Abstract
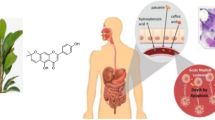
The present study envisages the cytotoxic potential of 3-butenyl isothiocyanate isolated from Brassica juncea L. Czern var. Pusa Jaikisan against the human cancer cell lines viz. prostate, bone osteosarcoma, cervical, liver, neuroblastoma and breast cancer. As the compound was observed to be more effective against prostate cancer cell line, therefore, this cell line was further used to study the mechanism of cell death using neutral red assay, reactive oxygen species assay, mitochondrial membrane potential assay, microscopic and cell cycle analysis. The mechanistic analysis indicated that it induced the cell death of prostate cancer cells via apoptosis and hence made it an excellent choice as an effective anticancer compound.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucosinolates are sulphur and nitrogen containing natural compounds, derived from glucose and amino acid. This organic compound in its intact form has low biological activity whereas its hydrolytic products formed by the action of myrosinase enzyme exert high biological activities (Arora et al. 2014a, b, 2016a; Bongoni et al. 2014). Among the variety of hydrolytic products formed, isothiocyanates (ITCs) emerges with unsurpassed activities (De Nicola et al. 2013; Arora et al. 2014c). They act as an external and important factor in maintaining cellular homeostasis in oxidative stress conditions (Ghasemzadeh and Ghasemzadeh 2011; Cabello-Hurtado et al. 2012). Since oxidative stress and cancer are correlated, so for the normal functioning of the body, both must be kept in control (Kryston et al. 2011). The cancer cells generated as a result of oxidative damage are mostly removed via xenobiotic metabolizing enzymes, which lead to the onset of apoptosis in these cells. The imbalanced enzyme system of body prevents the reduction of oxidative stress and hence alternate compounds are required to supplement the antioxidative and anticancer abilities of the body. Among the diverse range of ITCs, 3-butenyl ITC is an important hydrolytic product of gluconapin (parent glucosinolate). A thorough literature search has revealed that 3-butenyl ITC has not been explored for antiproliferative and anticancer activities. In line with these facts, the current study involves the evaluation of in vitro antiproliferative and anticancer activity of 3-butenyl ITC in human cancer cell lines.
Materials and methods
Plant material
Seeds of Brassica juncea (L.) Czern var. Pusa Jaikisan were procured from Indian Agricultural Research Institute (IARI), Karnal, India. The seeds were thoroughly cleaned for the removal of any pesticide or unwanted residue and were stored at −20 °C until further use.
Isolation and chemical characterization
The seeds after grinding were subjected to hydrodistillation with slight modifications (Arora et al. 2014b, 2016b). Extract was obtained in liquid form and was stored at −80 °C until further use. The liquid extract was processed for the isolation of 3-butenyl isothiocyanate (BITC) using silica gel 60–120 mesh size and with hexane: ethyl acetate as mobile phase.
The isolated compound BITC was characterized using gas chromatography–flame ionization detector (GC–FID), gas chromatography–mass spectrometer (GC–MS) and ultra high pressure liquid chromatography–photo diode array (UHPLC–PDA). For GC-FID, the compound was dissolved in 1 ml GC-grade methylene chloride and was analyzed using Shimadzu (QP2010 series) gas chromatography-flame ionization detector (Tokyo, Japan). The instrument was equipped with an AOC-20i autosampler, which was coupled to a DB-5 MS capillary column (30 m × 0.25 mm i.d., 0.25 μm). The analysis was started using the initial temperature gradient of 40 °C, held for 4 min and programmed to 230 °C at rate of 4 °C/min and was held for 15 min at 230 °C. The injection volume was 2 μl with the temperature of 40 °C. An inlet pressure of 97.1 kPa with helium as carrier gas (flow rate 1.1 ml/min in split mode 1:50). The total run time of GC–FID was 46 min.
The GC–MS analysis of the isolated compound was also performed using Shimadzu (QP2010 series) gas chromatography with mass spectrometer detector (Tokyo, Japan). The autosampler, column, temperature gradient and program were same as GC-FID. An inlet pressure of 97.1 kPa with helium as carrier gas (flow rate 1.1 ml/min in split mode 1:50). MS interface temperature was kept 250 °C at MS mode, detector voltage was set to 0.9 kV, mass range was 40–800 u and scan speed was 1666 u/s with an interval of 0.50 s (2 Hz).
The compound was also analyzed for its purity using UHPLC, Naxera model, Shimadzu Asia Pacific Ltd. The instrument was coupled with a PDA detector and a C18 column (150 mm × 5 μm i.d., 0.18 μm). The compound (2 μl) was dissolved in 1 ml HPLC grade acetonitrile. An injection volume of 2 μl and a gradient mobile phase (acetonitrile: water) was programmed. The total run time for the UHPLC analysis was 20 min.
Cell lines and culture
The anticancer activity of BITC was measured using in vitro assays involving human cancer cell lines. The human prostate cancer cell line (PC-3) and human bone osteosarcoma cell line (MG-63) were grown and maintained in RPMI-1640 medium supplemented with FBS (10 %), at 37 °C in a humidified incubator containing 5 % CO2. While, the human cervical cancer cell line (HeLa), human liver cancer cell line (HepG-2), human neuroblastoma cell line (IMR-32) and human breast cancer cell line (MCF-7) were grown and maintained in DMEM medium supplemented with FBS (10 %), at 37 °C in a humidified incubator containing 5 % CO2. All the cell lines were obtained from National Centre for Cell Science (NCCS), Pune and were passaged for less than 6 months in our laboratory. 3-Butenyl isothiocyanate was dissolved in DMSO for cellular treatment. All the cell lines were used for MTT assay, and prostate cancer cells (PC-3) were used for further mechanistic assays. The initial cytotoxicity assay involved seeding of 3 × 103 cells per well, while the mechanistic assay involved the use of 5 × 105 cells per well. The number of cells required for the experimentation was standardized in our laboratory.
Cytotoxic activity
The cytotoxic activity of the compound was assessed using MTT and neutral red assay.
MTT assay
It is a colorimetric assay employed for the determination of antiproliferative activity of BITC against a number of cell lines. The assay was performed using the protocol designed by Keepers et al. (1991). The cells were seeded at a density of 3 × 103 cells per well in a 96 well plate. The results obtained from MTT assay were used for the calculation of IC50 and IC70 concentration of BITC in PC-3 cells, which were used for further mechanistic studies.
Neutral red assay
This method involves the detection of intact lysosomes via neutral red uptake assay following the method given by Ohno et al. (1998). Briefly, PC-3 cells at a concentration of 5 × 105 were seeded in a 24 well plate and incubated for 24 h. Following incubation, the cells were washed with 0.1 M PBS and later treated with camptothecin (positive control) and BITC (IC50 and IC70) for 12–14 h. After incubation, the cells were again washed using PBS and were treated with neutral red dye (10 μg/ml) for 2–3 h. Later, the cells were washed using PBS and fixed using 4 % PFA for 30 min and then the extraction solution was added to the cells (1 % glacial acetic acid and 50 % ethanol). The supernatant was then analyzed using ELISA plate reader (Synergy HT Biotek instruments Inc, USA) at 540 nm.
Mechanistic studies
The following mechanistic assays were performed to ascertain the mode of cell death.
Mitochondrial membrane potential assay
The mitochondrial membrane potential (MMP) assay is an important method that indicates the changes in membrane integrity of cancer cells following the treatment of BITC. It was assayed following the method proposed by Deng et al. (2013). Briefly, the cells (5 × 105) were seeded in a 24 well plate. The cells were treated with BITC (IC50 and IC70) for 12–14 h. Following treatment, the dye rhodamine 123 (1 μM) was added in the wells and the fluorescence was measured using an excitation wavelength of 488 nm and emission wavelength 530 nm.
Reactive oxygen species assay
The reactive oxygen species generated by BITC (IC50 and IC70) and camptothecin were measured in a 24 well plate seeded with 5 × 105 cells per well. Following treatment, the dye 2,7- dichlorofluorescein diacetate (50 μM) was added in the wells and the fluorescence was measured using an excitation wavelength of 488 nm and emission wavelength 530 nm (Wang and Joseph 1999).
Microscopic studies
The microscopic studies involved the use of phase contrast microscopy to assess the change in surface morphology, scanning electron microscopy (SEM) for observing the surface morphology and confocal microscopy for determining nuclear changes. The imaging of cell by phase contrast microscopy was done using Nikon Eclipse TS100 inverted microscope. In this, the untreated cells and cells given treatment with camptothecin (positive control) and BITC (IC50 and IC70) were analyzed for structural changes following the treatment.
For SEM analysis, PC-3 cells were seeded in a 6-well plate at a concentration of 5 × 105 cells per well in RPMI medium. After 24 h incubation, the cells were washed with 0.1 M PBS followed by the treatment with camptothecin (positive control) and BITC (IC50 and IC70) for 12–14 h. Following incubation, the cells were fixed with 4 % osmium tetraoxide for 4 h and then the cells were dehydrated using ethanol and acetone ranging from 10 to 100 % for 10 min each. The cells were later freeze dried using lypholyser and subjected to a single gold coating using Quarum Q150R ES Rotary-Pumped Sputter Coater/Carbon Coater. Finally, the cells were examined in EVO LS 10 scanning electron microscope (Carl Zeiss, Germany) (Ye et al. 2012).
In confocal microscopic, PC-3 cells were seeded at a concentration of 5 × 105 cells per well in a 24 well plate for 24 h. Following incubation, the cells were washed with 0.1 M PBS and later treated with camptothecin (positive control) and BITC (IC50 and IC70) for 12–14 h. After the incubation, the cells were again washed with 0.1 M PBS and fixed using 4 % PFA for 30 min. Finally, the cells were stained with DAPI and the slides were prepared. The confocal fluorescence images were taken using Nikon AiR laser scanning confocal microscope system (Nikon Corp., Japan) fitted with Nikon 40 × 0.95 NAD-ICM/N2 Plan objectives. The confocal image acquisition and analysis was performed using the built-in Nikon NIS Element AR software and the fluorescence was observed with a long-pass 488 emission filter (Bhushan et al. 2007).
Cell cycle analysis
The cell cycle analysis was done using the method proposed by Hu et al. (2010) to ascertain the arrest of cancer cells at different phases after the treatment with BITC (IC50 and IC70). The cells in a density of 5 × 105 cells per well were incubated in a 24 well plate. Following 24 h incubation, the cells were treated with camptothecin (positive control) and BITC (IC50 and IC70) for 12–14 h. After the treatment, cells were trypsinized and harvested from plate by centrifugation. The pellet formed was resuspended in ice cold PBS and fixed with 70 % ethanol. The cells were fixed with propidium iodide and analyzed in FL-2 channel using BD Accuri C6 flow cytometer (BD Biosciences immunocytometry systems, San Jose, CA).
Caspase activity
Caspase 3 activity was analyzed using a kit based colorimetric assay with acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) as the substrate. This assay measures the amount of caspase released by cancer cells after BITC treatment, which is an indicator of early apoptosis.
Lactate dehydrogenase assay
The lactate dehydrogenase (LDH) assay measures the amount of lactate dehydrogenase released by the necrotic cells using the method proposed by Abe and Matsuki (2000). The cells in a density of 5 × 103 cells per well were seeded in a 96 well plate. After a 24 h incubation in a CO2 incubator, the supernatant (50 μl) was collected and then added 50 μl solution A [2.5 mg lithium lactate, 2.5 mg NAD, 100 μl of 2.5 mg/ml MTT, 1 μl MPMS in 900 μl tris–HCl (0.2 M, pH 8.2 with 0.1 % triton)]. The mixture was incubated for 2 h and the OD was taken in an ELISA reader (Synergy HT multi-mode microplate reader by BioTek Instruments Inc.) at 570 nm. Since, lactate dehydrogenase can only be released by necrotic cells and thus it differentiates between apoptosis and necrosis.
Annexin V-FITC/PI assay
Apoptosis was further confirmed using Annexin V-FITC apoptosis detection kit and using confocal microscopy. Briefly, cells in a density of 5 × 105 cells per well were incubated in a 24 well plate. Following 24 h incubation, the cells were treated with camptothecin (positive control) and BITC (IC50 and IC70) for 12–14 h. After the treatment, the cells were co-stained with Annexin V-FITC and PI for 15 min. Later, the cells were trypsanized and the slides of different treatments were analyzed using Nikon AiR laser scanning confocal microscope system (Nikon Corp., Japan) fitted with Nikon 40 × 0.95 NAD-ICM/N2 Plan objectives. The confocal image acquisition and analysis was performed using the built-in Nikon NIS Element AR software. This method further confirms the apoptotic mechanism of cell death and clearly differentiates it from necrosis. In this method, the viable cells are both annexin V-FITC and PI negative, early apoptotic cells are annexin V-FITC positive and PI negative and dead cells are both annexin V-FITC and PI positive.
Statistical analysis
The experiments was carried out in triplicate and expressed as mean ± SE of IC50 and IC70. One way analysis of variance (ANOVA) and Tukey’s HSD post hoc test were carried to determine significant differences between the mean at p ≤ 0.05 using SigmaStat v3.5.
Results
A compound 3-Butenyl ITC (BITC) was isolated from the extract of Brassica juncea (L.) Czern var. Pusa Jaikisan using hexane: ethyl acetate as solvent system. The purity of compound was confirmed by GC–MS, GC–FID and UHPLC–PDA and was found to be ≥90 % pure (Online resource 1a, b and c). Mass spectra of the compound was m/z 113, 72, 55, 39, which was further confirmed by NMR (1H-NMR (600 MHz, CDCl3): δ 5.75–5.82 (m, 1H), 5.16–5.20 (m, 2H), 3.51–3.56 (m, 2H), 2.41–2.45 (m, 2H); 13C NMR (400 MHz, CDCl3): 135.4, 131.9, 117.4, 43.2, 33.0).
After the chemical characterization and identification, the compound were analyzed its cytotoxic activity using MTT and neutral red assays. In MTT assay, seven different human cancer cell lines viz. prostate, bone, osteosarcoma, cervical, liver, neuroblastoma and breast cancer cell lines were used (Table 1). The IC50 and IC70 values of the compound were ascertained in each of the cell lines using MTT assay. It was observed that the compound was most effective against prostate cancer cell line (IC50: 0.041 μl/ml; IC70: 0.060 μl/ml). These values were found to be even lower than the positive control camptothecin (IC50: 121.597 μM). The antiproliferative activity was further confirmed using neutral red assay in prostate cancer cell line. The initial 100 % cell viability of PC-3 cells in control was reduced to 12 % at IC50 and 5 % at IC70 (Fig. 1a). Overall, the antiproliferative activity of BITC in both MTT and neutral red assay was found to be good in prostate cancer cell line.
To understand the mechanism of cell death as indicated by MTT and neutral red assay, a number of other assays viz. MMP, ROS, phase contrast microscopy, confocal microscopy, SEM, cell cycle analysis, caspase activity, LDH assay and annexin V-FITC/PI assay were also performed. In MMP assay, a better bioactivity in case of IC50 and IC70 concentration of BITC in PC-3 cells compared to positive control was observed. The BITC treatment in ROS assay caused an increased generation of reactive oxygen species in prostate cancer cells as seen by the IC50 and IC70 values thus showing a positive correlation with MMP assay (Fig. 1b, c).
The above studies were further strengthened by microscopic studies which indicated the changes in cell and nuclear morphology following the treatment with BITC. Online resource 2a clearly shows the rounding of PC-3 cells following the treatment with BITC (IC50 and IC70) and camptothecin (positive control) in phase contrast microscopy. The cancer cells began to die via apoptosis mechanism and the apoptotic bodies were formed. The surface morphology of PC-3 cells showed an enhanced apoptosis in BITC (IC50) treated cells as compared to positive control in SEM studies as seen in Online resource 2b. The formation of apoptotic bodies started, leading to cellular death (Online resource 2bIII). The confocal microscopic imaging of PC-3 cells treated with BITC (Fig. 2aIII, IV) showed changes in nuclear morphology compared to control (Fig. 2aI).
The cell cycle analysis using flow cytometry was also conducted. It was seen that the BITC treatment caused the arrest of PC-3 cells at hypo-diploid (sub Go) phase of cell cycle (78.7 and 87 % in IC50 and IC70 respectively) (Fig. 2bIII, IV). In contrast, the cells in G1 phase considerably decreased following the treatment with BITC (21.3 % at IC50 and 13.0 % at IC70). In addition, no cells were seen at S and G2/M phase, after the treatment with BITC. The positive control camptothecin on the other hand had 39.2 % cells at sub Go phase, 23.8 % cells at G1 phase, 8.5 % cells at S and 28.5 % cells at G2/M phase of cell cycle.
The apoptotic mechanism of cell death induced by BITC in PC-3 cells was further investigated using a battery of other assays in order to differentiate the necrotic and apoptotic mechanism of cell death. The caspase 3 activity was increased after the BITC (IC50: 185.19 % and IC70: 214.81 %) treatment as compared to the control (Fig. 3a). The LDH activity in PC-3 cells following BITC (IC50 and IC70) treatment was also studied. An increased LDH activity following the treatment with the compound was considered as the marker of tissue damage (Fig. 3b). This is an important marker for identifying the actual mechanism of cellular death. Finally, annexin V-FITC and PI assay was performed to confirm the apoptotic cellular death. The co-incubation of PC-3 cells and BITC with and without these dyes (IC50 and IC70) (Fig. 3cI) showed no red coloration, revealing an absence or decreased cellular death. On the other, hand BITC (IC50 and IC70) treatment showed cellular death via apoptosis at IC50 concentration, while slight necrosis could be seen at IC70 concentration (Fig. 3cIII, IV).
Discussion
Brassica juncea is a member of family Brassicaceae and is a rich source of glucosinolates (GSLs) (Fahey et al. 2001). The evaluation of hydrolytic products of Brassica juncea var. Pusa Jaikisan showed 3-butenyl ITC as the major product. It was then isolated using silica 60–120 mesh and ethyl acetate: hexane as solvent system. The isolated product BITC was obtained with a purity ≥90 %. The results were confirmed via a number of analytical instruments viz. GC–MS, GC–FID, UHPLC–PDA and NMR. All these instruments validated the compound along with its purity.
Although, BITC showed antiproliferative activity in all cell lines but it was found to be more effective in prostate cancer cells. It was seen that IC50 value of BITC was as low as 0.041 μl/ml in PC-3 cells compared to 167.49 μl/ml in A-549 cells. The antiproliferative activity points towards the ability of BITC to prevent/inhibit the cancer cell growth. A number of manuscripts have reported ITCs as potential antiproliferative agents (Ferrarini et al. 2011; Melchini et al. 2013). An initial test of cytotoxicity using neutral red assay was performed for reconfirming the activity of BITC. The test showed a low cellular viability at IC50 concentration. A potent antiproliferative activity plays a keystone for assessing the possible role of BITC as an anticancer agent. For this purpose, a number of assays were performed to confirm the apoptotic mechanism of cellular death and thus testing the anticancer activity of this compound. The mitochondrial membrane potential of PC-3 cells was reduced following the treatment with BITC. On the other hand, an increase in the reactive oxygen species was seen after the treatment. The decreased MMP and an increased mitochondrial ROS is often linked to initiation of apoptosis related cellular death (Gottlieb et al. 2003; Chen et al. 2010; Kwang-Youn et al. 2011).
Microscopic studies are another important visual study for proving the anticancer activity of BITC. The rounding and distortion in the normal shape of the cells along with the budding of apoptotic bodies in phase contrast microscope pointed towards the beginning of apoptosis (Chiang et al. 2011). This mechanism of apoptosis seen in phase contrast microscopy was further confirmed using scanning electron microscope. A clear and detailed formation and budding of apoptotic bodies was seen in the PC-3 cells treated with IC50 concentration of BITC.
The confocal microscopy of PC-3 cells was also conducted. This study revealed the change in nuclear morphology following the treatment with BITC. The cells showed swelling, clear morphological and nuclear deformation and shrinkage of nuclear material as compared to untreated control. A cell cycle analysis acts as another important factor adding to the apoptotic mechanism of cellular death. The PC-3 cells treated at IC50 concentration of BITC showed a restriction at sub Go (78.7 %) phase of cell division as compared to the untreated control (29.6 %). After the treatment with BITC, the cells in the G1 phase considerably decreased, while an increase in the cells at G0 phase was observed. The cells were therefore entering the quiescent state and thus an inhibition of cell cycle was observed. This was further confirmed by the absence of any cells in the S and G2/M phase of cell cycle. This increase in the number of cells at sub Go phase and decrease in the cells at G1, S and G2/M phase is an indicator that the cancer cells are prevented from undergoing cellular division and thus pushing these towards apoptotic cell death.
The difference between the apoptotic and necrotic cell death following the treatment with BITC was also confirmed using a number of other assays. Among these, caspase 3 is an important enzyme responsible for chromatin condensation and DNA fragmentation. An increase in caspase 3 activity was recorded in PC-3 cells treated with BITC. This increase showed the apoptotic cellular death occurring due to the compound in hand. LDH is another important marker of tissue damage (Gonzalez-Flecha et al. 1993). A low level of LDH in BITC treated cells is a clear indicator of the ongoing apoptotic cellular death (Wrobel et al. 2003). The confirmation was further strengthened by annexin-V FITC and PI assay. The IC50 dose and the positive control showed apoptotic cellular death (Fig. 4). Thus, BITC is an important and potent anticancer agent leading to cellular death by apoptosis.
Conclusion
Isothiocyanates have emerged as a ray of hope against a number of biological stresses including cancer. The current study involved the isolation of an important isothiocyanate viz. 3-butenyl isothiocyanate with a purity of ≥90 %. The compound exhibited exceptional anticancer activity among the different human cancer cell lines. Among these, the prostate cancer (PC-3) cells showed great sensitivity against this compound. The mechanistic pathway involved in the cellular death of the prostate cancer cells was evaluated using a number of assays and it was concluded that the cells were killed following apoptosis. This study exhibited the potent anticancer activity of 3-butenyl isothiocyanate and hence makes it a suitable candidate for animal model study.
Abbreviations
- ITCs:
-
Isothiocyanates
- BITC:
-
3-Butenyl isothiocyanate
- GC:
-
Gas chromatography
- FID:
-
Flame ionization detector
- MS:
-
Mass spectrometer
- UHPLC:
-
Ultra high pressure liquid chromatography
- PDA:
-
Photo diode array
- NMR:
-
Nuclear magnetic resonance
- PC-3:
-
Prostate cancer cell line
- MG-63:
-
Human bone osteosarcoma cell line
- HeLa:
-
Human cervical cancer cell line
- HepG-2:
-
Human liver cancer cell line
- IMR-32:
-
Human neuroblastoma cell line
- MCF-7:
-
Human breast cancer cell line
- FBS:
-
Fetal bovine serum
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MMP:
-
Mitochondrial membrane potential
- ROS:
-
Reactive oxygen species
- SEM:
-
Scanning electron microscopy
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- LDH:
-
Lactate dehydrogenase
References
Abe K, Matsuki N (2000) Measurement of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci Res 38:325–329
Arora R, Bhushan S, Kumar R, Mannan R, Kaur P, Singh AP, Singh B, Vig AP, Sharma D, Arora S (2014a) Hepatic dysfunction induced by 7,12-dimethylbenz(α)anthracene and its obviation with erucin using enzymatic and histological changes as indicators. PLoS ONE 9(11):e112614. doi:10.1371/journal.pone.0112614
Arora R, Sharma D, Kumar R, Singh B, Vig AP, Arora S (2014b) Evaluating extraction conditions of glucosinolate hydrolytic products from seeds of Eruca sativa (Mill.) Thell. using GC–MS. J Food Sci 79:C1964–C1969
Arora R, Vig AP, Arora S (2014c) Glucosinolates: transposing trends of identification methods from paper chromatography to microchip analysis. Int J Life Sci Biotechnol Pharma Res 3:42–61
Arora R, Bhushan S, Kumar R, Mannan R, Kaur P, Singh B, Sharma R, Vig AP, Singh AP, Arora S (2016a) To analyze the amelioration of phenobarbital induced oxidative stress by erucin, as indicated by biochemical and histological alterations. Anticancer Agents Med Chem 16. http://www.ncbi.nlm.nih.gov/pubmed/?term=To+Analyze+the+Amelioration+of+Phenobarbital+Induced+Oxidative+Stress+by+Erucin%2C+as+Indicated+by+Biochemical+and+Histological+Alterations
Arora R, Singh B, Vig AP, Arora S (2016b) Conventional and modified hydrodistillation method for the extraction of glucosinolate hydrolytic products: a comparative account. SpringerPlus 5:479
Bhushan S, Kumar A, Malik F, Andotra SS, Sethi VK, Kaur IP, Taneja SC, Qazi NG, Singh J (2007) A triterpenediol from Boswellia serrata induces apoptosis through both the intrinsic and extrinsic apoptotic pathways in human leukemia HL-60 cells. Apoptosis 12:1911–1926
Bongoni R, Verkerk R, Steenbekkers B, Dekker M, Stieger M (2014) Evaluation of different cooking conditions on broccoli (Brassica oleracea var. Italic) to improve the nutritional value and consumer acceptance. Plant Food Hum Nutr 69:228–234
Cabello-Hurtado F, Gicquel M, Esnault MA (2012) Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem 132:1003–1009
Chen Q, Wang Y, Xu K, Lu G, Ying Z, Wu L, Zhan J, Fang R, Wu Y, Zhou J (2010) Curcumin induces apoptosis in human lung adenocarcinoma A549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol Rep 23:397–403
Chiang JH, Yang JS, Ma CY, Yang MD, Huang HY, Hsia TC, Kuo HM, Wu PP, Lee TH, Chung JG (2011) Danthron, an anthraquinone derivative, induces DNA damage and caspase cascades-mediated apoptosis in SNU-1 human gastric cancer cells through mitochondrial permeability transition pores and bax-triggered pathways. Chem Res Toxicol 24:20–29
De Nicola GR, Bagatta M, Pagnotta E, Angelino D, Gennari L, Ninfali P, Rollin P, Iori R (2013) Comparison of bioactive phytochemical content and release of isothiocyanates in selected brassica sprouts. Food Chem 141:297–303
Deng S, Yuan H, Yi J, Lu Y, Wei Q, Guo C, Wu J, Yuan L, He Z (2013) Gossypol acetic acid induces apoptosis in RAW264. 7 cells via a caspase-dependent mitochondrial signaling pathway. J Vet Sci 14:281–289
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 569:5–51
Ferrarini L, Pellegrini N, Mazzeo T, Miglio C, Galati S, Milano F, Rossi C, Buschini A (2011) Antiproliferative activity and chemoprotective effects towards DNA oxidative damage of fresh and cooked brassicacea. Br J Nutr 107:1324–1332
Ghasemzadeh A, Ghasemzadeh N (2011) Flavonoids and phenolic acids: role and biochemical activity in plants and human. J Med Plant Res 5:6697–6703
Gonzalez-Flecha B, Cutrin JC, Boveris A (1993) Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest 91:456–464
Gottlieb E, Armour SM, Harris MH, Thompson CB (2003) Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ 10:709–717
Hu X, Zhang X, Qiu S, Yu D, Lin S (2010) Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem Biophys Res Commun 398:62–67
Keepers YP, Pizao PE, Peters GJ, Ark-Otte JV, Winogard B, Pinedo HM (1991) Comparison of the sulforhodamine b protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer 27:897–900
Kryston TB, Georgiev AB, Pissis P, Georgakilas AG (2011) Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res Fundam Mol Mech Mutagen 711:193–201
Kwang-Youn K, Yu SN, Lee SY, Chun SS, Choi YL, Park YM, Song CS, Chatterjee B, Ahn SC (2011) Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun 413:80–86
Melchini A, Traka MH, Catania S, Miceli N, Taviano MF, Maimone P, Fransico M, Mithen RF, Costa C (2013) Antiproliferative activity of the dietary isothiocyanate erucin, a bioactive compound from cruciferous vegetables, on human prostate cancer cells. Nutr Cancer 65:132–138
Ohno T, Futamura Y, Harihara A (1998) Validation study on five cytotoxicity assays by JSAAE-VIII. AATEX 5:131–145
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Wrobel A, Seltmann H, Fimmel S, Muller-Decker K, Tsukada M, Bogdanoff B, Mandt N, Blume-Peytavi U, Orfanos CE, Zouboulis CC (2003) Differentiation and apoptosis in human immortalized sebocytes. J Invest Dermatol 120:175–181
Ye LH, Li WJ, Jiang XQ, Chen YL, Tao SX, Qian WL, He JS (2012) Study on the autophagy of prostate cancer PC-3 cells induced by oridonin. Anat Rec 295:417–422
Acknowledgments
The authors thank financial support of Department of Science and Technology (DST) & University Grants Commission (UGC), New Delhi and Guru Nanak Dev University, Amritsar. Authors are thankful to UPE (under the university with potential for excellence), BSR and CPEPA programme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors pose no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online resource 1
Chemical characterization of 3-butenyl isothiocyanate using: a) GC–MS; b) GC-FID; c) UHPLC-PDA (TIFF 1003 kb)
Online resource 2
Microscopic analysis: a) phase contrast microscopy I) control, II) positive control, III) BITC (IC50), IV) BITC (IC70); the cells were observed at the magnification of 400× (40× objective and 10× eye piece) b) scanning electron microscopy I) control, II) positive control, III) BITC (IC50); the cells were observed at the magnification of 11 kx (TIFF 3471 kb)
Rights and permissions
About this article
Cite this article
Arora, R., Kumar, R., Mahajan, J. et al. 3-Butenyl isothiocyanate: a hydrolytic product of glucosinolate as a potential cytotoxic agent against human cancer cell lines. J Food Sci Technol 53, 3437–3445 (2016). https://doi.org/10.1007/s13197-016-2316-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2316-7