Abstract
Quorum sensing or cell to cell communication which includes inter- and intra-cellular communication has been implicated in the production of virulence factor and formation of biofilm in food-borne pathogens. In the present study, the effect of quorum sensing signals on the biofilms of food-borne pathogens has been elucidated. N-butryl homoserine lactone and N-hexanoyl homoserine lactone belonging to acyl homoserine lactone (AHL) family of signaling molecules were investigated for their effect on the biofilm formation (attachment and exopolymeric substance production) in the food-borne pathogens Escherichia coli, Salmonella enterica serovar Typhimurium and Vibrio parahemolyticus. The signaling molecules at a concentration of 1 µM were capable of increasing biofilm formation in all the tested pathogens. There was an increase in the attachment of the bacterial cells and biomass as observed by microtiter plate assay and exopolymeric substances production in the biofilms in presence of the AHLs. Further, it needs to be elucidated if the effect of AHLS on the biofilms of E. coli and S. enterica serovar Typhimurium is SdiA dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the food processing environments, the development of persistent biofilms harboring pathogens is a major problem as contamination of food-contact surfaces may cause significant impact on public health (Bridier et al. 2015). The first report on biofilm formation by food-borne pathogens defined the adhesive behaviour of Salmonella sp., and since then, pathogens including Listeria monocytogenes, Campylobacter jejuni, Escherichia coli O157:H7, Yersinia enterocolitica and Staphylococcus spp. were known to form biofilm in the food processing plants (Giaouris et al. 2012). Biofilm formation by the pathogens protects the bacteria from environmental stress, increases their tolerance to biocides which makes it difficult to remove them from food processing environments (Winkelstroter et al. 2014). Pathogenic Escherichia coli represents a significant risk to human health as these have specific biofilm formation factors which help in colonizing the gastrointestinal tract in hosts and also on abiotic surfaces (Yeom et al. 2012). Salmonella adheres to various surfaces in food processing and production environments and forms biofilms on food and organic matrices. The long term survival or persistence of Salmonella in food environments is attributed to its biofilm formation behaviour (Jaglic et al. 2014). Similarly, Vibrio species were known to cause diarrhoeal diseases in the humans, the ability to form strong biofilms is the key factor for environmental survival and transmission (Yildiz and Visick 2009). In the past years, substantial effort has been applied for a deeper understanding of the environmental parameters, architecture and physiology of biofilms. At the molecular level, the mechanism of signaling is involved in biofilms formation in the food-related spoilage and pathogenic bacteria.
Quorum sensing or cell to cell signaling systems are extensively distributed in Vibrios and involves population density dependent production of signal molecules or autoinducers, and its release and detection. The Vibrio species have two master QS regulators namely AphA and HMR [high cell density (HCD) master regulator] expressed at low cell density (LCD) as well as HCD, respectively (Rutherford et al. 2011). HMR is known as OpaR in V. parahaemolyticus, HapR in V. cholera, SmcR in V. vulnificus and LuxR in V. harveyi (Wang et al. 2013). Many members of the Vibrionaceae are known to regulate activities such as biofilm formation, virulence, and luminescence by quorum sensing (Ng and Bassler 2009). However, the quorum sensing mechanism in biofilm formation is not intensively investigated in Vibrio parahaemolyticus.
In many of the gram-negative bacteria, AHLs produced by the bacteria bind to the receptors which are transcriptional regulators resulting in repression or activation of target genes. However, certain bacteria including E. coli and S. enterica do not synthesizing AHL signals but possess the receptor for AHL known as SdiA. These SdiA are known to bind to AHLs of other bacterial species and regulates gene expression. The sdiA gene in Salmonella regulates the expression of rck gene, which is involved in the pathogen adhesion and invasion of epithelial cells as well as resistance to the host complement. SdiA regulates certain phenotypes in E. coli such as motility, biofilm formation, virulence, acid resistance, and transport and processing of autoinducer-2 (Smith et al. 2011). Though the involvement of AHL-receptor, SdiA in biofilm formation of pathogens E. coli and Salmonella has been investigated, yet there are no studies showing the role of the signaling molecules in cell attachment and surface colonization.
Thus, there are very few studies ascertaining the effect of acyl homoserine lactones in biofilm formation in the enteric pathogens E. coli, Salmonella and Vibrio in food environments. In the present study, the effect of the small chain AHLs N-butryl homoserine lactone and N-hexanoyl homoserine lactone were investigated on the biofilm formation (attachment and exopolymeric substance production) in E. coli, S. typhimurium and V. parahemolyticus.
Materials and methods
Bacterial strains
Food-borne pathogens Escherichia coli (MTCC 7410), Salmonella enterica serovar Typhimurium (MTCC 3224) and Vibrio parahemolyticus (MTCC 451) were used in the study. The bacteria were cultured and maintained in tryptic soy broth.
Biofilm assays
The effect of AHLs on the initial attachment and biofilm formation of bacteria at 30 °C was studied by crystal violet microtitre plate assay (O’Toole and Kolter 1998). 200 μL of the inoculum containing 108 cells/mL in tryptic soy broth (TSB) were pipetted into wells containing 1 µM concentrations of the signal molecules C4-HSL and C6-HSL and incubated at 37 °C for 48 h. The absorbance at 600 nm was measured for the bacterial growth. The loosely adherent bacteria from each well were removed by aspirating and washing thrice with sterile water (200 μL). After drying the plates in oven for 30 min, 125 μL of crystal violet dye (0.1 %) was pipetted to each well and incubated for 30 min at room temperature. Destaining was performed by washing with sterile distilled water (200 μL). The dye was solubilized by adding 200 μL of 95 % ethanol to each stained well. After incubating for 10–15 min at 4 °C, the contents of each well (the crystal violet/ethanol solution) were transferred to a separate well of an optically clear flat-bottom 96-well plate. The absorbance was measured at 595 nm to assay bacterial attachment and biofilm formation. The crystal violet microtitre plate assay was performed in triplicates.
Quantification of exopolysaccharide (EPS) production by ruthenium red staining
The EPS production in biofilm formation of the pathogens in the presence and absence of the signal molecules C4-HSL and C6-HSL were assayed by ruthenium red staining technique, as described previously (Bai and Rai 2014).
Effect of AHLs on bacterial growth
The effect of the signal molecules on the growth of bacteria were determined in sterile 96 well MTPs by culturing 200 μL of the inoculum (108 cells/mL) in TSB with 1000–100 nM concentrations of the signal molecules C4-HSL and C6-HSL in each well. The effect of AHLs on the growth of bacteria was determined by measuring absorbance of the culture at 600 nm at every 2 h interval until 36 h.
Statistical analysis
The experiments were performed in triplicates and the data were reported as means ± standard deviation. The differences between control and test was analyzed by Student’s t test. The differences in the various assays was analyzed by One-way analysis of variance (ANOVA). The p values <0.05 were statistically significant. IBM SPSS Statistics 18 software was used for the statistical analysis.
Results and discussion
Most of the food-borne pathogenic bacteria adhere to various materials and form biofilms on the surfaces in the food processing plants under all environmental conditions (Bridier et al. 2015). The microbial colonization of surfaces begins with initial cell attachment to the substratum and progresses to form a mature biofilm, wherein the complex matrix protects it from environmental stresses and biocidal agents (Flemming and Wingender 2010). Hence, the present study dealt with the effect of signaling molecules on initial adherence and EPS production in biofilms, which are the two major steps required for initiation of biofilms and its maturation into a three dimensional architecture. The signaling molecules used in the study were N-butryl homoserine lactone and N-hexanoyl homoserine lactone (varying in acyl chains of C4 and C6 in length, respectively) and investigated for their effect on the biofilms of the commonly encountered enteric pathogens Escherichia coli, Salmonella enterica serovar Typhimurium and Vibrio parahemolyticus. These two signaling molecules are short chained and therefore, capable of diffusing easily into a bacterial cell and are produced by the frequently occurring food spoilers and contaminants such as Pseudomonas, Serratia and Yersinia species.
The role of signaling molecules in the biofilm formation has been well established in certain bacteria. For instance, quorum sensing regulates the secretion of EPS required for biofilm formation in V. cholerae (Hammer and Bassler 2003). Similarly, signal molecule N-(3-oxododecanoyl)-L-homoserine lactone regulates biofilms in Pseudomonas aeruginosa (Davies et al. 1998) and the autoinducer—2 signal controls biofilm formation in E.coli through a motility quorum sensing regulator (González Barrios et al. 2006).
The bacteria Escherichia, Salmonella, Klebsiella, Enterobacter and Citrobacter lack the LuxI homolog AHL synthetase and do not produce AHLs. However, they have a LuxR homolog, SdiA which acts as a receptor for signal molecules (Patankar and Gonzalez 2009). The SdiA of Escherichia and Salmonella is capable of detecting AHLs synthesized by other bacterial species. The SdiA are capable of detecting a broad range of AHLs including those without modification at position 3 and having chain lengths ranging from C4, C6, C8, etc. The SdiA have detection sensitivity ranging from 1 nm to 1 µm of AHLs (Soares and Ahmer 2011).
In E. coli, the SdiA–AHL exhibits “folding switch” pattern wherein a significant fraction of the SdiA protein produced are in a folded and soluble form in the presence of C8-HSL. However, the protein is present as inclusion bodies in insoluble form in the absence of the signal molecules (Yao et al. 2006). Thus, SdiA uses various AHLs as switches for its folding. The bacteria possessing SdiA use the quorum sensing mechanism for inter species communication.
In vitro studies have shown that SdiA expressed from a high-copy-number plasmid showed decreased motility and reduced LEE (locus of enterocyte effacement) gene expression in E. coli O157:H7. Further, the deletion of sdiA increased motility, curli gene expression, and formation of biofilm (Sharma et al. 2010). It has also been reported that SdiA is essential for colonization of E. coli O157:H7 in the bovine intestine (Dziva et al. 2004). Similar studies have demonstrated that under in vitro conditions the sdiA gene represses the adherence of LEE and LEE-mediated factors to cultured cells, however, sdiA is essential for the colonization of E. coli in bovine large intestine (Sharma and Bearson 2013).
In our study, the signaling molecules C4-HSL and C6-HSL increased attachment of the E. coli bacterial cells in the CV-MTP assay. Biofilm formation was more pronounced for E. coli grown in C6-HSL supplemented TSB broth (p < 0.05). The addition of 1 µM of C6-HSL increased biofilm formation by 9 %, whereas in presence of C4-HSL the attachment of cells to the substratum increased by 4 %. However, Lee et al. (2009), have reported that E. coli uses SdiA for decreased biofilm formation in the presence of the signal molecule AHLs and indole. Exogenous addition of AHLs such as 10 μM of N-butyryl-, N-hexanoyl-, and N-octanoyl-DL-homoserine lactones inhibited biofilm formation in E. coli by 27 % without effecting its growth (Lee et al. 2007). However, in the SdiA2D10 variant, the mutations of SdiA2D10 increased biofilm formation by 1.9-fold on addition of 10 μM 3o-C8-L-HSL while in the wild-type SdiA, the exogenous addition of the same AHL reduced biofilm formation by 6 %. Hence, the four mutations of SdiA2D10 could reverse the effect of the AHLs on biofilm formation. The AHL-sensitive SdiA2D10 and wild-type SdiA were studied for biofilm formation with nine different AHLs varying in side chains from 4 to 14 carbons at 10 μM. Mutations of SdiA2D10 led to increase in biofilm formation by four- to sevenfold in presence C4-to C12-DL-HSL and 3o-C8- to 3o-C12-L-HSL in LB glu medium after 24 h. This is congruent with our studies wherein, both the tested signaling molecules increased biofilm formation. Recent studies, have shown that in E. coli, there was large difference between the response of genes to plasmid-based expression of sdiA and chromosomal sdiA as well as to exogenous AHLs. These results have significant implications for defining the true function of AHL signal molecule detection by E. coli (Dyszel et al. 2010).
In the present study, the signaling molecules also influenced EPS production in the E. coli biofilms. Again, EPS production was higher for C6-HSL treated biofilms than the C4-HSL treated cells and the control (p < 0.05). The increase in EPS production in presence of 1 µM C4-HSL and C6-HSL was 2 and 4 %, respectively. The signaling molecules did not have any effect on the growth of the E. coli strain used in our study. Similar, studies on the effect of signaling molecules on E. coli growth have been carried out. The analysis of growth curves of E. coli MG1655 in presence and absence of 0.5 μM C6-HSL showed that addition of the AHL did not affect the growth of the bacteria in LB broth (Van Houdt et al. 2006). Therefore, the increased biofilm formation in E. coli in presence of the signal molecules AHLs showed that, the biofilm formation is not due to increased growth but as a response to gene regulation. Thus, it can be concluded that the AHLs do influence attachment and biofilm formation in the E. coli. However, it has to be elucidated if the effect of AHLs on biofilm formation is SdiA independent or it depends on sdiA variants in E. coli strains.
In S. enterica serovar Typhimurium (S. Typhimurium), the sdiA gene regulates two loci, the rck operon and the srgE gene. The rck operon on the 90 kb virulence plasmid contains the six genes pefI, srgA srgB, srgC, srgD and rck. Rck is an outer membrane protein involved in resistance to host complement mediated killing, adhesion of cells to fibronectin and laminin, and the host cell invasion by a zippering-type (Rosselin et al. 2010). The srgE gene is a single gene in the chromosome likely encoding a Type III secreted effector molecule (Samudrala et al. 2009). Interestingly, the rck operon is not expressed below 37 °C whereas srgE is expressed at low temperatures (Smith and Ahmer 2003). The temperature requirement of rck operon and the host interaction functions of the SdiA regulon implies that SdiA has an important role in the host than the external environment. Salmonella detects the AHL molecule production in different bacterial species under in vitro conditions, however, the bacteria that are detected, the environmental settings and consequences of such interactions are not determined (Soares and Ahmer 2011).
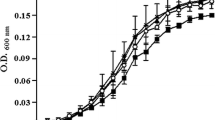
There are no reports on the role of the SdiA in Salmonella biofilms. As Rck promotes adherence to extracellular matrix proteins and epithelial cells apart from resistance to complement mediated killing, the role for SdiA in Salmonella biofilm formation can be postulated. Also, pef1 and srgA of the rck operon, affect the expression of the pef operon which codes for the plasmid-encoded fimbriae. Thus, probably SdiA indirectly affects the expression as well as the assembly of the plasmid-encoded fimbriae on cell surface and also affects biofilm formation (Steenackers et al. 2012). In our study, it was observed that, in S. typhimurium biofilms, both the exogenously added signaling molecules C4-HSL and C6-HSL increased biofilm formation as well as EPS secretion (Fig. 1). However, the effect of C6-HSL was significant than C4-HSL in increasing cell attachment and producing exopolymeric substances in S. typhimurium biofilms. The addition of 1 µM C6-HSL increased EPS production in S. typhimurium biofilms by 12 %. Thus, it can be inferred that in S. typhimurium the signaling molecules influence both the cell adherence required for biofilm initiation and EPS production required for biofilm maturation. It was also seen that the signaling molecules had no effect on the growth of S. typhimurium at 37 °C.
In a previous study, it was shown that the quorum sensing inhibitors belonging to the class of brominated furanones inhibited biofilm formation in Salmonella by interfering with flagellar biosynthesis (Janssens et al. 2008). The brominated furanones inhibited Salmonella biofilm formation without interference with the SdiA and AI-2 quorum sensing systems of S. enterica serovar Typhimurium (Janssens et al. 2008). Thus, it is evident from the above studies that, both the quorum sensing signals belonging to AHLs and the quorum sensing inhibitors influence biofilm formation. However, unlike the furanones, it is not clear for AHLs if their effect on biofilm formation is SdiA dependent. Therefore, in case of Salmonella species, the increased biofilm formation in the presence of the AHLs signal molecules may be SdiA dependent or due to the combination of effects on a number of different targets. Thus, further investigation on the specific targets of the signaling molecules in S. enterica serovar Typhimurium is required.
In V. parahemolyticus, at low cell density or at initial stages of colonization, the master regulator, AphA is expressed abundantly. It activates the genes involved in virulence factor synthesis and biofilm formation, resulting in bacterial colonization and infection. However, at high cell density the regulator OpaR is produced in abundance resulting in inhibition of the transcription of biofilm and virulence genes. Thus, AphA was involved in biofilm, motility, and virulence in pandemic V. parahaemolyticus (Zhang et al. 2012). OpaR was the LuxR ortholog in V. parahemolyticus and was known to repress the surface sensing and was involved in colony opacity. Therefore, quorum effects in this bacterium was limited to the induction of capsule and repression of swarming (Gade-Portratz and McCarter 2011).
In a recent study, it was demonstrated that wildtype V. parahaemolyticus was capable of forming strong biofilms and exhibited a rugose colony morphology which is due to the synthesis of abundant EPS, while aphA mutant exhibited smooth colony. A decreased production of biofilm EPS in aphA mutant relative to wildtype accounted for the reduced biofilm formation of this mutant (Wang et al. 2013). This clearly implies the role of AphA in V. parahaemolyticus biofilm. However, there are no studies that have shown any evidence of acyl homoserine lactone mediated signaling in V. parahaemolyticus biofilms.
In the present study, there was no significant difference in biofilm formation in the untreated control and C4-HSL treated V. parahemolyticus (p < 0.05). However, V. parahemolyticus grown in TSB with 1 µM C6-HSL showed an increased attachment to the substratum resulting in significant biomass formation (Fig. 1). V. parahemolyticus biofilm formation increased by 29 % in presence of C6-HSL. The effect of the signaling molecules on the EPS production in biofilm formation was contrary, as C4-HSL treated cells produced more EPS in the biofilms than the untreated control and C6-HSL treated cells. The exogenous addition of 1 µM C4-HSL increased EPS production by 17 %, whereas in presence of C6-HSL, EPS production increased by 12 %. It is probable that, in the V. parahemolyticus biofilms, C4-HSL influences only EPS production and not cell attachment whereas C6-HSL increases biofilm formation by biomass production than EPS synthesis. The studies on the effect of the signaling molecules on the growth kinetics of the tested pathogen revealed that the difference in the growth rate of the AHL treated and untreated control cells was not significant (p < 0.05). Therefore, in V. parahemolyticus, AHLs positively influence biofilm formation either by promoting cell attachment or by increasing EPS production. However, it is not clear if they bind to any regulator and act on specific genes, implying that the relevant molecular mechanisms need to be elucidated.
Conclusion
Salmonella species and E. coli do not possess a luxI gene coding for AHL synthase and thus are not capable of synthesizing AHLs. However, these organisms have the LuxR homolog, SdiA, which detects signals produced by other bacterial species. Though V. parahemolyticus uses quorum sensing mechanism for biofilm formation, the signaling molecules employed by it are not known. The effect of the signaling molecules C4-HSL and C6-HSL were studied for their effect on growth kinetics and biofilm formation in food-borne pathogens. The signaling molecules increased attachment and EPS production in all the tested bacteria. Thus, the food-borne pathogens can utilize the signaling molecules produced by the food spoilers for biofilm formation in food processing environments. This clearly implies the possibility of cross communication between the food spoilers and food borne pathogens in food ecosystem. Therefore, anti-biofilm strategies can be developed based on targeting the quorum sensing mechanism in controlling the biofilms of food-borne pathogens in food processing sectors.
References
Bai JA, Rai VR (2014) Quorum sensing inhibitory and anti-biofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotech 28:269–292
Bridier A, Sanchea-Vizuete P, Guilbaud M, Piard JC, Naitali M, Briandet R (2015) Biofilm-associated persistence of food-borne pathogens. Food Microbiol 45:167–178
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298
Dyszel JL, Soares JA, Swearingen MC, Lindsay A, Smith JN et al (2010) E coli K-12 and EHEC Genes regulated by SdiA. PLoS ONE 5:e8946
Dziva F, van Diemen PM, Stevens MP, Smith AJ, Wallis TS (2004) Identification of Escherichia coli O157: H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 150:3631–3645
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Gade-Portratz CJ, McCarter LL (2011) Quorum Sensing and Silencing in Vibrio parahaemolyticus. J Bacteriol 193:4224–4237
Giaouris E, Chorianopoulos N, Skandamia P, Nychas GJ (2012) Attachment and biofilm formation by salmonella in food processing environments. In: Mahmoud BSM (ed) Salmonella—A Dangerous Foodborne Pathogen. InTech, pp. 157–180
González Barrios AF, Zuo R, Hashimoto Y, Yang L, Bentley WE, Wood TK (2006) Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum sensing regulator (MqsR, B3022). J Bacteriol 188:305–316
Hammer BK, Bassler BL (2003) Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol 50:101–104
Jaglic Z, Desvaux M, Weiss A, Nesse LL, Meyer RL, Demnerova K, Schimdt H, Giaouris E, Sipailiene A, Teixeira P, Kacaniova M, Riedel CU, Knochel S (2014) Surface adhesins and exopolymers of selected foodborne pathogens. Microbiol 160:2561–2582
Janssens JCA, Steenackers H, Robijns S, Gellens E, Levin J, Zhao H, Hermans K, De Coster D, Verhoeven TL, Marchal K, Vanderleyden J, De Vos DE, De Keersmaecker SCJ (2008) Brominated Furanones Inhibit Biofilm Formation by Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 74:6639–6648
Lee J, Page R, Garcia-Contreras R, Palermino JM, Zhang XS, Doshi O, Wood TK, Peti W (2007) Structure and function of the Escherichia coli protein YmgB: a protein critical for biofilm formation and acid-resistance. J Mol Biol 373:11–26
Lee J, Maeda T, Hong SH, Wood TK (2009) Reconfiguring the quorum sensing regulator SdiA of Escherichia coli to control biofilm formation via indole and N-acylhomoserine lactones. Appl Environ Microbiol 75:1703–1716
Ng WL, Bassler BL (2009) Bacterial quorum sensing network architectures. Annu Rev Genet 43:197–222
O’Toole GA, Kolter R (1998) Initiation of biofilm formation in Pseudomonas fluorescens CS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol Microbiol 28:449–461
Patankar AV, Gonzalez JE (2009) Orphan luxr regulators of quorum sensing. FEMS Microbiol Rev 33:739–756
Rosselin M, Virlogeux-Payant I, Roy C, Bottreau E, Sizaret PY, Mijouin L, Germon P, Caron E, Velge P, Wiedemann A (2010) Rck of Salmonella enterica, subspecies enterica serovar enteritidis, mediates zipper-like internalization. Cell Res 20:647–664
Rutherford ST, van Kessel JC, Shao Y, Bassler BL (2011) AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev 25:397–408
Samudrala R, Heffron F, McDermott JE (2009) Accurate prediction of secreted substrates and identification of a conserved putative secretion signal for type III secretion systems. PLOS Pathogen 5:1000375
Sharma VK, Bearson SM (2013) Evaluation of the impact of quorum sensing transcriptional regulator SdiA on long-term persistence and fecal shedding of Escherichia coli O157:H7 in weaned calves. Microb Pathog 57:21–26
Sharma VK, Bearson SM, Bearson BL (2010) Evaluation of the effects of sdiA, a luxR homologue, on adherence and motility of Escherichia coli O157: H7. Microbiol 156:1303–1312
Smith JM, Ahmer BM (2003) Detection of other microbial species by Salmonella: expression of the sdia regulon. J Bacteriol 185:1357–1366
Smith JL, Fratamico PM, Yan X (2011) Eavesdropping by bacteria: the role of SdiA in Escherichia coli and Salmonella enterica serovar Typhimurium quorum sensing. Foodborne Pathogen Dis 8:169–178
Soares JA, Ahmer BMM (2011) Detection of acyl-homoserine lactones by Escherichia and Salmonella. Curr Opin Microbiol 14:188–193
Steenackers H, Hermans K, Vanderleyden J, De Keersmaecker SCJ (2012) Salmonella biofilms: an overview on occurrence, structure, regulation and eradication. Food Res Int 45:502–531
Van Houdt R, Aertsen A, Moons P, Vanoirbeek K, Michiels CW (2006) N-acyl-l-homoserine lactone signal interception by Escherichia coli. FEMS Microbiol 256:83–89
Wang L, Ling Y, Jiang H, Qiu Y, Qiu J, Chen H, Yang R, Zhou D (2013) AphA is required for biofilm formation, motility, and virulence in pandemic Vibrio parahemolyticus. Int J Food Microbiol 160:245–251
Winkelstroter LK, Teixeira FBR, Silva EP, Alves VF, De Martinis ECP (2014) Unraveling microbial biofilms of importance for food microbiology. Microbial Eco. 68:35–46
Yao Y, Martinez-Yamout MA, Dickerson TJ, Brogan AP, Wright PE, Dyson HJ (2006) Structure of the Escherichia coli Quorum Sensing Protein SdiA: activation of the folding switch by Acyl Homoserine Lactones. J Mol Biol 355(2):262–273
Yeom J, Lee Y, Park W (2012) Effects of non-ionic solute stresses on biofilm formation and lipopolysaccharide production in Escherichia coli O157:H7. Res Microbiol 163:258–267
Yildiz FH, Visick KL (2009) Vibrio biofilms: so much the same yet so different. Trends Microbiol 17:109–118
Zhang Y, Qiu Y, Tan Y, Guo Z, Yang R, Zhou D (2012) Transcriptional regulation of opaR, qrr2-4and aphA by the master quorum sensing regulator OpaR in Vibrio parahaemolyticus. PLoS ONE 7:34622
Acknowledgments
This work was supported by the Indian Council of Medical Research through the Senior Research Fellowship (F.No. 80/709/2011-ECD-I dated 21/06/2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
A., J.B., V., R.R. Effect of small chain N acyl homoserine lactone quorum sensing signals on biofilms of food-borne pathogens. J Food Sci Technol 53, 3609–3614 (2016). https://doi.org/10.1007/s13197-016-2346-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2346-1