Abstract
Cancer is a leading cause of death worldwide. In our continuing search for new anticancer agents, four Malaysian Calophyllum species, namely C. castaneum, C. teysmannii, C. canum, and C. sclerophyllum, had been phytochemically studied to give compounds 1–12. All the isolated compounds were evaluated for their antiproliferative activity against nasopharyngeal (SUNE1, TW01, CNE1, HK1) and breast (HCC38, MDA-MB-231, MDA-MB-468, SKBR3) cancer cell lines via methyl thiazolyl tetrazolium cell viability assay. Among the tested compounds, isodispar B (1) showed a promising dose-dependent and a broad spectrum of cytotoxic effects on all the tested cancer cell lines; in particular, potent inhibitory activities were observed on nasopharyngeal cancer cell lines (SUNE1, TW01, CNE1, HK1), with IC50 values ranging from 3.8 to 11.5 µM. In comparison with 5-fluorouracil as positive control, compound 1 was found to exhibit at least sixfold much higher activity than the standard drug used against the nasopharyngeal cell lines. Compound 1 was later found to induce apoptotic cell death in nasopharyngeal cancer cells, as evidenced by ‘Cell Death Detection’ ELISAPLUS kit, and exhibited good cancer-specific cytotoxicity when tested with noncancerous NP460 cells. Meanwhile, compounds 2–12 displayed moderate to weak activities against the tested cancer cell lines. The findings have highlighted the therapeutic potential of compound 1 against nasopharyngeal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants from the genus Calophyllum are found to be a valuable source of bioactive chromanones, coumarins, xanthones, biflavonoids, and triterpenoids (Oliveira et al., 2014). Ever since the discovery of (+)-calanolide A as an anti-HIV agent from C. lanigerum in the early 1990s, there has been a growing interest shown by global scientists in Calophyllum species in the search for new chemotherapeutic leads from these plants due to their promising pharmacological properties. (+)-Calanolide A has been reported to exhibit potent activity against human immunodeficiency virus type-1 (HIV-1), and is currently tested in human clinical trials (Cragg and Newman, 2003). Apart from that, preliminary studies had also revealed that plants from this genus exhibited a wide range of biological activities, including antiviral (Ito et al., 1999; Brahmachari and Jash, 2014), cytotoxic (Mah et al., 2015), antimalarial (Hay et al., 2004), antibacterial, (Cuesta-Rubio et al., 2015) and antioxidant activities (Taher et al., 2010).
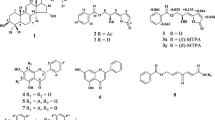
Cancer is a leading cause of death worldwide. According to estimates from the International Agency for Research on Cancer, there were 14.1 million new cancer cases and 8.2 million cancer deaths reported in 2012, and the number of cancer deaths is expected to increase to 13.2 million by 2030 (GLOBOCAN, 2012). Although a number of plant-derived anticancer drugs such as vinblastine, vincristine, epipodophyllotoxin, and paclitaxel have been successfully developed over the years, the ability of cancer cells to develop resistance to the drugs during the course of treatment has evoked the need for a continuous search for new drugs with a better efficacy to overcome the drug-resistant problem (Cragg and Newman, 2003). In conjunction with this, investigation has been undertaken by our team on the four Malaysian Calophyllum species, namely C. sclerophyllum, C. teysmannii, C. castaneum, and C. canum. This work has successfully yielded 12 isolated compounds, including two phenylcoumarins, five chromanone acids, two xanthones, and three triterpenoids. C. sclerophyllum afforded isodispar B (1), 5,7-dihydroxy-6-(3-methylbutyryl)-4-phenylcoumarin (2), and friedelin (3); C. teysmannii gave caloteysmannic acid (4), calolongic acid (5), isocalolongic acid (6), and stigmasterol (7); C. castaneum yielded blancoic acid (8), isoblancoic acid (9), euxanthone (10), friedelinol (11), and friedelin (3); C. canum gave ananixanthone (12), euxanthone (10), friedelinol (11), and friedelin (3). All these compounds (Fig. 1) were screened for their antiproliferative activities against nasopharyngeal (SUNE1, TW01, CNE1, HK1) and breast (HCC38, MDA-MB-231, MDA-MB-468, SKBR3) cancer cell lines. Interestingly, some of these compounds, particularly with isodispar B (1), showed prominent and a broad spectrum of activity against the tested cancer cell lines. The present paper describes the bioactivity-screening results of isolated compounds 1–12 from the four Calophyllum species.
Materials and methods
Chemicals
All reagents were of analytical quality and used without further purification unless otherwise specified. Column chromatography (CC) was performed on silica gel 60 (230–300 mesh, Merck) and Sephadex LH-20 (GE Healthcare). Analytical thin-layer chromatography was performed on precoated silica gel 60 F254 (Merck). 5-Fluorouracil (purity ≥ 99 %) was purchased from Sigma-Aldrich.
Plant materials
The stem bark materials of C. sclerophyllum, C. teysmannii, C. castaneum, and C. canum were collected in April 2013, from the jungle in Landeh district of Sarawak, Malaysia, and the authentication was carried out by Mr. Tinjan Anak Kuda, botanist from the Forest Department, Sarawak. Voucher specimens (UITM 3008, UITM 3006, UITM 3001, and UITM 3007) were deposited at the herbarium of Universiti Teknologi MARA, Sarawak.
Extraction
The air-dried and powdered stem bark material of C. sclerophyllum (1.5 kg), C. teysmannii (2.0 kg), C. castaneum (2.0 kg), and C. canum (2.6 kg) was separately extracted at room temperature with dichloromethane (2 × 10 L) for 72 h. Removal of the solvent under reduced pressure by a rotary evaporator at 40 °C yielded 52, 298, 41, and 125 g of dichloromethane extracts, respectively.
Isolation of compounds from C. sclerophyllum
About 50 g of dichloromethane extract was subjected to Si gel CC (40–63 µm, 8.5 × 50 cm, 600 g) packed in n-hexane and eluted with n-hexane-dichloromethane mixtures of increasing polarity (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 1 L, 25 mL/min) followed by increasing concentration of acetone in dichloromethane (10:90, 20:80, 30:70, 40:60, 50:50, 60:40, 70:30, 80:20, 90:10, 100:0, each 1 L, 25 mL/min) to give 20 fractions (CSA1–20). From fractions CSA9–10, isodispar B (1, 121 mg) was obtained. Fractions CSA12–13 (3.9 g) were combined based on a similar TLC pattern (spots were detected on TLC under ultraviolet (UV) light and in an iodine chamber) and fractionated by Si gel CC (40–63 µm, 3.0 × 50 cm, 110 g) with a gradient of n-hexane–EtOAc (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to give 20 subfractions (CSB1-20). Subfractions CSB2–3 afforded friedelin (3, 107 mg). Meanwhile, fractions CSA15–17 (4.2 g) were pooled and subjected to Si gel CC (40–63 µm, 3.0 × 50 cm, 110 g), and eluted with n-hexane–acetone (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to give 20 subfractions (CSC1–20). Subfractions CSC12–14 yielded 5,7-dihydroxy-6-(3-methylbutyryl)-4-phenylcoumarin (2, 136 mg).
Isolation of compounds from C. teysmannii
About 100 g of dichloromethane extract was subjected to Si gel CC (40–63 µm, 8.5 × 50 cm, 600 g) packed in n-hexane and eluted with n-hexane–dichloromethane mixtures of increasing polarity (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 1 L, 25 mL/min), followed by increasing concentration of EtOAc in dichloromethane (10:90, 20:80, 30:70, 40:60, 50:50, 60:40, 70:30, 80:20, 90:10, 100:0, each 1 L, 25 mL/min) to give 20 fractions (CTDA1–20). Fraction CTDA14 (6.2 g) was fractionated by Si gel CC (40–63 µm, 3.5 × 50 cm, 150 g) with a gradient of n-hexane–acetone (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to give 20 subfractions (CTDB1–20). Subfractions CTDB14–15 (0.75 g) were combined and further recrystallized in MeOH to afford caloteysmannic acid (1, 696 mg) as yellow cubic crystals. From subfractions CTDB4–7, isocalolongic acid (3, 1250 mg) was obtained. Meanwhile, fraction CTDA12 (4.8 g) was rechromatographed over Si gel CC (40–63 µm, 3.5 × 50 cm, 150 g) eluted with n-hexane–EtOAc (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to afford 20 subfractions (CTDC1–20). Subfraction CTDC14 (0.4 g) was further fractionated by Si gel CC (40–63 µm, 2.0 × 50 cm, 50 g) with a gradient of n-hexane–acetone (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.25 L, 6 mL/min) to give 20 subfractions (CTDD1–20). Subfraction CTDD10 afforded calolongic acid (2, 9 mg). Fractions CTDA3–4 (0.5 g) were combined and purified by Si gel CC (40–63 µm, 2.0 × 50 cm, 50 g), and eluted with n-hexane–EtOAc (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.25 L, 6 mL/min) to yield stigmasterol (4, 7 mg).
Isolation of compounds from C. castaneum
About 35 g of dichloromethane extract was subjected to Si gel CC (40–63 µm, 8.5 × 50 cm, 600 g) packed in n-hexane and eluted with n-hexane-dichloromethane mixtures of increasing polarity (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 1 L, 25 mL/min), followed by increasing concentration of EtOAc in dichloromethane (10:90, 20:80, 30:70, 40:60, 50:50, 60:40, 70:30, 80:20, 90:10, 100:0, each 1 L, 25 mL/min) to give 20 fractions (CCA1–20). Fractions CCA8–9 (4.0 g) were combined and fractionated by Si gel CC (40–63 µm, 3.0 × 50 cm, 110 g) with a gradient of n-hexane-EtOAc (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to give 20 subfractions (CCB1–20). Subfractions CCB5–6 yielded friedelin (3, 163 mg) as colorless needle-like crystals. From subfraction CCB9, friedelinol (11, 74 mg) was obtained. Meanwhile, fractions CCA14–15 (4.8 g) were combined and purified by Si gel CC (40–63 µm, 3.0 × 50 cm, 110 g) and eluted with n-hexane-acetone (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to afford 20 subfractions (CCC1–20). Subfractions CCC9–10 (0.5 g) were combined and subjected to Si gel CC (40–63 µm, 2.0 × 50 cm, 50 g), eluted with n-hexane–acetone (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.25 L, 6 mL/min) to give 20 subfractions (CCD1–20). Subfractions CCD11–14 (0.03 g) were pooled and purified by sephadex LH-20 CC (2.0 × 50 cm) eluted with dichloromethane–MeOH (10:90, 1 mL/min) to yield euxanthone (10, 15 mg). Subfractions CCC12–15 (0.8 g) were combined and rechromatographed over Si gel CC (40–63 µm, 2.0 × 50 cm, 50 g) with a gradient of n-hexane-acetone (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.25 L, 6 mL/min) to give 20 subfractions (CCE1–20). Subfractions CCE9–10 yielded blancoic acid (8, 283 mg). Lastly, subfractions CCC17–18 (0.7 g) were combined and subjected to sephadex LH-20 CC (3.0 × 50 cm) eluted with dichloromethane–MeOH (10:90, 2 mL/min) to give isoblancoic acid (9, 427 mg).
Isolation of compounds from C. canum
About 120 g of dichloromethane extract was subjected to Si gel CC (40–63 µm, 8.5 × 50 cm, 600 g) packed in n-hexane and eluted with n-hexane-dichloromethane mixtures of increasing polarity (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 1 L, 25 mL/min), followed by increasing concentration of acetone in dichloromethane (10:90, 20:80, 30:70, 40:60, 50:50, 60:40, 70:30, 80:20, 90:10, 100:0, each 1 L, 25 mL/min) to give 20 fractions (CDA1–20). Fractions CDA7–8 (6.5 g) were combined and fractionated by Si gel CC (40–63 µm, 3.5 × 50 cm, 150 g) and eluted with n-hexane-EtOAc (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to give 20 subfractions (CDB1–20). Subfractions CDB 5–6 afforded friedelin (3, 1062 mg). From subfractions CDB8–10, friedelinol (11, 125 mg) was obtained. Meanwhile, fractions CDA10–13 (7.2 g) were combined and purified by Si gel CC (40–63 µm, 3.5 × 50 cm, 150 g) with a gradient of n-hexane–EtOAc (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to give 20 subfractions (CDC1–20). Subfractions CDC9–11 (0.1 g) were pooled and rechromatographed over sephadex LH-20 CC (2.5 × 50 cm) eluted with dichloromethane–MeOH (10:90, 1 mL/min) to give ananixanthone (12, 21 mg) as yellowish needles. Fractions CDA15–18 (6.1 g) were combined and subjected to Si gel CC (40–63 µm, 3.5 × 50 cm, 150 g) and eluted with n-hexane–acetone (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, each 0.5 L, 15 mL/min) to give 20 subfractions (CDD1–20). Subfractions CDD11–13 (0.1 g) were pooled and purified by sephadex LH-20 CC (2.5 × 50 cm) eluted with 100 % MeOH at 1 mL/min to yield euxanthone (10, 12 mg).
Cell lines and cell culture
The nasopharyngeal cancer cells (HK1, CNE1, TW01, and SUNE1) and breast cancer cells (HCC38, MDA-MB-231, MDA-MB-468, and SKBR3) were maintained in RPMI 1640 medium supplemented with 100 IU/mL of penicillin and 100 µg/mL of streptomycin (Sigma–Aldrich). The immortalized normal nasopharyngeal epithelial cells NP460 were kindly provided by Dr. George Tsao, Department of Anatomy, Hong Kong University. NP460 cells were maintained in keratinocyte-SFM containing epidermal growth factor (EGF 1-53) and bovine pituitary extract (BPE) (Invitrogen). All cells were maintained at 37 °C under 5 % CO2 in a humidified incubator.
Cell proliferation assay
Inhibition of cell proliferation by the isolated compounds was determined by using the methyl thiazolyl tetrazolium (MTT) cell viability assay, as described previously with slight modification (Tan et al., 2013; Low et al., 2012). 5-Fluorouracil was used as a positive control in the assay. Briefly, all isolated compounds and positive control were reconstituted using dimethylsulfoxide (DMSO) (Sigma-Aldrich) to 100 mM and further diluted to the desirable concentrations using ultra purified sterile water just prior to the assays. Cancerous cells (5 × 103 cells/well) were plated in sterile 96-well plates for 24 h. Cells were treated with the isolated compounds in a dose-dependent manner for 72 h. The cell growth and anticancer effects were recorded at a test wavelength of 570 nm and a reference wavelength of 630 nm using the Tecan® Infinite F200 plate reader. The results were compiled in a dose-response curve to enable the quantification of IC50, or the concentration of the isolated compounds that inhibits cell proliferation by 50 %. In order to further assess the selectivity of the isolated compounds toward cancerous and noncancerous cells, the above process was also repeated on the noncancerous nasopharyngeal cells (NP460).
Detection of mode of cancer cells deaths by quantitative sandwich enzyme immunoassay (ELISA)
The degree of mode of cancer cell deaths induced by isodispar B (1) was quantified using the Cell Death Detection ELISAPLUS kit (Roche Diagnostics) as per the manufacturer’s instruction and as in our previous studies (Mai et al., 2009; Mai et al., 2014). Since nasopharyngeal cancer cells were most sensitive to the treatment, TW01, CNE1, HK1, and SUNE1 cells were seeded at a density of 5 × 103 cells/well on 96-well plates and treated with 1 % DMSO, 1 µM or 10 µM of isodispar B (1) for 3 days. The absorbances were measured at 405 nm using Tecan® Infinite F200 plate reader. Enrichment factors were calculated based on the absorbance of cells treated with compound 1 over absorbance of cells treated with 1 % DMSO.
Results and discussion
The structures of isolated compounds 1–12 (Fig. 1) were established by spectroscopic methods and comparison with literature data (Plattner et al., 1974; Guilet et al., 2001; Lin et al., 2006; Bayma et al., 1998; Dharmaratne et al., 2009; Sousa et al., 2012; Lim et al., 2015). Isolation of isodispar B (1) was previously reported from C. dispar (Guilet et al., 2001). Compound 1 has been shown to be a HIV transcription inhibitor by displaying anti-NF-κB and anti-Tat activities (Bedoya et al., 2005). It displayed cytotoxic activities against human SF-268, H-460, MCF-7, and KB cancer cell lines (Guilet et al., 2001; López-Pérez et al., 2005), and antifungal activities (Sandjo et al., 2012). 5,7-Dihydroxy-6-(3-methylbutyryl)-4-phenylcoumarin (2) was previously synthesized and assayed to show anti-inflammatory activity by inhibiting NO production in LPS-induced RAW 264.7 cells (Lin et al., 2006). Friedelin (3), stigmasterol (7), and friedelinol (11) were ubiquitous compounds commonly found in higher plants, which have been extensively studied to show antibacterial (Viswanathan et al., 2012), antifungal (Jain et al., 2001), and cytotoxic (Csupor-Löffler et al., 2011; Shen et al., 2012) activities. Tesymannic acid (4) isolated from C. teysmannii was found to exhibit potent inhibitory activity against HeLa cancer cells (IC50 value of 7.3 µM) (Lim et al., 2015). Calolongic acid (5) and isocalolongic acid (6) previously isolated from C. caledonicum were both reported to exert strong antifungal activities against Aspergillus fumigatus, showing MIC80 values of 4 and 2 µg/mL, respectively (Hay et al., 2004). Blancoic acid (8) and isoblancoic acid (9) were previously isolated from C. brasiliense (Plattner et al., 1974) and, to our knowledge, there has been no biological activity result reported on these two compounds by far. Euxanthone (10) isolated from Harungana madagascariensis was strongly active against the Gram-positive Bacillus megaterium (Kouam et al., 2007), and was reported to inhibit HIV-1 reverse transcriptase (Reutrakul et al., 2006) and cytotoxic against human MCF-7, TK-10, and UACC-62 cancer cell lines (Pedro et al., 2002). Ananixanthone (12) previously isolated from C. caledonicum has been found to exhibit antifungal activity against A. fumigatus (Morel et al., 2002) and demonstrated anti-tobacco mosaic virus (anti-TMV) activities with inhibition rates above 10 % (Wu et al., 2013).
In this study, the in vitro antiproliferative activities of coumarins 1, 2, chromanone acids 4, 5, 6, 8, 9, xanthones 10, 12, triterpenoids 3, 7, 11, and positive control 5-fluorouracil were measured against a panel of nasopharyngeal (SUNE1, TW01, CNE1, HK1) and breast (HCC38, MDA-MB-231, MDA-MB-468, SKBR3) cancer cell lines. The cell viability was assessed using the MTT-dye reduction assay and the corresponding IC50 values were calculated as the concentrations of tested compounds leading to 50 % decrease of cell survival (Table 1).
The cytotoxic results indicated that most of the tested compounds showed selective antiproliferative activity against the tested cancer cell lines except for isodispar (1), which displayed a broad spectrum of antiproliferative activities (> 90 % inhibition) against all tested cancer cell lines. More interestingly, compound 1 exerted the highest cytotoxicity among the tested compounds, against nasopharyngeal cancer cell lines (SUNE1, TW01, CNE1, HK1), with IC50 values ranging from 3.8 to 11.5 µM (Table 1). This compound demonstrated a greater cytotoxic potency, with at least sixfold much higher activity than the positive control used in the assay. The IC50 values of compound 1 against normal nasopharyngeal epithelial cells, NP460, was about 3.8 to 11.5-fold higher than that of nasopharyngeal cancer cells (Table 2). The dose–response curves (Fig. 2a) showed that compound 1 exhibited lower average percentages of cell viability in all nasopharyngeal cancer cells at all concentrations as compared to the normal nasopharyngeal epithelial cells. The cytotoxic effects exhibited by compound 1 were also found to be dose-dependent. Microscopic observation (Fig. 2b) showed no significant morphological change in NP460 cells treated with 10 µM of compound 1, as compared to NP460 cells treated with negative control (1 % DMSO). These results further confirmed that compound 1 induced cancer-specific cytotoxicity, sparing the noncancer cells. In addition, a great reduction in the number of viable nasopharyngeal cancer cells treated with compound 1 was observed (Fig. 2b). Comparing with nasopharyngeal cancer cells treated with 1 % DMSO, cancer cells treated with compound 1 was shrunken and round in shape (Fig. 2b). This microscopic observation suggests that the shrunken and round-shaped cancer cells were the apoptotic bodies, which resulted from the preferred apoptotic program cell death (Ziegler and Groscurth, 2004; Elmore, 2007; Saraste and Pulkki, 2000). In order to confirm the mode of cell death as observed in Fig. 2b, nasopharyngeal cells were treated with 1 % DMSO, 1 and 10 µM of isodispar B (1) for 72 h. Apoptosis inductions by compound 1 were measured using the Cell Death Detection ELISAPLUS (Roche, Germany). The results showed that compound 1 induced a significantly higher percentage of apoptosis (p < 0.05) as compared with cells treated with 1 % DMSO in all nasopharyngeal cancer cells. The effects were also dose-dependent. The apoptotic induction effects were most significant in SUNE1 cells, followed by HK1, CNE1, and TW01 (Fig. 3). These results correlated with the IC50 induced by compound 1 on nasopharyngeal cancer cells, in which SUNE1 cells were also the most sensitive nasopharyngeal cancer cells (Table 2). Apart from that, ananixanthone (12) and isoblancoic acid (9) also displayed significant cytotoxicity against nasopharyngeal cancer cell lines with IC50 values of less than 40 µM.
a The dose-response curve of isodispar B (1) against nasopharyngeal cancer cells (TW01, CNE1, HK1, and SUNE1) and noncancerous nasopharyngeal cells (NP460). Cell viability was determined 72 h after treatment using MTT assay. Points represent mean ± standard deviation from minimum three independent experiments. Statistical analysis was performed using one-way analysis of variance (ANOVA) post hoc Dunnett t-test using SPSS 18.0. Statistically significant differences (p < 0.05) are expressed as * as compared to control (1 % DMSO). b Morphological changes in all the cells upon treatment with 1 using inverted phase-contrast microscopy (10×) 72 h after treatment with 10 µM of 1
Apoptotic induction effect of isodispar B (1) on nasopharyngeal cancer cells (TW01, CNE1, HK1, and SUNE1). Cells were treated with compound 1 with 1 % DMSO, 1 or 10 µM for 72 h. Apoptosis induction by compound 1 was measured using the Cell Death Detection ELISAPLUS (Roche, Germany). All data were reported as mean ± standard deviation from minimum three independent experiments. Statistical analysis was performed using one-way analysis of variance (ANOVA) post hoc Dunnett t-test using SPSS 18.0. Statistically significant differences (p < 0.05) are expressed as * as compared to control (1 % DMSO)
SAR study revealed that the presence of isovaleryl group at different key positions on coumarin nucleus imparts determinant effect on antiproliferative activities against the nasopharyngeal cancer cell lines. Compound 1 with an isovaleryl group linked at C-8 position showed potent inhibitory activities against the cancer cell lines. On the contrary, the presence of isovaleryl moiety at C-6 position in compound 2 was found to be totally devoid of activity against the same panel of cancer cell lines. In the present study, the biological role of isovaleryl group in compound 1 remained unknown. However, the presence of isovaleryl group was found to be essential for cytotoxicity. Apart from that, ananixanthone (12) was reported to trigger a much greater cytotoxic potency (IC50 values of 20.7–29.5 µM) than euxanthone (10) (IC50 values of 66.1 µM and above) against the nasopharyngeal cancer cell lines, suggesting the importance of prenyl and pyrano moieties in compound 12 for the studied effect on the cancer cell lines, and this was in agreement with the literature (Lim et al, 2011).
Among chromanone acids 4, 5, 6, 8, and 9 tested, isoblancoic acid (9) showed the highest cytotoxicity toward HK1, TW01, and SUNE1 cancer lines, giving IC50 values below 30 µM. On the other hand, blancoic acid (8), which is the stereoisomer of compound 9, exhibited a much weaker inhibitory activity against the cancer cell lines, indicating the substantial role played by the stereochemistry of compounds on growth inhibition. Compound 8 showed trans-2,3-dimethyl substitution on the chomanone ring, which was different from compound 9 with a cis-2,3-dimethyl substitution. In the case of triterpenoids 3, 7, and 11 tested, stigmasterol (7) with a steroidal skeleton demonstrated a more selective activity than those of friedelane triterpenoids 3 and 11 in the assay. In comparison with the phenolic compounds tested, friedelin (3) gave the highest inhibitory activity against MDA-MB-468 cancer cells with IC50 values of 20.6 µM. However, these triterpenoids were not suitable for drug development because of their poor pharmacodynamic and pharmacokinetic properties.
Conclusions
Twelve chemical constituents, 1–12, isolated from four Malaysian Calophyllum species were evaluated for their cytotoxic activity against a panel of nasopharyngeal (SUNE1, TW01, CNE1, HK1) and breast (HCC38, MDA-MB-231, MDA-MB-468, SKBR3) cancer cell lines, and were found to exhibit strong to weak inhibitory activities in the assay. Among these compounds, isodispar B (1) showed the most promising result; in particular, potent inhibitory activities were observed on nasopharyngeal cancer cell lines (SUNE1, TW01, CNE1, HK1), with IC50 values ranging from 3.8 to 11.5 µM. This compound was found to induce apoptotic cell death in nasopharyngeal cancer cells and exhibited good cancer-specific cytotoxicity when tested with noncancerous NP460 cells. The findings in the present study might be important in development of a new drug lead against nasopharyngeal cancer.
References
Bayma JC, Arruda MSP, Neto MS (1998) A prenylatedxanthone from the bark of Symphonia globulifera. Phytochemistry 49:1159–1160
Bedoya LM, Beltrán M, Sancho R, Olmedo DA, Sánchez-Palomino S, Olmo E, López-Pérez JL, Muñoz E, Feliciano AS, Alcamí J (2005) 4-Phenylcoumarins as HIV transcription inhibitors. Bioorg Med Chem Lett 15:4447–4450
Brahmachari G, Jash SK (2014) Naturally occurring calanolides: an update on their anti-HIV potential and total syntheses. Recent Pat Biotechnol 8:3–16
Cragg GM, Newman DJ (2003) Plants as a source of anti-cancer and anti-HIV agents. Ann Appl Biol 143:127–133
Csupor-Löffler B, Hajdú Z, Zupkó I, Molnár J, Forgo P, Vasas A, Kele Z, Hohmann J (2011) Antiproliferative constituents of the roots of Conyza canadiensis. Planta Med 77:1183–1188
Cuesta-Rubio O, Oubada A, Bello A, Maes L, Cos P, Monzote L (2015) Antimicrobial assessment of resins from Calophyllum Antillanum and Calophyllum Inophyllum. Phytother Res 29:1991–1994
Dharmaratne HRW, Napagoda MT, Tennakoon SB (2009) Xanthones from roots of Calophyllum thwaitesii and their bioactivity. Nat Prod Res A: Struct Synth 23:539–545
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
GLOBOCAN (2012) Cancer incidence and mortality worldwide: IARC CancerBase No. 11. http://globocan.iarc.fr. Accessed 16 July 2015
Guilet D, Séraphin D, Rondeau D, Richomme P, Bruneton J (2001) Cytotoxic coumarins from Calophyllum dispar. Phytochemistry 58:571–575
Hay AE, Hélesbeux JJ, Duval O, Labaïed M, Grellier P, Richomme P (2004) Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci 75:3077–3085
Ito C, Itoigawa M, Furukawa H, Tokuda H, Okuda Y, Mukainaka T, Okuda M, Nishino H (1999) Anti-tumor-promoting effects of 8-substituted 7-methoxycoumarins on Epstein-Barr virus activation assay. Cancer Lett 138:87–92
Jain SC, Singh B, Jain R (2001) Antimicrobial activity of triterpenoids from Heliotropium ellipticum. Fitoterapia 72:666–668
Kouam SF, Yapna DB, Krohn K, Ngadjui BT, Ngoupayo J, Choudhary MI, Schulz B (2007) Antimicrobial prenylatedanthracene derivatives from the leaves of Harungana madagascariensis. J Nat Prod 70:600–603
Lim CK, Subramaniam H, Say YH, Jong VY, Khaledi H, Chee CF (2015) A new chromanone acid from the stem bark of Calophyllum teysmannii. Nat Prod Res 29:1970–1977
Lim CK, Tho LY, Lim CH, Lim YM, Shah SAA, Weber JFF (2011) Synthesis and SAR study of prenylatedxanthone analogues as HeLa and MDA-MB-231 cancer cell inhibitors. Lett Drug Des Disc 8:523–528
Lin CM, Huang ST, Lee FW, Kuo HS, Lin MH (2006) 6-Acyl-4-aryl/alkyl-5,7-dihydroxycoumarins as anti-inflammatory agents. Bioorg Med Chem 14:4402–4409
López-Pérez JL, Olmedo DA, Olmo E, Vásquez Y, Solís PN, Gupta MP, Feliciano AS (2005) Cytotoxic 4-phenylcoumarins from the leaves of Marila pluricostata. J Nat Prod 68:369–373
Low SY, Tan BS, Choo HL, Tiong KH, Khoo AS, Leong CO (2012) Suppression of BCL-2 synergizes cisplatin sensitivity in nasopharyngeal carcinoma cells. Cancer Lett 314:166–175
Mah SH, Ee GCL, Teh SS, Sukari MA (2015) Calophyllum inophyllum and Calophyllum soulattri source of anti-proliferative xanthones and their structure–activity relationships. Nat Prod Res 29:98–101
Mai CW, Pakirisamy P, Tay EF, Subramaniam S, Shamsuddin ZH, Pichika MR (2009) Nasopharyngeal carcinoma cell proliferation and apoptosis induced by the standardised ethanolic extracts of Mucuna bracteata. Malays J Chem 11:143–148
Mai CW, Yaeghoobi M, Abd-Rahman N, Kang YB, Pichika MR (2014) Chalcones with electron-withdrawing and electron-donating substituents: anticancer activity against TRAIL resistant cancer cells, structure-activity relationship analysis and regulation of apoptotic proteins. Eur J Med Chem 77C:378–387
Morel C, Séraphin D, Teyrouz A, Larcher G, Bouchara JP, Litaudon M, Richomme P, Bruneton J (2002) New and antifungal xanthones from Calophyllum caledonicum. Planta Med 68:41–44
Oliveira MC, Lemos LMS, de Oliveira RG, Dall’Oglio EL, de Sousa Júnior PT, de Oliveira Martins DT (2014) Evaluation of toxicity of Calophyllum brasiliense stem bark extract by in vivo and in vitro assays. J Ethnopharmacol 155:30–38
Pedro M, Cerqueira F, Sousa ME, Nascimentoa MSJ, Pinto M (2002) Xanthones as inhibitors of growth of human cancer cell lines and their effects on the proliferation of human lymphocytes in vitro. Bioorg Med Chem 10:3725–3730
Plattner RD, Spencer GF, Weisleder D, Kleiman R (1974) Chromanone acids in Calophyllum brasiliense seed oil. Phytochemistry 13:2597–2602
Reutrakul V, Chanakul W, Pohmakotr M, Jaipetch T, Yoosook C, Kasisit J, Napaswat C, Santisuk T, Prabpai S, Kongsaeree P, Tuchinda P (2006) Anti-HIV-1 constituents from leaves and twigs of Cratoxylum arborescens. Planta Med 72:1433–1435
Sandjo LP, Foster AJ, Rheinheimer J, Anke H, Opatz T, Thines E (2012) Coumarin derivatives from Pedilanthus tithymaloides as inhibitors of conidial germination in Magnaporthe oryzae. Tetrahedron Lett 53:2153–2156
Saraste A, Pulkki K (2000) Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res 45:528–537
Shen T, Zhang L, Wang YY, Fan PH, Wang XN, Lin ZM, Lou HX (2012) Steroids from Commiphora mukul display antiproliferative effect against human prostate cancer PC3 cells via induction of apoptosis. Bioorg Med Chem Lett 22:4801–4806
Sousa GF, Duarte LP, Alcantara AFC, Silva GDF, Vieira-Filho SA, Silva RR, Oliveira DM, Takahashi JA (2012) New triterpenes from Maytenus robusta: structural elucidation based on NMR experimental data and theoretical calculations. Molecules 17:13439–13456
Taher M, Attoumani N, Susanti D, Ichwan SJA, Ahmad F (2010) Antioxidant activity of leaves of Calophyllum rubiginosum. Am J Appl Sci 7:1305–1309
Tan BS, Kang O, Mai CW, Tiong KH, Khoo AS, Pichika MR, Bradshaw TD, Leong CO (2013) 6-Shogaol inhibits breast and colon cancer cell proliferation through activation of peroxisomal proliferator activated receptor gamma (PPARgamma). Cancer Lett 336:127–139
Viswanathan MB, Ananthi JDJ, Kumar PS (2012) Antimicrobial activity of bioactive compounds and leaf extracts in Jatropha tanjorensis. Fitoterapia 83:1153–1159
Wu YP, Zhao W, Xia ZY, Kong GH, Lu XP, Hu QF, Gao XM (2013) Three new xanthones from the stems of Garcinia oligantha and their anti-TMV activity. Phytochem Lett 6:629–632
Ziegler U, Groscurth P (2004) Morphological features of cell death. News Physiol Sci 19:124–128
Acknowledgments
This work was financially supported by the UTAR Research Fund (Project No. IPSR/RMC/UTARRF/2013-C2/L09). The authors would also like to thank Mr. Tinjan Anak Kuda for authentication of the plant material, and the Cancer and Stem Cell Research Centre of International Medical University (IMU) for technical supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Lim, C.K., Hemaroopini, S., Gan, S.Y. et al. In vitro cytotoxic activity of isolated compounds from Malaysian Calophyllum species. Med Chem Res 25, 1686–1694 (2016). https://doi.org/10.1007/s00044-016-1606-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1606-y