Abstract
Quorum sensing regulates a variety of phenotypes in bacteria including the production of virulence factors. Salmonella spp. have quorum sensing systems mediated by three autoinducers (AI-1, AI-2, and AI-3). The AI-1-mediated system is incomplete in that the bacterium relies on the synthesis of signaling molecules by other microorganisms. This study aimed to evaluate the influence of the AI-1 N-dodecanoyl-DL-homoserine lactone (C12-HSL) on the growth, motility, adhesion, and biofilm formation of Salmonella enterica serovar Enteritidis PT4 578 on a polystyrene surface. Experiments were conducted at 37 °C in anaerobic tryptone soy broth supplemented with C12-HSL and/or a mixture of four synthetic furanones, at the concentration of 50 nM each. The planktonic growth, adhesion, swarming, and twitching motility were not altered in the presence of C12-HSL and/or furanones under anaerobic conditions. However, C12-HSL induced biofilm formation after 36 h of cultivation as determined by quantification of biofilm formation, by enumeration of adhered cells to polystyrene coupons, and finally by imaging the presence of multilayered cells on an epifluorescence microscope. When furanones were present in the medium, an antagonistic effect against C12-HSL on the biofilm development was observed. The results demonstrate an induction of biofilm formation in Salmonella Enteritidis by AI-1 under anaerobic conditions. Considering that Salmonella does not produce AI-1 but respond to it, C12-HSL synthesized by other bacterial species could trigger biofilm formation by this pathogen in conditions that are relevant for its pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonella is one of the most common foodborne pathogens frequently associated with illness, hospitalization, and deaths worldwide (Finstad et al. 2012; Nunes et al. 2013; Chironna et al. 2014). In Brazil, the phage type 4 is the most prevalent in infections caused by Salmonella enterica serovar Enteritidis, commonly transmitted by poultry meat and eggs (Irino et al. 1996; Nunes et al. 2003; Santos et al. 2003). The high virulence of this pathogen is related to the presence of up to 23 pathogenicity islands (SPIs), coding for many virulence factors (Humphrey et al. 1996, 1998; Hensel 2004; Ong et al. 2010; Hayward et al. 2013). Some of these factors such as those related to invasion and type III secretion system have been described as being regulated by the mechanism of cell–cell communication known as quorum sensing (Fuqua et al. 1994; Choi et al. 2007).
Quorum sensing (QS) leads to differential gene expression in response to changes in the population density among microbial cells or microbial and host cells (Fuqua et al. 1996, 2001; Keller and Surette 2006). In Salmonella spp., the QS mechanism can be mediated by three types of autoinducers (AI), called AI-1, AI-2, and AI-3 (Walters and Sperandio 2006). The AI-1 communication system is used by gram-negative bacteria, and in Salmonella, this system is incomplete since it does not contain a homologous luxI gene which codes for the AI-1 synthase, and hence, the bacterium is unable to produce its own acyl homoserine lactone (AHL) signal. However, the pathogen presents a protein known as SdiA, a transcriptional regulator homologous to LuxR, which detects AHLs produced by other microorganisms that are able to freely diffuse through membranes (Michael et al. 2001; Smith and Ahmer 2003; Parker and Sperandio 2009; LaSarre and Federle 2013; Papenfort and Bassler 2016). The interaction between SdiA of Salmonella and the AHLs produced by enterobacterial species in an animal model has already been tested. Smith et al. (2008) showed that SdiA of Salmonella Typhimurium was activated during the transit through the gastrointestinal tract of turtles colonized with Aeromonas hydrophila, an AHL producer bacterium. However, this protein was not activated in chicken, cow, guinea pig, mice, pig, and rabbit for reasons that may be related to the microbiota of these animals which may not produce appropriate AHLs able to activate SdiA. On the other hand, Dyszel et al. (2010a) showed that SdiA of Salmonella Typhimurium was activated during the transit through mice colonized with Yersinia enterocolitica, another AHL producer. Cloning of yenI gene of Y. enterocolitica, a luxI homologue, in Salmonella Typhimurium provided a greater and immediate fitness advantage to the pathogen in mice (Dyszel et al. 2010a). Although it responds to the presence of AHLs, the function of SdiA is not completely known in Salmonella (Dyszel et al. 2010a, b; Sabag-Daigle et al. 2012; Steenackers et al. 2012).
The AI-2 system, which is used by both gram-positive and gram-negative bacteria, is found in Salmonella where the signal molecule is synthesized by LuxS and internalized via products of the lsr operon (Xavier and Bassler 2005). The AI-3 system allows communication between bacteria and their mammalian hosts, and in Salmonella the signal molecule AI-3 is sensed by the products of the qseBC operon and qseE gene (Hughes and Sperandio 2008). Both systems are involved in the regulation of pathogenicity factors in Salmonella (Widmer et al. 2007; Moreira et al. 2010; Liu et al. 2014). The influence of QS on adhesion and biofilm formation has been studied in different serovars of S. enterica such as Salmonella Typhi (Prouty et al. 2002; Liu et al. 2014), Salmonella Enteritidis (Chorianopoulos et al. 2010) and nine other serovars (Wang et al. 2013). However, conflicting and incomplete results have been observed as highlighted next.
The establishment of a biofilm involves cell motility, adhesion, cell growth and microcolony formation, synthesis of exopolysaccharides, and other extracellular substances which are governed by a complex regulatory network according to the surfaces, environmental conditions, and signals, as well as biofilm maturation which is a process regulated by QS (Stepanović et al. 2003; Kearns 2010; Steenackers et al. 2012; Kalai Chelvam et al. 2014). Stepanović et al. (2003) showed that strains of Salmonella Enteritidis were able to form stronger biofilms on polystyrene surface when incubated for 24 h at 35 °C under 5–8% of CO2 in comparison with other atmospheric conditions. On the other hand, biofilm formation was lower under anaerobic conditions. These data corroborate with those found by Lamas et al. (2016) in which the biofilms formed on polystyrene by Salmonella Enteritidis, Salmonella Infantis, Salmonella Newport, and Salmonella Typhimurium were smaller in anaerobiosis after 24 h of incubation at 37 °C when compared to these structures formed in microaerobiose and aerobiose conditions. Besides, the expression of genes involved in the biofilm formation such as csg D and adr A and in the quorum sensing mechanism such as sdi A and lux S was reduced under microaerobiosis and anaerobiosis. However, Campos-Galvão et al. (2015b) found that under anaerobic condition, Salmonella Enteritidis was able to form more biofilm in the presence of AHLs on polystyrene with increased expression of biofilm-related genes such as lpfA, fimF, fliF, and glgC.
Biofilm formation in Salmonella is an important pathogenicity mechanism that represents great concern for the food industry, once cells from the biofilm can contaminate foods during processing due to difficulties related to its eradication (Burmølle et al. 2010; Høiby et al. 2010; Jensen et al. 2010; Srey et al. 2013). The search for inhibitors of biofilm formation or development has been the target of several studies, and furanones are considered as potential agents against Salmonella biofilms. The inhibitory effect of different furanones on biofilm formation by Salmonella Typhimurium (Janssens et al. 2008), Salmonella Agona (Vestby et al. 2013) and Salmonella Enteritidis (Campos-Galvão et al. 2015b) was already reported. Furanones are antagonistic compounds to QS AI-1 and AI-2 in gram-negative bacteria, since they present structural similarity to the autoinducers due to the homoserine lactone ring, but hinder transcriptional regulation by a mechanism still not fully understood (Rasmussen et al. 2000; Zhu and Winans 2001; Hentzer et al. 2002, 2003; Manefield et al. 2002; Janssens et al. 2008; Vestby et al. 2013). Although the antagonistic effect of furanones on AI-1 QS system was observed on the biofilm formation by Salmonella Enteritidis, the initial stages of adhesion as well as some important aspects of the biofilm development over time were not evaluated (Campos-Galvão et al. 2015b).
Thus, our study aimed to evaluate the influence of the AI-1 and its combination with furanones on planktonic growth, swarming and twitching motility, as well as adhesion and biofilm formation by S. enterica under anaerobic conditions in order to mimic a more relevant condition, such as the gut environment, in which this microorganism expresses pathogenesis.
Materials and methods
Bacterial strain
Salmonella enterica serovar Enteritidis phage type 4 (PT4) 578, isolated from chicken meat, was provided by Fundação Oswaldo Cruz (FIOCRUZ, Rio de Janeiro, Brazil) (Campos-Galvão et al. 2015a, b). Cultures were stored at −20 °C in Luria-Bertani (LB) broth (Miller 1972) supplemented with 20% (v/v) of sterilized glycerol.
Preparation of inoculum
Tryptone soy broth (TSB; Merck, Germany) was prepared under O2-free conditions with a CO2 filled dispensed into anaerobic bottles that were sealed with butyl rubber stoppers and then autoclaved (anaerobic TSB) (Lima et al. 2009). Before each experiment, cells were cultivated into anaerobic bottles containing 20 mL of anaerobic TSB for 24 h at 37 °C in a static-model anaerobic chamber (Coy Laboratory, USA) containing a mixture of H2 (3.0–5.0%) and CO2 (95.0–97.0%). Then, 1.0 mL was transferred into 10 mL of anaerobic TSB and incubated at 37 °C in anaerobic chamber. After 4 h of incubation, exponentially growing cells were harvested by centrifugation at 5000g at 4 °C for 10 min (Sorvall, USA), washed with 0.85% saline, and the pellet resuspended in 0.85% saline. The inoculum was standardized to 0.1 of optical density at 600 nm (OD600nm) (approximately 107 CFU mL−1) using a spectrophotometer (Thermo Fisher Scientific, Finland).
HSL and mixture of furanones
N-dodecanoyl-DL-homoserine lactone (C12-HSL; PubChem CID: 11565426; Fluka, Switzerland) was suspended in acetonitrile (PubChem CID: 6342; Merck, Germany) at a concentration of 10 mM and further diluted to a working solution of 10 µM in acetonitrile. A mixture of 3-methyl-2(5H)-furanone (PubChem CID: 30945; Sigma-Aldrich, Germany), 2-methyltetrahydro-3-furanone (PubChem CID: 18522; Sigma-Aldrich, Germany), 2(5H)-furanone (PubChem CID: 10341; Sigma-Aldrich, Germany), and 2,2-dimethyl-3(2H)-furanone (PubChem CID: 147604; Sigma-Aldrich, Germany) was suspended in acetonitrile at a concentration of 10 mM each and further diluted to a working solution of 10 µM in acetonitrile. Control experiments were performed using acetonitrile, since C12-HSL and furanones were suspended in this solvent. The final concentration of acetonitrile in the media was always less than 1% (v/v) to avoid interference in the growth and response of Salmonella to C12-HSL or furanones (Michael et al. 2001). The C12-HSL and TSB were chosen based on the results of Campos-Galvão et al. (2015b) who have shown a better effect of this AHL on biofilm formation by Salmonella Enteritidis, over other AHLs and culture media that were tested.
Effect of HSL and furanones on the planktonic growth of Salmonella
To evaluate the effect of C12-HSL alone and combined with the mixture of furanones on the planktonic growth of Salmonella, bottles containing 20 mL of anaerobic TSB supplemented with 110 µL of C12-HSL and/or the mixture of furanones were inoculated with 2 mL of the standardized inoculum. The final concentration of C12-HSL and the mixture of furanones in the anaerobic TSB was 50 nM. Bottles were incubated at 37 °C for up to 8 h in anaerobic chamber. In established time points, the optical density of the cell suspension was determined at 600 nm using a spectrophotometer (Thermo Fisher Scientific, Finland).
Determination of swarming and twitching motility
The swarming and twitching motility were performed on anaerobic TSB added with 0.7 or 1% (w/v) of agar, respectively (Reimmann et al. 2002; Sperandio et al. 2002; Kearns 2010). Twenty milliliters of the media were supplemented with 100 µL of C12-HSL and/or the mixture of furanones. The final concentration of C12-HSL and the mixture of furanones in media was 50 nM. For swarming motility, 5 µL of the standardized inoculum were placed on the center of the media, and for twitching motility, an inoculum of 10 µL was used. After the aliquot had dried, plates were incubated at 37 °C in anaerobic chamber. The diameter of the colonies was determined after 2, 7, 12, 18, 24, 36, 42, and 48 h of incubation.
Quantification of adhesion potential and biofilm formation on polystyrene
In order to quantify the adhesion potential and biofilm formation of Salmonella, 2 mL of anaerobic TSB supplemented with 11 µL of C12-HSL and/or the mixture of furanones was inoculated with 200 µL of the standardized inoculum and transferred to a 96-well microtiter plate. The final volume at each well was 200 µL, and the final concentration of C12-HSL and the mixture of furanones in the anaerobic TSB was 50 nM. Plates were incubated at 37 °C for 36 h in anaerobic chamber with replacement of the medium, inoculum and tested solutions every 10 h (Ryall et al. 2008; Guerrieri et al. 2009). After 26 and 36 h of incubation, the optical density of total cells was determined at 600 nm. Then, the culture supernatant was discarded, and the surface-attached cells were stained with 200 μL of 0.1% (w/v) crystal violet for 30 min. Subsequently, the crystal violet was removed and the plate was washed three times with water. After air drying for 15 min at 40 °C, the attached cells were determined at 590 nm with the microtiter plate reader by addition of 200 μL of 95% (v/v) ethanol (Pimentel-Filho et al. 2014). Data were expressed as the ratio between the absorbance of crystal violet extract of adhered cells (ACVEAC) and the optical density of total cells (ODTC) (Viana et al. 2009).
Quantification of planktonic and adhered cells on polystyrene
Polystyrene coupons (18 mm × 10 mm × 1 mm) and stainless steel rods were first cleaned by washing with liquid neutral detergent and water followed by rinsing with distilled water and then immersing in 1% (w/v) sodium hydroxide in ultrasound bath for 1 h, in order to remove fat, and rinsed again with sterile distilled water. Subsequently, they were immersed in 92% (v/v) ethanol in ultrasound bath for 30 min, rinsed with sterile distilled water and air-dried under UV light. Sterility of coupons was assessed by submerging a coupon in anaerobic TSB and checking medium turbidity after 48 h of incubation at 37 °C.
Polystyrene coupons were fixed by metal rods at the edge of anaerobic bottles containing 10 mL of anaerobic TSB supplemented with 55 µL of C12-HSL and/or the mixture of furanones. The final concentration of C12-HSL and the mixture of furanones in anaerobic TSB was 50 nM. Medium was inoculated with 1 mL of the standardized inoculum and then incubated at 37 °C for 36 h in anaerobic chamber with replacement of the culture medium, inoculum, and tested solutions every 10 h (Ryall et al. 2008; Guerrieri et al. 2009). For planktonic cells quantification, after 2, 7, 26, and 36 h of incubation, 1 mL of the supernatant was diluted, plated on plate count agar (PCA; Himedia, India), and colonies were enumerated following incubation at 37 °C in anaerobic chamber. For adherent cells quantification, the polystyrene coupons were removed from the anaerobic bottles after 2, 7, 26, and 36 h of incubation and washed with 1 mL of phosphate-buffered saline (PBS, pH 7.4) in order to remove the loosely attached cells. Then, the coupons were placed in a tube containing 2 mL of sterile PBS (pH 7.4) and subjected to the action of ultrasound for 30 min to remove the strongly attached cells. The suspension was homogenized, and an aliquot was diluted, plated, and incubated as previously described.
Monitoring of adhesion and biofilm formation on polystyrene by microscopy
To monitor Salmonella adhesion and biofilm formation on polystyrene surface, assays were carried out using the same experimental design as previously described for quantification of planktonic and adhered cells. After 2, 7, 26, and 36 h of incubation, coupons were removed from the anaerobic bottles, washed with 1 mL of PBS (pH 7.4), air-dried at room temperature, and fixed for 3 min with Kirkpatrick’s solution (isopropyl alcohol:chloroform:formaldehyde—6:3:1). Then, coupons were washed again with PBS, stained with 0.04% (w/v) of acridine orange solution for 5 min, washed, and dried at room temperature in the dark. After 1 h of staining in the dark, coupons were observed using epifluorescence microscope (Olympus, model BX50, Japan) at 1000 times amplification using WB2 filter (460–490 nm).
Molecular docking of SdiA protein of Salmonella Enteritidis PT4 578
The structural modeling of SdiA protein of Salmonella Enteritidis PT4 578 as well as the molecular docking with potential ligands was performed by using the CLC Drug Discovery Workbench 2.5 (http://www.clcbio.com/products/clc-drug-discovery-workbench/) software as described by Almeida et al. (2016). In order to generate the structure, the sequence of SdiA protein deposited on the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/; GenBank: AGZ95694.1) was modeled on the tridimensional structure of SdiA protein “4Y13” from Enterohemorrhagic Escherichia coli (EHEC) (Nguyen et al. 2015) deposited on RCSB Protein Data Bank (RCSB PDB; http://www.rcsb.org/pdb/home/home.do). Then, the molecular docking of SdiA of Salmonella Enteritidis PT4 578 was performed with the putative ligands C6-HSL (PubChem CID: 3462373), C12-HSL (PubChem CID: 11565426), 3-methyl-2(5H)-furanone (PubChem CID: 30945), 2-methyltetrahydro-3-furanone (PubChem CID: 18522), 2(5H)-furanone (PubChem CID: 10341), and 2,2-dimethyl-3(2H)-furanone (PubChem CID: 147604). Each ligand generates a score which mimics the potential energy change when the protein and the ligand come together. Lower scores (more negative ones) correspond to stronger binding while less negative or even positive scores indicate weak or non-existing bonds.
Statistics
Experiments were conducted in three biological replicates. All data were subjected to analysis of variance (ANOVA) followed by Tukey’s test using the Statistical Analysis System and Genetics Software® (Ferreira 2011). A p value less than 0.05 (p < 0.05) was considered to be statistically significant.
Results and discussion
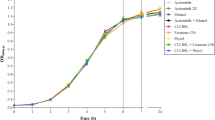
The effect of 50 nM of C12-HSL and/or furanone mix on the planktonic growth of Salmonella Enteritidis PT4 578 in TSB was evaluated anaerobically at 37 °C for 8 h (Fig. 1). The presence of C12-HSL in the medium did not interfere on the pathogen’s growth during 8 h of incubation. The maximal optical density at 600 nm in the presence of C12-HSL (0.173 ± 0.014) did not differ (p > 0.05) to the control treatment (0.169 ± 0.004). Similar results were found by Campos-Galvão et al. (2015a) for Salmonella Enteritidis grown in LB broth in the presence or absence of different AHLs at a concentration of 100 nM, such as N-hexanoyl-DL-homoserine lactone (C6-HSL), N-octanoyl-DL-homoserine lactone (C8-HSL), N-decanoyl-DL-homoserine lactone (C10-HSL), and C12-HSL. The concentration of C12-HSL used in this study is much higher than the required to induce the expression of sdiA, the transcriptional regulator which detects AHLs produced by other microorganisms. According to Michael et al. (2001), in Salmonella Typhimurium, the concentration can be as low as 1 nM depending on the AHL evaluated. On the other hand, the cell-free supernatants (CFS) of Y. enterocolitica and Serratia proteamaculans containing AHLs, AI-2 among other unknown metabolites affected the growth of different phage types of Salmonella Enteritidis and Salmonella Typhimurium in aerobic conditions (Dourou et al. 2011). Wang et al. (2013) also showed that the growth rate of nine serovar of S. enterica during the exponential phase decreased in the presence of the CFS of Pseudomonas aeruginosa containing AHLs and other unknown metabolites.
Growth of Salmonella Enteritidis PT4 578 in the presence of C12-HSL and/or a mixture of furanones. Salmonella was anaerobically cultivated in TSB at 37 °C for 8 h in the presence of acetonitrile (open cycle); 50 nM of C12-HSL (crossed line); 50 nM of C12-HSL combined with a mixture of four furanones at 50 nM each (closed square); and only in the presence of the mixture of furanones (closed triangle). Error bars indicate standard error
Aiming to study the phenotype regulated by AI-1, the planktonic growth of Salmonella Enteritidis PT4 578 was also evaluated in the presence of a mixture of furanones. Furanones are structurally analogous to AHLs but able to inhibit quorum sensing gene regulation (Rasmussen et al. 2000; Zhu and Winans 2001; Hentzer et al. 2002, 2003; Manefield et al. 2002).The mixture of furanones composed of 50 nM of each did not interfere on the bacterial growth, and the maximal optical density at 600 nm (0.175 ± 0.006) was similar (p > 0.05) to the control treatment (0.169 ± 0.004) (Fig. 1). Janssens et al. (2008) also reported that the growth of Salmonella Typhimurium was not influenced by addition of 50, 60, and 100 µM of different furanones after 12 h of incubation in TSB at 16 °C.
The combination of C12-HSL and furanones was able to reduce (p < 0.05) the maximal optical density to 0.150 ± 0.003 after 8 h of incubation, when compared to the other evaluated treatments (Fig. 1). However, this treatment did not differ (p > 0.05) when other cultivation technique as well as other times was evaluated (Fig. 4) which leads us to conclude that there is no significant difference among the treatments.
The effect of C12-HSL and furanones, added individually or combined, was evaluated on the swarming and twitching motility of Salmonella Enteritidis PT4 578 in anaerobic conditions. These tests were performed since bacterial motility is an important virulence factor and the initial adhesion of bacteria to the surface can be facilitated by cell motility (Kearns 2010; Kalai Chelvam et al. 2014). Neither swarming nor twitching motilities were observed following the applied treatments (Fig. 2); therefore, no difference in the colony diameter was detected which indicates that C12-HSL has no effect on motility of Salmonella Enteritidis PT4 578 under the evaluated conditions. In E. coli K-12, the overproduction of the quorum-regulated transcriptional regulator sdiA repressed genes involved in flagellum biosynthesis, chemotaxis, and motility (Wei et al. 2001). Moreover, the presence of synthetic brominated furanone reduced swimming and swarming motility of Salmonella Agona in aerobic conditions without changes in the expression of flagella genes (Vestby et al. 2013).
Swarming motility (a–d) and twitching motility (e–h) of Salmonella Enteritidis PT4 578 after 48 h at 37 °C in anaerobic chamber. Salmonella was inoculated on anaerobic TSB added with 0.7% (w/v) (a–d) and 1.0% (w/v) (e–h) of agar supplemented with acetonitrile (a, d); 50 nM of C12-HSL (b, f); 50 nM of C12-HSL combined with the mixture of four furanones at 50 nM of each one (c, g); or the mixture of four furanones at 50 nm of each one (d, h)
The effect of C12-HSL and/or the mixture of furanones on the adhesion potential and biofilm formation on polystyrene surface is shown in Fig. 3. Compared to the control treatment, no difference (p > 0.05) was observed on the adhesion and biofilm formation on the polystyrene surface by Salmonella Enteritidis PT4 578 during the first 26 h of incubation in the presence of C12-HSL and with this signaling molecule combined with furanones. Contrasting with this finding, after 36 h of incubation in the presence of 50 nM of C12-HSL, an induction on biofilm formation was observed (p < 0.05) (Fig. 3). When the mixture of furanones was added, in addition to C12-HSL, a reduction in the biofilm of Salmonella was observed following this incubation period, indicating an antagonist effect by furanones (Fig. 3). These results showed that biofilm formation was stimulated by C12-HSL only after 36 h. Campos-Galvão et al. (2015b) have shown that the addition of the 50 nM of the C6-HSL, C8-HSL, C10-HSL, and C12-HSL in minimal salts medium or TSB increased the adhesion by Salmonella Enteritidis in polystyrene after 24, 48, 72, and 96 h of incubation in anaerobic conditions, which is in agreement with our results. However, Campos-Galvão et al. (2015b) did not evaluate the initial stages of adhesion as was performed in the present study. According to Steenackers et al. (2012), the influence of AI on the initial cell adhesion must be evaluated, since this step may be decisive for biofilm formation, even though in our study it did not present a measurable effect.
Effect of C12-HSL and/or the mixture of furanones on the adhesion potential and biofilm formation on polystyrene surface. Ratio between the absorbance of crystal violet extract of adhered cells (ACVEAC) and the optical density of total cells (ODTC) of Salmonella Enteritidis PT4 578 exposed to C12-HSL and/or a mixture of furanones. Salmonella was anaerobically cultivated in TSB at 37 °C in the presence of acetonitrile (white bars); 50 nM of C12-HSL (gray bars); 50 nM of C12-HSL combined with a mixture of four furanones at 50 nM of each one (black bars); and only in the presence of the mixture of furanones (scratched bars). Error bars indicate standard error, and *means significant difference (p < 0.05)
The effect of C12-HSL and furanones on the growth, initial adhesion, and biofilm formation was also evaluated by the enumeration of planktonic cells (Fig. 4) and adhered cells to polystyrene surface (Fig. 5). The cultivation of Salmonella Enteritidis PT4 578 during 2, 7, 26, and 36 h in anaerobic conditions showed no difference (p > 0.05) on the planktonic cell number among the evaluated treatments (Fig. 4). The number of adhered cells of Salmonella on the polystyrene coupons was not different (p > 0.05) at 2, 7, and 26 h of incubation, showing that C12-HSL has no influence on initial adhesion. Chorianopoulos et al. (2010) also showed that the addition of the 10 µM or 100 µM of the N-3-oxo-hexanoyl-L-homoserine lactone (3-oxo-C6-HSL) or mixture of N-butyryl-DL-homoserine lactone (C4-HSL), 3-oxo-C6-HSL, C8-HSL, and C12-HSL in brain heart infusion (BHI) broth did not influence the adhesion by Salmonella Enteritidis on stainless steel. On the other hand, Liu et al. (2014) reported that the presence of C8-HSL along with plasmid pRST98, which contains the rck virulence gene, increased the adhesion of Salmonella Typhi in HeLa cells after 1 h of incubation at 37 °C with 5% CO2 and also increased biofilm formation on polystyrene after 24 h of incubation. In other bacterial species, the initial adhesion on the surface is not dependent on the QS system, but QS is decisive for biofilm maturation (Parsek and Greenberg 2000; Huber et al. 2001; Lynch et al. 2002).
Planktonic growth of Salmonella Enteritidis PT4 578 exposed to C12-HSL and/or a mixture of furanones. Salmonella was anaerobically cultivated in TSB at 37 °C in the presence of acetonitrile (white bars); 50 nM of C12-HSL (gray bars); 50 nM of C12-HSL combined with a mixture of four furanones at 50 nM of each one (black bars); and only in the presence of the mixture of furanones (scratched bars). Error bars indicate standard error
Adhered cells of Salmonella Enteritidis PT4 578 on polystyrene surface exposed to C12-HSL and/or a mixture of furanones. Salmonella was anaerobically cultivated in TSB at 37 °C in the presence of acetonitrile (white bars); 50 nM of C12-HSL (gray bars); 50 nM of C12-HSL combined with a mixture of four furanones at 50 nM of each one (black bars); and only in the presence of the mixture of furanones (scratched bars). Error bars indicate standard error, and *means significant difference (p < 0.05)
However, C12-HSL induced biofilm formation on polystyrene surface following 36 h of incubation in anaerobic conditions corroborating the findings from Fig. 3. On this condition, an increase of approximately one log cycle (p < 0.05) in the number of adhered cells to the surface, compared to the control and the other treatments, was observed (Fig. 5). It is important to highlight that the increase in adhesion is not related to an enhanced number of planktonic cells growing in the presence of C12-HSL, as already shown on Fig. 4. On the other hand, the CFS of Hafnia alvei containing AHLs, among other unknown metabolites, negatively influenced the biofilm formation by Salmonella Enteritidis on stainless steel after 12, 24, 48, and 72 h of incubation in aerobic conditions (Chorianopoulos et al. 2010). Wang et al. (2013) also showed that the CFS of P. aeruginosa containing AHLs and other unknown metabolites decreased the biofilm formation by nine serovar of S. enterica on polystyrene surfaces after 1, 3, and 5 days of incubation in aerobic conditions. The reason for the differences observed between our study and the above mentioned is possibly explained by the use of culture supernatant by those investigators instead of purified AHL which likely generates more conclusive results.
The data of quantification of adhered cells on polystyrene also show an antagonist effect of the furanones along the 36-h cultivation period, even in the presence of C12-HSL, further confirming our previous results (Fig. 3). Moreover, the adhesion potential and biofilm formation in the presence of furanones alone did not differ (p > 0.05) with the control treatment, highlighting that furanones had no influence in this process, under the evaluated conditions. However, Janssens et al. (2008) showed that the presence of 25, 50, and 100 µM of different brominated furanones inhibited the biofilm formation by Salmonella Typhimurium on polystyrene after 48 h of incubation at 16 °C in aerobic conditions, when compared to the control treatment. Contrasting with our findings, the authors reported that there was no evidence that furanones acted on any of the known QS systems reported to be present in Salmonella. Conversely, Vestby et al. (2013) reported the inhibitory effect of a synthetic furanone on the biofilm formation by Salmonella Agona on polystyrene without bactericidal effect, but the mode of action has not been established. These results are possibly explained by the differences among the evaluated strains and conditions used in these studies; however, a comparative work using different serovars and the same conditions used in the present study awaits further research.
On the polystyrene surfaces immersed in anaerobic TSB inoculated with Salmonella Enteritidis PT4 for 2, 7, and 26 h of incubation, no difference in adhesion was observed by fluorescence microscopy following the treatments evaluated (Fig. 6), confirming the results found in previous experiments (Figs. 3, 5). In these time points, only cells individually adhered to the surface or in small clusters were observed, without overlapping layered cells or the presence of a fully formed biofilm structure. Images of adhered cells on polystyrene coupons treated for 2 h with C12-HSL and/or furanones are shown as a representative result of the three time points which presented very similar results (Fig. 6).
Adhesion by Salmonella Enteritidis PT4 578 on polystyrene surface immersed for 2 h at 37 °C in anaerobic TSB supplemented with acetonitrile (control treatment) (a); 50 nM of C12-HSL (b); 50 nM of C12-HSL combined with the mixture of four furanones at 50 nM of each one (c); or the mixture of four furanones at 50 nm of each one (d). Epifluorescence microscopy images are shown at ×1000 magnification
In the control treatment (Fig. 7a), as well as in the presence of furanones alone (Fig. 7d) or combined with C12-HSL (Fig. 7c), only cells individually adhered or in small clusters on the surface were observed after 36 h of incubation. However, when exposed to C12-HSL for 36 h (Fig. 7b), Salmonella was able to form biofilm which can be observed as multilayered cells indicating the presence of exopolysaccharide (Fig. 7a). These results corroborate with Campos-Galvão et al. (2015b) and confirm all of our previous findings demonstrating the stimulatory effect of C12-HSL on the biofilm formation by Salmonella in anaerobic conditions and reveal that the presence of furanones inhibits the biofilm development stimulated by C12-HSL (Fig. 7c), possibly by interfering with SdiA-mediated signaling.
Adhesion and biofilm formation by Salmonella Enteritidis PT4 578 on polystyrene surface immersed for 36 h at 37 °C in anaerobic TSB supplemented with acetonitrile (control treatment) (a); 50 nM of C12-HSL (b); 50 nM of C12-HSL combined with the mixture of four furanones at 50 nM of each one (c); or the mixture of four furanones at 50 nm of each one (d). Epifluorescence microscopy images are shown at ×1000 magnification
In order to gain insights into the molecular mechanisms which would explain the observed phenotypes and the nature of interactions between AHLs and furanones with SdiA from Salmonella Enteritidis PT4 578, we took advantage of the recently published crystal structure of SdiA from Enterohemorrhagic E. coli (EHEC) (Nguyen et al. 2015). In addition, it is important to highlight that the sdiA gene (GenBank: KF381283) of Salmonella Enteritidis PT4 578 showed 100% similarity with Salmonella Enteritidis PT4 P125109 (Campos-Galvão et al. 2015a) and more than 69% identity with EHEC (Almeida et al. 2016). The results from the molecular docking showed a stronger binding of C12-HSL (a score of −65.28) than with C6-HSL (a score of −56.50). We hypothesize that the stronger effect of C12-HSL on biofilm formation of Salmonella Enteritidis PT4 578 is due to the better binding of this ligand with SdiA, but this assertion awaits biochemical confirmation. As it can be observed in Fig. S1A and B, tryptophan 67 (W67) is a common residue for interaction with AHLs and furanones. However, AHLs can make additional bonds with SdiA, while furanones can only bind W67. This observation explains the lower binding affinity observed in the furanones scores −35.02, −31.35, and −29.99 (Fig. S1C, D, and E, respectively), while the 2,2-dimethyl-3(2H)-furanone supposedly does not bind SdiA (Fig. S1F). The antagonistic effect of furanones due to their binding to W67 in SdiA of Salmonella Enteritidis PT4 578 is a potential target for future mutagenic studies and so they are the additional residues that potentially bind AHLs. It is likely that the signaling interference observed for furanones against C12-HSL is due to a competitive binding for SdiA.
Conclusion
The presence of exogenous C12-HSL induced biofilm formation on polystyrene by Salmonella Enteritidis PT4 578 under anaerobic conditions, and quorum sensing influences biofilm maturation rather than initial adherence. This is an indication that in the presence of another bacterium able to synthesize this AHL, biofilm can be established by Salmonella as a response to the quorum sensing signals. This change in life style, from the planktonic to sessile state, can confer a competitive advantage to the pathogen, such as survival and adaptation to stress conditions in the environment of the gut for instance. On the other hand, another important result to be considered is the antagonistic effect of the furanones since the combination of furanones with C12-HSL inhibited biofilm formation. This antagonistic effect caused by furanones can be explored as a potential strategy in the food industry since these compounds compete with the AHLs produced by some microorganisms present in the food processing environment, preventing biofilm formation.
References
Almeida FA, Pinto UM, Vanetti MCD (2016) Novel insights from molecular docking of SdiA from Salmonella Enteritidis and Escherichia coli with quorum sensing and quorum quenching molecules. Microb Pathog 99:178–190. doi:10.1016/j.micpath.2016.08.024
Burmølle M, Thomsen TR, Fazli M, Dige I, Christensen L, Homøe P et al (2010) Biofilms in chronic infections—a matter of opportunity—monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol 59(3):324–336. doi:10.1111/j.1574-695X.2010.00714.x
Campos-Galvão MEM, Leite TDS, Ribon AOB, Araújo EF, Vanetti MCD (2015a) A new repertoire of informations about the quorum sensing system in Salmonella enterica serovar Enteritidis PT4. Genet Mol Res 14(2):4068–4084. doi:10.4238/2015.April.27.22
Campos-Galvão MEM, Ribon AOB, Araújo EF, Vanetti MCD (2015b) Changes in the Salmonella enterica Enteritidis phenotypes in presence of acyl homoserine lactone quorum sensing signals. J Basic Microbiol 55:1–9. doi:10.1002/jobm.201500471
Chironna M, Tafuri S, Gallone MS, Sallustio A, Martinelli D, Prato R et al (2014) Outbreak of Salmonella infantis gastroenteritis among people who had eaten at a hash house in southern Italy. Public Health 128(5):438–443. doi:10.1016/j.puhe.2014.02.002
Choi J, Shin D, Ryu S (2007) Implication of quorum sensing in Salmonella enterica serovar Typhimurium virulence: the luxS gene is necessary for expression of genes in pathogenicity island 1. Infect Immun 75(10):4885–4890. doi:10.1128/IAI.01942-06
Chorianopoulos NG, Giaouris ED, Kourkoutas Y, Nychas GJE (2010) Inhibition of the early stage of Salmonella enterica serovar Enteritidis biofilm development on stainless steel by cell-free supernatant of a Hafnia alvei culture. Appl Environ Microbiol 76(6):2018–2022. doi:10.1128/AEM.02093-09
Dourou D, Ammor MS, Skandamis PN, Nychas GJE (2011) Growth of Salmonella Enteritidis and Salmonella Typhimurium in the presence of quorum sensing signalling compounds produced by spoilage and pathogenic bacteria. Food Microbiol 28(5):1011–1018. doi:10.1016/j.fm.2011.02.004
Dyszel JL, Smith JN, Lucas DE, Soares JA, Swearingen MC, Vross MA et al (2010a) Salmonella enterica serovar Typhimurium can detect acyl homoserine lactone production by Yersinia enterocolitica in mice. J Bacteriol 192(1):29–37. doi:10.1128/JB.01139-09
Dyszel JL, Soares JA, Swearingen MC, Lindsay A, Smith JN, Ahmer BM (2010b) E. coli K-12 and EHEC genes regulated by SdiA. PLoS ONE 5:e8946. doi:10.1371/journal.pone.0008946
Ferreira DF (2011) SISVAR: a computer statistical analysis system. Cienc e Agrotecnol 35(6):1039–1042. doi:10.1590/s1413-70542011000600001
Finstad S, O’Bryan CA, Marcy JA, Crandall PG, Ricke SC (2012) Salmonella and broiler processing in the United States: relationship to foodborne salmonellosis. Food Res Int 45(2):789–794. doi:10.1016/j.foodres.2011.03.057
Fuqua WC, Winans SC, Greenberg EP (1994) Quorum sensing in bacteria: the LuxR-LuxI family of cell density- responsive transcriptional regulators. J Bacteriol 176(2):269–275
Fuqua C, Winans SC, Greenberg EP (1996) Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol 50:727–751. doi:10.1146/annurev.micro.50.1.727
Fuqua C, Parsek MR, Greenberg EP (2001) Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet 35:439–468. doi:10.1146/annurev.genet.35.102401.090913
Guerrieri E, de Niederhäusern S, Messi P, Sabia C, Iseppi R, Anacarso I et al (2009) Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small-scale model. Food Control 20(9):861–865. doi:10.1016/j.foodcont.2008.11.001
Hayward MR, Jansen VAA, Woodward MJ (2013) Comparative genomics of Salmonella enterica serovars Derby and Mbandaka, two prevalent serovars associated with different livestock species in the UK. BMC Genom 14(1):365. doi:10.1186/1471-2164-14-365
Hensel M (2004) Evolution of pathogenicity islands of Salmonella enterica. Int J Med Microbiol 294(2–3):95–102. doi:10.1016/j.ijmm.2004.06.025
Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR et al (2002) Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148(1):87–102. doi:10.1099/00221287-148-1-87
Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N et al (2003) Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22:3803–3815. doi:10.1093/emboj/cdg366
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35(4):322–332. doi:10.1016/j.ijantimicag.2009.12.011
Huber B, Riedel K, Hentzer M, Heydorn A, Gotschlich A, Givskov M et al (2001) The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 147(9):2517–2528. doi:10.1099/00221287-147-9-2517
Hughes DT, Sperandio V (2008) Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6(2):111–120. doi:10.1038/nrmicro1836
Humphrey TJ, Williams A, McAlpine K, Lever MS, Guard-Petter J, Cox JM (1996) Isolates of Salmonella enterica Enteritidis PT4 with enhanced heat and acid tolerance are more virulent in mice and more invasive in chickens. Epidemiol Infect 117(1):79–88. doi:10.1017/S0950268800001151
Humphrey TJ, Williams A, McAlpine K, Jørgensen F, O’Byrne C (1998) Pathogenicity in isolates of Salmonella enterica serotype Enteritidis PT4 which differ in RpoS expression: effects of growth phase and low temperature. Epidemiol Infect 121(2):295–301. doi:10.1017/S0950268898001162
Irino K, Fernandes SA, Tavechio AT, Neves BC, Dias AMG (1996) Progression of Salmonella Enteritidis phage type 4 strains in São Paulo state, Brazil. Rev Inst Med Trop Sao Paulo 38(3):193–196. doi:10.1590/S0036-46651996000300005
Janssens JCA, Steenackers H, Robijns S, Gellens E, Levin J, Zhao H et al (2008) Brominated furanones inhibit biofilm formation by Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 74(21):6639–6648. doi:10.1128/AEM.01262-08
Jensen PO, Givskov M, Bjarnsholt T, Moser C (2010) The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol 59(3):292–305. doi:10.1111/j.1574-695X.2010.00706.x
Kalai Chelvam K, Chai LC, Thong KL (2014) Variations in motility and biofilm formation of Salmonella enterica serovar Typhi. Gut Pathog 6(1):2. doi:10.1186/1757-4749-6-2
Kearns DB (2010) A field guide to bacterial swarming motility. Nat Rev Microbiol 8(9):634–644. doi:10.1038/nrmicro2405
Keller L, Surette MG (2006) Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol 4(4):249–258. doi:10.1038/nrmicro1383
Lamas A, Miranda JM, Vázquez B, Cepeda A, Franco CM (2016) Biofilm formation, phenotypic production of cellulose and gene expression in Salmonella enterica decrease under anaerobic conditions. Int J Food Microbiol 238:63–67. doi:10.1016/j.ijfoodmicro.2016.08.043
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77:73–111. doi:10.1128/MMBR.00046-12
Lima JR, Ribon ADOB, Russell JB, Mantovani HC (2009) Bovicin HC5 inhibits wasteful amino acid degradation by mixed ruminal bacteria in vitro. FEMS Microbiol Lett 292(1):78–84. doi:10.1111/j.1574-6968.2008.01474.x
Liu Z, Que F, Liao L, Zhou M, You L, Zhao Q et al (2014) Study on the promotion of bacterial biofilm formation by a Salmonella conjugative plasmid and the underlying mechanism. PLoS ONE 9(10):e109808. doi:10.1371/journal.pone.0109808
Lynch MJ, Swift S, Kirke DF, Keevil CW, Dodd CER, Williams P (2002) The regulation of biofilm development by quorum sensing in Aeromonas hydrophila. Environ Microbiol 4(1):18–28. doi:10.1046/j.1462-2920.2002.00264.x
Manefield M, Rasmussen TB, Henzter M, Andersen JB, Steinberg P, Kjelleberg S et al (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148(4):1119–1127. doi:10.1099/00221287-148-4-1119
Michael B, Smith JN, Swift S, Heffron F, Ahmer BM (2001) SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J Bacteriol 183:5733–5742. doi:10.1128/JB.183.19.5733-5742.2001
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harb. Lab. Press, Cold Spring Harbour
Moreira CG, Weinshenker D, Sperandio V (2010) QseC mediates Salmonella enterica serovar Typhimurium virulence in vitro and in vivo. Infect Immun 78(3):914–926. doi:10.1128/IAI.01038-09
Nguyen Y, Nguyen NX, Rogers JL, Liao J, MacMillan JB, Jiang Y et al (2015) Structural and mechanistic roles of novel chemical ligands on the SdiA quorum-sensing transcription regulator. mBio 6(2):1–10. doi:10.1128/mBio.02429-14
Nunes IA, Helmuth R, Schroeter A, Mead GC, Santos MAA, Solari CA et al (2003) Phage typing of Salmonella Enteritidis from different sources in Brazil. J Food Prot 66(2):324–327
Nunes MM, Mota ALAA, Caldas ED (2013) Investigation of food and water microbiological conditions and foodborne disease outbreaks in the Federal District, Brazil. Food Control 34(1):235–240. doi:10.1016/j.foodcont.2013.04.034
Ong SY, Ng FL, Badai SS, Yuryev A, Alam M (2010) Analysis and construction of pathogenicity island regulatory pathways in Salmonella enterica serovar Typhi. J Integr Bioinform. doi:10.2390/biecoll-jib-2010-145
Papenfort K, Bassler BL (2016) Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol 14:576–588. doi:10.1038/nrmicro.2016.89
Parker CT, Sperandio V (2009) Cell-to-cell signalling during pathogenesis. Cell Microbiol 11:363–369. doi:10.1111/j.1462-5822.2008.01272.x
Parsek MR, Greenberg EP (2000) Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci USA 97(16):8789–8793. doi:10.1073/pnas.97.16.8789
Pimentel-Filho NDJ, Martins MCDF, Nogueira GB, Mantovani HC, Vanetti MCD (2014) Bovicin HC5 and nisin reduce Staphylococcus aureus adhesion to polystyrene and change the hydrophobicity profile and Gibbs free energy of adhesion. Int J Food Microbiol 190:1–8. doi:10.1016/j.ijfoodmicro.2014.08.004
Prouty AM, Schwesinger WH, Gunn JS (2002) Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun 70(5):2640–2649. doi:10.1128/IAI.70.5.2640-2649.2002
Rasmussen TB, Manefield M, Andersen JB, Eberl L, Anthoni U, Christophersen C et al (2000) How Delisea pulchra furanones affect quorum sensing and swarming motility in Serratia liquefaciens MG1. Microbiology 146:3237–3244. doi:10.1099/00221287-146-12-3237
Reimmann C, Ginet N, Michel L, Keel C, Michaux P, Krishnapillai V et al (2002) Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148(4):923–932. doi:10.1099/00221287-148-4-923
Ryall B, Lee X, Zlosnik JEA, Hoshino S, Williams HD (2008) Bacteria of the Burkholderia cepacia complex are cyanogenic under biofilm and colonial growth conditions. BMC Microbiol 8:108. doi:10.1186/1471-2180-8-108
Sabag-Daigle A, Soares JA, Smith JN, Elmasry ME, Ahmer BMM (2012) The acyl homoserine lactone receptor, SdiA, of Escherichia coli and Salmonella enterica serovar Typhimurium does not respond to indole. Appl Environ Microbiol 78(15):5424–5431. doi:10.1128/AEM.00046-12
Santos LR, Nascimento VP, Oliveira SD, Rodrigues DP, Reis EMF, Seki LM et al (2003) Phage types of Salmonella Enteritidis isolated from clinical and food samples, and from broiler carcasses in southern Brazil. Rev Inst Med Trop Sao Paulo 45(1):1–4. doi:10.1590/S0036-46652003000100001
Smith JN, Ahmer BMM (2003) Detection of other microbial species by Salmonella: expression of the SdiA regulon. J Bacteriol 185(4):1357–1366. doi:10.1128/JB.185.4.1357-1366.2003
Smith JN, Dyszel JL, Soares JA, Ellermeier CD, Altier C, Lawhon SD et al (2008) SdiA, an N-acylhomoserine lactone receptor, becomes active during the transit of Salmonella enterica through the gastrointestinal tract of turtles. PLoS ONE 3(7):e2826. doi:10.1371/journal.pone.0002826
Sperandio V, Torres AG, Kaper JB (2002) Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43(3):809–821. doi:10.1046/j.1365-2958.2002.02803.x
Srey S, Jahid IK, Ha S-D (2013) Biofilm formation in food industries: a food safety concern. Food Control 31(2):572–585. doi:10.1016/j.foodcont.2012.12.001
Steenackers H, Hermans K, Vanderleyden J, De Keersmaecker SCJ (2012) Salmonella biofilms: an overview on occurrence, structure, regulation and eradication. Food Res Int 45(2):502–531. doi:10.1016/j.foodres.2011.01.038
Stepanović S, Ćirković I, Mijač V, Švabić-Vlahović M (2003) Influence of the incubation temperature, atmosphere and dynamic conditions on biofilm formation by Salmonella spp. Food Microbiol 20:339–343. doi:10.1016/S0740-0020(02)00123-5
Vestby LK, Johannesen KCS, Witsø IL, Habimana O, Scheie AA, Urdahl AM et al (2013) Synthetic brominated furanone F202 prevents biofilm formation by potentially human pathogenic Escherichia coli O103: H2 and Salmonella ser. Agona on abiotic surfaces. J Appl Microbiol 116(2):258–268. doi:10.1111/jam.12355
Viana ES, Campos MEM, Ponce AR, Mantovani HC, Vanetti MCD (2009) Biofilm formation and acyl homoserine lactone production in Hafnia alvei isolated from raw milk. Biol Res 42(4):427–436. doi:10.4067/S0716-97602009000400004
Walters M, Sperandio V (2006) Quorum sensing in Escherichia coli and Salmonella. Int J Med Microbiol. doi:10.1016/j.ijmm.2006.01.041
Wang HH, Ye KP, Zhang QQ, Dong Y, Xu XL, Zhou GH (2013) Biofilm formation of meat-borne Salmonella enterica and inhibition by the cell-free supernatant from Pseudomonas aeruginosa. Food Control 32(2):650–658. doi:10.1016/j.foodcont.2013.01.047
Wei Y, Lee JM, Smulski DR, LaRossa RA (2001) Global impact of sdiA amplification revealed by comprehensive gene expression profiling of Escherichia coli. J Bacteriol 183(7):2265–2272. doi:10.1128/JB.183.7.2265-2272.2001
Widmer KW, Jesudhasan PR, Dowd SE, Pillai SD (2007) Differential expression of virulence-related genes in a Salmonella enterica serotype Typhimurium luxS mutant in response to autoinducer AI-2 and poultry meat-derived AI-2 inhibitor. Foodborne Pathog Dis 4(1):5–15. doi:10.1089/fpd.2006.40
Xavier KB, Bassler BL (2005) Regulation of uptake and processing of the quorum-sensing autoinducer AI-2 in Escherichia coli. J Bacteriol 187(1):238–248. doi:10.1128/JB.187.1.238-248.2005
CLC Drug Discovery Workbench 2.5. http://www.clcbio.com/products/clc-drug-discovery-workbench/
Zhu J, Winans SC (2001) The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci USA 98(4):1507–1512. doi:10.1073/pnas.98.4.1507
Acknowledgements
Felipe Alves de Almeida was supported by a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and this research has been supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Brazil and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). UMP acknowledges funding from CNPq for his research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jorge Membrillo-Hernández.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Fig. S1
Molecular docking of SdiA protein of Salmonella Enteritidis PT4 578 to ligands such as C12-HSL (A), C6-HSL (B), 3-methyl-2(5H)-furanone (C), 2(5H)-furanone (D), 2-methyltetrahydro-3-furanone (E), and 2,2-dimethyl-3(2H)-furanone (F). The white arrows indicate the residues of the SdiA protein that bind to ligands. W67 = Tryptophan 67, Y63 = Tyrosine 63, D80 = Aspartate 80. (JPEG 120 kb)
Rights and permissions
About this article
Cite this article
Almeida, F.A., Pimentel-Filho, N.J., Pinto, U.M. et al. Acyl homoserine lactone-based quorum sensing stimulates biofilm formation by Salmonella Enteritidis in anaerobic conditions. Arch Microbiol 199, 475–486 (2017). https://doi.org/10.1007/s00203-016-1313-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1313-6