A new bioactive naphthalene glucoside, named neanoside C (1), was isolated from the aerial part of Neanotis wightiana. The structure of neanoside C (1) was elucidated as 1,4-dihydroxy-2-(6′-Omethylglucopyranosyloxymethyl)-naphthalene-3-vinyloxyglucopyranoside by interpretation of the spectroscopic data, including 2D NMR and chemical studies. Neanoside C (1) was further subjected to screening for anticancer potential using MTT based cytotoxicity assay, LDH leakage assay, and estimation of intracellular ROS production in two human breast cancer cell line. The compound exhibited promising anti-proliferative and cytotoxic effect against MCF-7 and MDA-MB-231 cell lines with an IC50 value of 22.77 nM and 35.71 nM, respectively. Neanoside C (1) also exhibited concentration-dependent intracellular LDH leakage and ROS production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Neanotis, a class of shrubs belonging to the Rubiaceae family, is distributed in tropical and subtropical Asian countries [1]. Neanotis wightiana (Wall. ex Wight & Arn.) W.H. Lewis [syn. N. wightiana var. compressa (Wall. ex G. Don) W. H. Lewis, Hedyotis wightiana Wall.], a perennial herb, grows wildly in the hilly areas of eastern Himalaya, Assam, Arunachal Pradesh, and Tripura in India [1]. Traditionally, the plant has been used for the treatment of liver disorders [1]. Earlier studies on the aerial part of the plant reported the presence of a hepatoprotective triterpene glycoside named neanoside A along with oleanolic acid, ursolic acid, β-sitosterol and its glucoside, stigmasterol and its glucoside, and hexacosanoic acid [2], and a hepatoprotective naphthalene diglucoside named neanoside B along with the known triterpenoids oleanolic acid and ursolic acid [3]. In continuation of our search for more bioactive constituents of this plant, we have isolated a new bioactive naphthalene glucoside, named neanoside C (1), from the n-BuOH fraction of the MeOH extract from the aerial part of the plant. A few reports have so far been published on the bioactivity of compounds from the naphthalene glucoside group. Naphthalene glucosides isolated from Rumex dentatus have shown anti-proliferative activities against four different cancer cell lines [4]. They have shown growth inhibitory activity against oral pathogens [5]. Nevertheless, this communication exclusively focused on the structure elucidation of neanoside C (1) and screening of its antiproliferative and cytotoxic potential against different cancer cell lines. It is believed that the results of this study will open up new directions for further investigations on the development of novel pharmacophores.
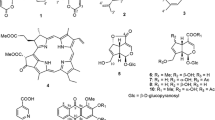
The n-BuOH fraction was subfractionated on Diaion HP 20 CC; the subfractions H2O–MeOH (1:1, 1:3) were almost identical in TLC. The subfractions were mixed together and subjected to repeated CC over silica gel to get 1 (55 mg) as light orange needles from EtOAc–MeOH (10:1) eluate, mp 246–247°C. The HR-ESI-MS of compound 1 (Fig. 1) exhibited a sodium adduct [M + Na]+ ion at m/z 593.1845 (calcd for C26H34O14Na, 593.1846), from which 10 degrees of unsaturation were deduced. The UV spectrum in MeOH showed absorption maxima at λmax 271, 282 sh, 307, and 320 nm, characteristic of the naphthalene chromophore [6]. The IR spectrum showed absorptions for hydroxyl (3380 cm–1), olefinic (1650 cm–1), aromatic (1605 and 1550 cm–1), and glycosidic (1070 and 1055 cm–1) functional groups. The 1H NMR (Table 1) showed an AA′BB′ coupling system [δ 8.48 (br.d, J = 7.8 Hz), 8.42 (br.d, J = 7.8 Hz), 7.45 (td, J = 7.8, 1.2 Hz), and 7.41 (td, J = 7.8, 1.2 Hz)] assigned to a 1,2-disubstituted phenyl group and ortho-coupled proton signals of a vinyloxy system [δ 6.70 and 5.78 (each 1H, d, J = 8.0 Hz)] [7]. These observations suggested that one of the aromatic rings of the naphthalene system is unsubstituted and the other ring contains a vinyloxy substituent. One oxygen bearing methylene proton signal at δ 5.37 and 5.20 (each 1H, d, J = 14.2 Hz) and two anomeric sugar proton signals at δ 4.56 and 4.64 (each 1H, d, J = 7.8 Hz) indicated the presence of a hydroxymethyl group attached to a sugar moiety. The anomeric protons [δH 4.56 and 4.64 (each 1H, d, J = 7.8 Hz)] and other methine proton signals (δ 3.03–3.46 m) were characteristic of glucose. The high chemical shifts of oxymethylene protons (δ 5.37 and 5.20) suggested its location in naphthalene ring adjacent to a hydroxyl or oxygen bearing substituent.
The two hydroxyl proton signals at δ 5.72 and 5.80 (each 1H, s) disappeared on addition of D2O, indicating the presence of two nonchelated hydroxyl groups in the naphthalene nucleus. All the 1H NMR spectral data suggested that one of the aromatic rings of the naphthalene system is unsubstituted and another is tetrasubstituted having two hydroxyl, one glucosyloxymethyl, and one glucosyloxyvinyl substituent. The 13C NMR distortionless enhancement by polarization transfer (DEPT) and heteronuclear single quantum coherence (HSQC) spectrum showed 26 carbon signals (Table 1). Out of the 26 carbon signals, DEPT experiments revealed one methoxy, three methylenes, 16 methines, and six quaternary carbons. The carbon signals with chemical shifts of δ 100.7, 105.0, 76.8, 76.7, 76.5, 76.4, 74.2, 74.0, 69.9, 69.7 (each methine carbon), 63.7, and 61.0 (each methylene carbon) indicated the presence of two glucosyl moieties [8]. One methoxy carbon signal at δ 56.6 (3H, s) and methylene protons signals at δ 4.97 and 4.10 suggested the presence of a methyl ether function at the C-6 position of a glucose moiety. The cross-peaks between oxymethylene protons (δ 4.97 and 4.10) with methoxy carbon (δ 56.6) in the heteronuclear multiple bond connectivity (HMBC) spectrum also supported this assignment. Furthermore, the strong cross-peaks of one anomeric proton (δ 4.56) with oxymethylene carbon (δ 61.2) and another anomeric proton (δ 4.64) with a vinyloxy carbon (δ 146.8) in the HMBC spectrum suggested that the compound contains two sugar moieties and the connectivity of one glucose moiety to oxymethylene carbon and another to the vinyloxy carbon (Fig. 1). The two glucose units were deduced to be in the β-configuration on the basis of their large coupling constants (3J = 7.8 Hz, each) from H-1′ to H-2′ and from H-1′′′ to H-2′′′. ESI-MS of 1 recorded strong mass ions at m/z 561 [M + Na – MeOH]+ and 416 [593 – 177]+ by the facile loss of methanol and glucosyl methyl ether, supporting the presence of a glucosyl methyl ether moiety in the molecule. The mass spectrum also recorded a mass ion at m/z 430 [593 – 163]+, corroborating the presence of another glucosyl moiety in 1. The geometrical configuration of 1 was determined by nuclear Overhauser effect spectroscopy (NOESY). From the NOESY spectrum, the oxymethylene proton δ 5.37 (H-11) displayed a nuclear Overhauser effect (NOE) with the phenolic hydroxyl proton δ 5.72 (1-OH), and this confirmed the position of the hydroxymethyl group at C-2 of the naphthalene nucleus. Similarly, the NOESY correlation between the oxymethylene proton δ 5.37 (H-11) and anomeric proton δ 4.56 (H-1′) confirmed the presence of a glucose moiety. Moreover, the NOESY correlation between the vinylic proton δ 6.70 (H-2′′) and anomeric proton δ 4.64 (H-1′′′) also supported the presence of another glucose moiety. Acid hydrolysis of compound 1 with 2 M methanolic HCl afforded D-glucose, detected by co-TLC with an authentic sample and study of the positive specific rotation. The 1H and 13C NMR data of compound 1 were very similar to the previously reported compound 1,4-dihydroxy-2-(methoxymethyl)-naphthalen-3-yl-methyl-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside [3], except for the presence of a vinyl group located at C-1′′ (Fig. 1). On the basis of the above evidence, the structure of 1 was assigned as 1,4-dihydroxy-2-(6′-O-methylglucopyranosyloxymethyl)-naphthalene-3-vinyloxyglucopyranoside, named neanoside C. It is a new metabolite. This is the first report of a 1,4-naphthoquinol glucoside having a vinyloxy substituent from the genus Neanotis.
The cytotoxic property of neanoside C (1) was assessed by the MTT assay. In this assay the reduction of cationic tetrazolium salt by the cellular oxidoreductase enzyme of viable cells produces a purple color that can be measured spectrophotometrically [9]. It was observed that proliferation of MCF-7 and MDA-MB-231 cells treated with various concentrations of the test compound neanoside C (1) was inhibited in a concentration-dependent manner (Fig. 2). After 24 h incubation of neanoside C with MCF-7 and MDA-MB-231 cell lines, a significant reduction in the number of living cells was found.
The highest concentration of neanoside C 100 nM killed almost 84.34 ± 3.51% cells of MCF-7 and 76.34 ± 5.033% cells of MDA-MB-231, respectively, after 24 h incubation, which was significant (*p < 0.001) when compared with control. However, the cytotoxic effect of neanoside C (1) at a concentration of 100 nM was found to be comparable to the standard drug cisplatin (10 μM) with no significant (*p < 0.001) difference (Fig. 2). The IC50 value of the isolated compound neanoside C (1) against MCF-7 and MDA-MB-231 cell lines was found to be 22.77 nM and 35.71 nM, respectively.
LDH leakage assay was performed to give complementary support to prove the cytotoxic and growth inhibitory effect of neanoside C (1) against MCF-7 and MDA-MB-231 cell lines. Intracellular lactate dehydrogenase is a cytosolic enzyme that is a stable marker of cellular damage [10]. In the current study, neanoside C (1) exhibited concentration- dependent (6.25–100 nM) LDH release after incubation for 24 h in the case of both MCF-7 and MDA-MB-231 cell lines (Fig. 3). Compared to control cells, neanoside C (1) treated cells demonstrated substantial LDH leakage, which was found statistically significant (**p < 0.001). The LDH leakage in neanoside C (1) at a concentration of 100 nM was found to be almost similar to the standard drug cisplatin (10 μM) in the treated cells (Fig. 3). Significant LDH leakage observed against both breast cancer cell lines confirms the cytotoxicity.
Intracellular ROS is known to be one of the most important mediators of cell signalling cascades. Cellular dysfunction and apoptosis can be triggered with the excessive generation of ROS, which induces oxidative stress [11]. Here, in our study we have treated the breast cancer cells (MCF-7 and MDA-MB-231) with different concentrations (6.25–100 nM) of the test compound neanoside C (1) for 24 h and estimated the level of ROS by measuring the intensity of DCF, which is the degradation product of the DCF-DA fluorescence probe. We observed a significant (**p < 0.001) concentration-dependent increase in the intensity of DCF fluorescence, which indicated the generation of cellular oxidative stress that leads to cytotoxicity (Fig. 4). However, the detailed mechanism and pathways involved in the cell death needs to be studied to confirm the molecular insight.
Experimental
General Experimental Procedures. Melting point, Kofler type melting point apparatus and uncorrected; UV-visible absorption spectra, Perkin Elmer Lambda 25 spectrometer; IR spectra, Perkin Elmer FTIR-100 spectrometer; 1D (1H and 13C NMR) and 2D NMR (COSY, NOESY, HSQC, and HMBC) spectra, Bruker 600 AVANCE spectrometer; Chemical shifts, ppm, with TMS as internal standard; ESI-MS spectra, Jeol JMS-HX 110 instrument; CC, silica gel (60–120 mesh, Merck, India) and Diaion HP-20 (Sigma-Aldrich, India); TLC, silica gel G (Merck, India).
Plant Material. The aerial part of Neanotis wightiana was re-collected from Kalsi (Jolaibari), South Tripura in March, 2017. The plant was identified by Prof. B. K. Datta, Plant Taxonomist, Department of Botany, Tripura University on the basis of the study of the morphology of floral and other aerial parts as well as DNA sequencing study of ovule cells.
A voucher specimen (No. BD/02/08) has been deposited at the National Herbarium, Shibpur Botanical Garden, Howrah, India, at the time of previous investigation of this plant [2].
Extraction and Isolation. Air-dried powder aerial part of N. wightiana (3 kg) was extracted three times with MeOH (10 L × 3) for 1 week each at room temperature. The MeOH extract was concentrated to a semisolid mass (~ 400 g). The residue was suspended in H2O (~ 125 mL) and extracted with hexane, chloroform, ethyl acetate, and n-BuOH (each 3 × 150 mL). After partitioning, we obtained 50 g hexane, 50 g chloroform, 100 g ethyl acetate, and 100 g n-BuOH extracts. The n-BuOH fraction (100 g) was subfractionated on Diaion HP 20 CC with the solvent systems H2O, H2O–MeOH (3:1, 1:1, 1:3), and MeOH (each 300 mL for three times). The residues obtained from H2O–MeOH (1:1, 1:3) were almost identical in TLC. These subfractions were mixed together and subjected to repeated CC over silica gel to get 1 (55 mg) as light orange needles from the EtOAc–MeOH (10:1) eluate, mp 246–247°C.
Neanoside C (1) [1,4-dihydroxy-2-(6′-O-methylglucopyranosyloxymethyl)-naphthalene-3-vinyloxyglucopyranoside]. Light orange needles, mp 246–247°C. IR (KBr, νmax, cm–1): 3380, 1650, 1615, 1550, 1070, 1055. UV (MeOH, λmax, nm): 271, 285 sh, 303, 317. For 1H and 13C NMR data, see Table 1. HR-ESI-MS m/z 593.1845 [C26H34O14Na]+, 561, 430, 416.
Cell Culture. Breast cancer cell lines (MCF-7 and MDA-MB-231) were obtained from National Cell Repository, NCCS, Pune, India and routinely cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin as well as streptomycin in a humidified incubator at 37°C along with 5% CO2.
Cell Growth Inhibition Assay. The cell growth inhibition potential of neanoside C (1) was measured using MTT assay [12]. Briefly, MCF-7 and MDA-MB-231 cells were cultured in 96-well plates at a density of 1 × 104 cells per well. Neanoside C (1) was dissolved in DMSO (10 mmol stock solution) and further diluted to make different concentrations (6.25–100 nM) in serum-free culture media. In the working test compound solution, the DMSO concentration was kept less than 0.5%. Cisplatin was used as a standard anticancer drug to compare the effect of neanoside C (1). Cells were incubated with different concentrations of neanoside C (1) for 24 h in a humidified CO2 incubator. After the incubation period, the supernatant media was flicked off, 20 μL of freshly prepared MTT (2.5 mg/mL in phenol red-free media) solution was added, and the cells were incubated for 4 h at 37°C. After incubation, 100 μL of DMSO was added to solubilize the MTT crystals, and the absorbance was measured at 570 nm in a UV-based six-well plate reader (Multiskan GO, Thermo, Germany).
LDH Leakage Assay. LDH release assay was performed according to the established method using an LDH assay kit [13]. Briefly, MCF-7 and MDA-MB-231 cells (1 × 104 cells/well) were pretreated with different concentrations of neanoside C (1) (6.25–100 nM prepared as above) for 24 h, and 100 μL of cell-free supernatant was then transferred into 96-well culture plates. Then 100 μL of substrate mixture from the kit containing catalyst diaphorase, NAD+, and dye solution (iodonitrotetrazolium chloride and sodium lactate) was mixed into each well and the whole warmed for an additional 25 min at 37°C. Absorbance was measured at 440 nm, which was directly proportional to the LDH released in culture media and is expressed as LDH activity (IU/L). The standard anticancer drug cisplatin (10 μM) was taken as positive control. LDH leakage with cisplatin was considered as 100% and compared with the LDH leakage effect of the test compound neanoside C (1).
Estimation of Intracellular ROS. The amounts of ROS were measured using the fluorescent probe DCFH-DA [14]. The cultured cells MCF-7 and MDA-MB-231 were plated in 96-well plates at a concentration of 1 × 104 cells/well to each well and incubated with various concentrations (6.25–100 nM) of neanoside C (1) for 24 h. After the incubation period, 20 μM of DCFH-DA was added to each well at 37°C for 1 h. The fluorescence intensities of the intracellular ROS were measured by a fluorescence plate reader (Spectramax I3x, Molecular Devices, USA) at excitation wavelength 485 nm and emission wavelength 530 nm.
Statistical Analysis. All statistical analyses were performed using the GraphPad Prism 5.0 statistical software. The significance was calculated using one-way analysis of variance (ANOVA) followed by Tukey′s test. A value of *p < 0.05, ***p < 0.001 was considered statistically significant. Results were expressed as means ± SD of three repeated experiments.
In the present study neanoside C (1), a compound of naphthalene glucoside group, was isolated from the MeOH extract of the aerial part of Neanotis wightiana. It was also demonstrated for the first time that neanoside C (1) exerts significant anti-proliferative and cytotoxic activity against two breast cancer cell lines, MCF-7 and MDA-MB-231, in a concentration-dependent manner. These findings indicate that neanoside C (1) may cause simultaneous injury of both cell compartments, thus producing anti-proliferative and cytotoxic activity against MCF-7 and MDA-MB-231 cancer cells. From the experimental evidence it is clearly seen that neanoside C (1) has significant cytotoxic and growth inhibitory potential against breast cancer cell lines, namely MCF-7 and MDA-MB-231, which can be further studied in-depth to understand the molecular mechanism involved. This molecule may also promote a newer arena of naphthalene glucoside-based synthetic research with respect to their anticancer activity in both cell and animal models against cancer and can be the subject of further study toward the development of anticancer molecules for breast cancer therapy.
References
D. B. Deb, The Flora of Tripura State, Vol. II, Today & Tomorrow′s Printers and Publishers, New Delhi, 1983, p. 72.
N. Das, P. S. Ghosh, M. C. Das, and B. Dinda, Phytochem. Lett., 6, 270 (2013).
N. Das, A. G. Atanasov, P. K. Deb, A. Mocan, S. M. Nabavi, R. Ghosh, and B. Dinda, Phytomedicine, 33, 14 (2017).
H. Zhang, Z. Guo, N. Wu, W. Xu, L. Han, N. Li, and Y. Han, Molecules, 17, 843 (2012).
J. F. Rivero-Cruz, M. Zhu, A. D. Kinghorn, and C. D. Wua, Phytochem. Lett., 1, 151 (2008).
C. N. Lin and B. L. Wei, Phytochemistry, 33, 905 (1993).
H. A. Hassanean, Z. Z. Ibraheim, K. Takeya, and H. Itorawa, Pharmazie, 55, 317 (2000).
K. Kamiya, W. Hamabe, S. Tokuyama, and T. Satake, Fitoterapia, 80, 196 (2009).
S. Edrini, A. Rahmat, P. Ismail, and T. Y. Y. Hin, J. Med. Sci., 2, 194 (2002).
D. B. Mitchell, K. S. Santone, and D. Acosta, J. Tissue Cult. Meth., 6, 113 (1980).
A. F. G. Slater, C. Stefan, I. Nobel, D. J. Van Den Dobbelsteen, and S. Orrenius, Toxicol. Lett., 82, 149 (1995).
T. Mosmann, J. Immunol. Meth., 65, 55 (1983).
E. Y. Kim, T. W. Chung, C. W. Han, S. Y. Park, K. H. Park, S. B. Jang, and K. T. Ha, Sci. Rep., 9, 3969 (2019).
D. Wu and P. Yotnda, J. Vis. Exp., 57, 3357 (2011).
Acknowledgment
The authors are thankful to Prof. B. K. Datta, Department of Botany, Tripura University, India for identification of the plant. The authors wish to thank Prof. B. Dinda (Retd.), Department of Chemistry, Tripura University, India for support, co-operation and encouragement during the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2022, pp. 23–27.
Rights and permissions
About this article
Cite this article
Das, N., Deb, P.K., Ali, E.S. et al. Anti-Proliferative Naphthalene Glucoside from Aerial Part of Neanotis wightiana. Chem Nat Compd 58, 21–26 (2022). https://doi.org/10.1007/s10600-022-03591-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03591-3