was carried out for 30 min in each experimental run. Grape seeds extract was obtained at a CO2 pressure level where oil fraction was not extracted from grape seeds and the final extract was collected in a flask connected to the back pressure regulator. The solvent was evaporated by drying under vacuum using rotary evaporator; the extract was weighed to obtain the yield (calculated as percentage of 3 g grape seeds sample converted into extract by SFE) and it was stored at −20 °C before further analysis of bioactive components and antiradical properties. For chemical analysis these extracts were dissolved in ethanol and a total volume of each extract solution was made (100 ml). These extracts solutions were further diluted and properly filtered before carrying out each analytical procedure.
Experimental Design
The experiments, carried out to optimize SFE process for bioactive compounds from grape seeds, were based on an orthogonal array design (OAD). The effects of extraction temperature, extraction pressure and modifier concentration on the bioactive components of extracts were investigated. An orthogonal matrix with three factors, each factor containing four levels was selected to arrange the experiments. Extraction temperatures were 37, 40, 43 and 46 °C, pressures were 137, 147, 157 and 167 bar and modifiers were 5, 6, 7 and 8 % ethanol. These ranges of process variables were based on the results of our preliminary trials (data not presented) and available reports [14]. Much higher ranges of pressures are needed to extract oil from grape seed [15]; however, we kept pressure low enough to avoid extraction of oil. CO2 + 2–8 % ethanol was also previously used by other researchers while carrying out SFE from plant seeds [10], hence we kept the concentration of ethanol at lower level to study the effects of major SFE parameters. Regression analysis was performed on the data from triplicate measurements of each dependent variable. Response surface analysis was applied on the experimental data from orthogonal array design for calculations and prediction of optimum conditions for SFE of total phenols, antioxidants and extract yields from grape seeds. The prediction of the optimum aqueous extraction condition was done according to the following equation:
where Y is the predicted response and β 0 is the offset term. β 1 , β 2 and β 3 are the regression coefficients for linear; β 11 , β 22 and β 33 are quadratic and β 12 , β 13 and β 23 are the interaction terms. X 1 , X 2 and X 3 are the independent variables i.e., SFE temperature, pressure and ethanol concentration, respectively.
Analysis for Total Phenolic Compounds
The total phenolic compounds were analyzed using the Folin Ciocalteau method with some modifications [17]. A 200 μl properly diluted sample of grape seeds extract was mixed with 400 μl Folin Ciocalteu reagent. The solution was diluted to a total volume of 4.6 ml using deionized water then thoroughly mixed. After incubation for 10 min at room temperature, 1 ml of 20 % Na2CO3 solution was mixed followed by incubation for 2 h at room temperature. The absorbance was read at 765 nm on a spectrophotometer (TU-1800; Human Corporation, Seoul, Korea). The calibration curve was obtained by using gallic acid and the total phenolic compounds of the samples were expressed in milligram gallic acid equivalent per ml of extract (mg GAE/ml).
Determination of Total Antioxidants
Total antioxidants in grape seeds extracts were evaluated by the phosphomolybdenum complex method [18]. In brief, 0.4 ml of sample solution (100 μl/ml methanol) was combined with 4 ml of reagent solution containing 0.6 M sulphuric aicd, 2 mM sodium phosphate and 4 mM ammonium molybdate. The blank solution contained 4 ml of reagent solution and 1 mL of methanol. Test tubes were caped and placed in hot water (95 °C) for 90 min. Absorbance was measured at 695 nm against blank. Ascorbic acid was used for preparing calibration curve and total antioxidants were expressed as milligram ascorbic acid equivalent per ml of extract (mg AAE/ml).
1, 1-Diphenyl-2-Picrylhydrazyl Antiradical Activity
The free radical activity of the grape seeds extract was determined by using 1, 1-diphenyl-2-picrylhydrazyl (DPPH) [19]. Briefly, 1 ml of grape seeds extract was mixed with 2 ml of 10 mg/l solution of DPPH in methanol. The mixture was shaken vigorously, allowed to stand at room temperature for 5 min and a decline in absorbance was recorded at 517 nm using methanol as control. The antiradical activity (%) of the grape seeds extract on DPPH radicals was calculated as follows:
Analysis of Phenolic Acids using High Performance Liquid Chromatography
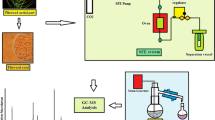
The HPLC system consisted of a Hewlett Packard 1100 series system with pump, UV detector, auto sampler and degasser. Data processing was performed by using the software HPcore chemstation (Hewlett Packard, Germany). Separation was performed on an YMC pack pro C18 RS column (250, 4.6 mm ID, S-5 μm, 8 nm, YMC Inc., USA) at room temperature. Injection volume was 10 μl filtered (using 0.45 μm filter) sample solution (100 μl of grape seeds extract dissolved in 1 ml of methanol) and flow rate was set at 1 ml/min. Solvent used were 5 % acetic acid (A) and 50 % acetonitrile (B). 1 ml of extract was diluted with 5 ml of methanol and filtered through 0.45 μm filter before injection into the HPLC. The column was eluted under a linear gradient from 5 % mobile phase B to 75 % over 20 min, to 100 % over 5 min, isocratic for 5 min, to 25 % over 5 min and to 5 % over 5 min. Compounds were detected at 290 nm with UV detector. Analytical standards of gallic, protocatechuic and p-hydroxybenzoic acids were used for preparing calibration curves and the quantities of detected compounds which were expressed as μg/ml of grape seeds extract.
Statistical Analysis
All the analysis were carried out in triplicate and the experimental results obtained were expressed as means ± SD. Statistical analysis was performed by using the statistical analysis system (SAS, version 9.1). Data were analyzed by the analysis of variance and the mean values were considered significantly different when p < 0.05. The optimal extraction conditions were estimated through regression analysis and three dimensional response surface plots of the independent variables and each dependent variable.
Results and Discussion
Extraction Process Model
Table 1 presents the orthogonal array design (OAD) for different sets of process variables (temperature, CO2 pressure and ethanol concentration) used in supercritical fluid extraction (SFE) along with the experimental values of extract yields, total phenols and antioxidants from grape seeds. The results of analysis of variance and goodness of fit of the models are summarized in Table 2. The data showed a good fit with p < 0.05. The model was used for the construction of three dimensional response surface plots to predict the relationships between independent variables and the dependent variables.
Effect of SFE Variables on the Yield of Grape Seeds Extract
The regression analysis (modeling and analyzing several variables, when the focus is on the relationship between a dependent variable and one or more independent variables) of the data (extract yield) obtained by SFE from grape seeds revealed that the yield was significantly (p < 0.05) affected by SFE temperature and pressure. The relationship of the extract yield and that of extraction temperature and pressure is depicted in Fig. 1 and it was linear with R 2 value of 0.987. By increasing either temperature or pressure, while the modifier concentration remains constant, results in enhancement of the extract yield. The relationship between significant process variables and the extract yield is presented in Eq. 2.
Y 1 is the yield (%) of grape seeds extract, X 1 is the extraction temperature (°C) and X 2 the extraction pressure (bar). The equation was based on the data of regression coefficients using the values of significant linear terms only (Table 2).
Effect of SFE Variables on Total Phenolics in Grape Seeds Extract
The regression analysis of total phenolics from grape seeds showed that the effect of SFE temperature was highly significant (p < 0.001) on the extraction of phenolic compounds. The effect of CO2 pressure was also significant (p < 0.05) while that of ethanol was non-significant. This is because high pressure and temperature increase the solvating power of the CO2 [20]. The relationship between total phenols of grape seeds extract and significant process variables is depicted in Fig. 2. Response surface analysis of data in Table 1 demonstrates that the relationship between total phenols and the operating parameters was linear with a good regression coefficient (R 2 = 0.993). The relationship between temperature and pressure during extraction of total phenolic compounds from grape seeds is presented as
where Y 2 represents total phenols in grape seeds extract. The equation was based on the values of significant regression terms (p values presented in Table 2). Although an increase in temperature can favor extraction of phenolic compounds from plant materials, it cannot be increased indefinitely; since the structural deformation of phenolic compounds may take place at temperatures above 50 °C [21]. Gong et al. [22] observed a decline in the total phenolic compounds analyzed in the extract of marigold when the temperature increased above 60 °C in a conventional solvent extraction system. SFE allowed the extraction of phenolic compounds at lower temperatures (46 °C), hence it is also believed to preserve the quality of the extracted phenolic compounds.
Effect of SFE Variables on Antioxidants in Grape Seeds Extract
The analytical results of antioxidant compounds in grape seeds extract obtained by SFE were also evaluated by regression analysis. The main SFE parameters for antioxidant compounds from grape seeds were temperature and pressure, former being highly significant (p < 0.001). The effect of ethanol concentration was not significant. SFE of bioactive compounds and antioxidants is believed to be affected mainly by temperature, pressure and dynamic extraction time [23]. The relationship between the extraction of antioxidant components, temperature and pressure is depicted in Fig. 3. Passos et al. [14] observed during extraction of oil from grape seeds that the antioxidant activity was mainly affected by temperature and pressure of SFE. Antioxidant activities of the grape seeds extracts in our study were affected by the linear, quadratic and interaction terms of SFE temperature and pressure and R 2 value was 0.998. The relationship between significant extraction variables and antioxidants is represented in Eq. 4 where non-significant regression terms involving X 3 (ethanol concentration) were neglected.
Y 3 represents the antioxidant compounds in grape seeds extract. X 1 2 , X 2 2 and X 1 X 2 represent the quadratic and interaction relation of SFE temperature and pressure.
Optimization of SFE Process
Process optimization has been the key issue in biochemical processes to increase product yield and to ensure product quality. Different statistical approaches are usually applied to achieve this objective. The optimum SFE conditions for the extract yields, total phenols and total antioxidants from grape seeds obtained by using OAD and response surface methodology (RSM) are presented in the Table 3. SFE temperature (44–46 °C), pressure (153–161 bar) and lower concentrations (5.8–6.8 %) of ethanol as modifier, can result in optimal extract yield (12.09 %), total phenols (2.41 mg GAE/ml) and total antioxidants (7.08 mg AAE/ml) from grape seeds. The experimental results (12.32 % extract yield, 2.45 mg GAE/ml total phenols and 7.08 mg/mL antioxidants) obtained using optimum SFE conditions matched with predicted results. Therefore, the RSM model was validated with a good correlation (R 2 and R 2–adjusted values are presented in Table 3).
Antiradical Activities of Grape Seeds Extracts
The antiradical activities of the 16 grape seeds extracts obtained by SFE were assessed using DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging assay and they are presented in Fig. 4. All the extracts showed high DPPH radical scavenging activities and the maximum antiradical activity of 92.3 (Fig. 4a) or 32.2 % (as a function of total phenolics, Fig. 4b) was observed for the extract obtained using 46 °C temperature and 167 bar CO2 pressure. Growing evidence suggests that phytochemicals such as phenolics, thiols and carotenoids, present in most plant species, can protect the human body against oxidative damage from free radicals through a variety of mechanisms. Plant-derived antioxidants could function as singlet and triplet oxygen quenchers, peroxide decomposers, enzyme inhibitors or synergists [24]. We also observed that there was a close relation between total phenols, antioxidants and antiradical activities of grape seeds extracts obtained by SFE. Similar correlations between phenolic compounds and bioactivities of medicinal plant extracts have been reported previously by others [25].
Hydroxybenzoic Acids in Grape Seeds Extracts
The phenolic acids are categorized as hydroxybenzoic and hydroxycinnamic acids due to difference in molecular structures. Grape seeds extracts obtained by SFE were analyzed for gallic acid, protocatechuic acid and p-hydroxybenzoic acid and the analytical results for 16 extracts, obtained according to OAD (Table 1), are presented in Table 4. The data revealed that p-hydroxybenzoic acid contents (206.72–688.18 μg/ml) were highest followed by those of protocatechuic acid (3.57–11.78 μg/ml) and gallic acid (1.21–3.84 μg/ml) among different SFE extracts of grape seeds. These hydroxybenzoic acids are important bioactive compounds having antimicrobial and biological properties. They have been previously detected in seeds of four red grape varieties (Cencibel, Cabernet Sauvignon, Merlot and Shiraz) and their quantities ranged from 3.3 to 7.3 mg/kg of fresh grapes [26]. We also observed that seeds of a red grape variety (Vitis labrusca B.) are good source of these important non-anthocyanic polyphenols and SFE is an effective method to recover considerable quantities of these polyphenols. Gallic acid and protocatechuic acid are phenolic compounds often detected in grape wines and also have reported pharmacological activities [27]. Alkyl esters of p-hydroxybenzoic are widely used as antimicrobial agents in foodstuffs, cosmetics, toiletries and pharmaceuticals. They are reported to have broad spectrum of antimicrobial activity including fungicidal activity [28].
We obtained extracts of grape seeds by using SFE (137–167 bar, 37–46 °C and 5–8 % ethanol) with the aim to maximize the recovery of phenolic and antioxidant compounds. The quantities of total phenolics and antioxidants recovered from grape seeds using SFE (Table 1) are comparable with those obtained by organic solvent extraction methods using higher temperatures. We obtained 85 mg GAE/g of phenolic compounds and 242.33 mg AAE/g of antioxidants (extract # 16) from grape seeds using modest SFE conditions (167 bar, 46 °C and 5 % ethanol). In a study carried out by Bucic-Kojic et al. [3], 94 mg GAE/g of total phenolics were extracted from grape seeds using 96 % ethanol and extraction temperature was 80 °C in a conventional system. Another study [29] reported antiradical activities of grape seeds extracts in the range of 74–83 %, when they were obtained by heating at 100 °C for 10–120 min in 70 % ethanol. Grape seed extracts, obtained using SFE in our designed experiments, showed 86–92.3 % antiradical activities. This shows that extracts of grape seeds obtained by SFE have better functional quality which is due to high quality phenolic and compounds.
Conclusion
Phenolic compounds and antioxidants can be effectively extracted from grape seeds using green SFE technology by employing modest conditions of CO2 pressure, temperature and modifier. Optimization of SFE process parameters can enhance the recovery of these compounds from grape seeds. Pressure and temperature were found to be the main variables for SFE from grape seeds. These extracts were found to be excellent scavengers of DPPH radicals and good sources of phenolic acids such as gallic acid, protocatechuic acid and p-hydroxybenzoic acid. Our findings and further studies for recovery of individual bioactives from grapes using SFE can be valuable for producing high quality health products.
References
Sreelatha S, Padma PR (2009) Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr 64:303–311
FAO Stat (2012) http://faostat.fao.org
Bucic-Kojic A, Planinic M, Tomas S, Jakobek L, Seruga M (2009) Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int J Food Sci Technol 44:2394–2401
Yilmaz Y, Toledo RT (2004) Health aspects of functional grape seed constituents. Trends Food Sci Technol 15:422–433
Hernández L, Afonso D, Rodríguez EM, Díaz C (2011) Phenolic compounds in wheat grain cultivars. Plant Foods Hum Nutr 66:408–415
Meyer AS, Yi OS, Pearson DA, Waterhouse AL, Frankel EN (1997) Inhibition of low-density lipoprotein oxidation in relation to composition of phenolic antioxidants in grapes (Vitis vinifera). J Agric Food Chem 45:1638–1643
Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Martin PM, Castanas E (2000) Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem 78:429–441
Kaur M, Agarwal C, Agarwal R (2009) Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr 139:1806S–1812S
Spigno G, Tramelli L, De-Faveri DM (2008) Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng 81:200–208
Salgin U (2007) Extraction of jojoba seed oil using supercritical CO2 + ethanol mixture in green and high-tech separation process. J Supercrit Fluids 39:330–337
Babovic N, Djilas S, Jadranin M, Vajs V, Ivanovic J, Petrovic S, Zizovic I (2010) Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov Food Sci Emerg Technol 11:98–107
Leitner W (2000) Green chemistry: designed to dissolve. Nature 405:129–130
Friedrich JP, List GR (1982) Characterization of soybean oil extracted by supercritical carbon dioxide and hexane. J Agric Food Chem 30:192–193
Passos CP, Silva RM, Da Silva FA, Coimbra MA, Silva CM (2010) Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil. Effect of the operating conditions upon oil composition and antioxidant capacity. Chem Eng J 160:634–640
Sovová H, Kučera J, Jež J (1994) Rate of the vegetable oil extraction with supercritical CO2-II. Extraction of grape oil. Chem Eng Sci 49:415–420
Bail S, Stuebiger G, Krist S, Unterweger H, Buchbauer G (2008) Characterization of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem 108:1122–1132
Kim DK, Jeong SC, Gorinstein S, Chon SU (2012) Total polyphenols, antioxidant and antiproliferative activities of different extracts in mungbean seeds and sprouts. Plant Foods Hum Nutr 67:71–75
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 269:337–341
Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Games EJC, Mehta RG (1998) Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen 1:35–46
Liu W, Fu YJ, Zu YG, Tong YG, Wu N, Liu XL, Zhang S (2009) Supercritical carbon dioxide extraction of seed oil from Opuntia dillenii Haw. and its antioxidant activity. Food Chem 114:334–339
Pinelo M, Arnous A, Meyer AS (2006) Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci Technol 17:579–590
Gong Y, Hou Z, Gao Y, Xue Y, Liu X, Liu G (2012) Optimization of extraction parameters of bioactive components from defatted marigold (Tagetes erecta L.) residue using response surface methodology. Food Bioprod Process 90:9–16
Solati Z, Baharin BS, Bagheri H (2012) Supercritical carbon dioxide (SC-CO2) extraction of Nigella sativa L. oil using full factorial design. Ind Crop Prod 36:519–523
Larson RA (1988) The antioxidants of higher plants. Phytochemistry 4:969–978
Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184
Montealegre RR, Peces RR, Vozmediano JLC, Gascueña JM, Romero EG (2006) Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J Food Compos Anal 19:687–693
La Torre GL, Saitta M, Vilasi F, Pellicano T, Dugo D (2006) Direct determination of phenolic compounds in Sicilian wines by liquid chromatography with PDA and MS detection. Food Chem 94:640–650
Oishi S (2004) Lack of spermatotoxic effects of methyl and ethyl esters of p-hydroxybenzoic acid in rats. Food Chem Toxicol 42:1845–1849
Kim SY, Jeong SM, Park WP, Nam KC, Ahn DU, Lee SC (2006) Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem 97:472–479
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghafoor, K., AL-Juhaimi, F.Y. & Choi, Y.H. Supercritical Fluid Extraction of Phenolic Compounds and Antioxidants from Grape (Vitis labrusca B.) Seeds. Plant Foods Hum Nutr 67, 407–414 (2012). https://doi.org/10.1007/s11130-012-0313-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-012-0313-1