Abstract
Conventional urothelial carcinoma is the most common histological type of urinary bladder carcinoma. The latest edition of the WHO classification of tumours of the urothelial tract lays special emphasis on the ability of urothelial tumours to exhibit divergent differentiation with multiple histologic variants and a diverse genomic landscape. The presence of a micropapillary component (MPC) in urothelial carcinoma is associated with high-grade disease and poor response to intravesical chemotherapy. The present study aims to enumerate the clinicohistological features of urothelial carcinomas with micropapillary differentiation. Slides from 144 radical cystectomy specimens received over 6 years were reviewed independently by two pathologists. A predominant histological pattern along with co-existing pathology was noted. Of these, five cases were pure micropapillary carcinomas, four had conventional urothelial carcinoma with a MPC, one had a microscopic tumour at the mucosal surface, and two cases showed micropapillary histology in the lymph node metastasis, following transurethral resection of bladder tumour and Bacillus Calmette-Guerin therapy. The tumours with pure micropapillary carcinoma presented with a higher pathological stage and poor overall survival. Organ and lymph node metastasis was noted in five and eight cases, respectively, of which six showed a micropapillary pattern in the lymph nodes. Micropapillary urothelial carcinoma is a rare and aggressive variant of urothelial carcinoma with unique histologic features. This variant is often missed and underreported in biopsy and surgical resection specimens. Since the presence of MPC confers a poorer prognosis, the identification and reporting of this entity are important.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary bladder carcinoma is the tenth most commonly diagnosed malignancy worldwide, accounting for approximately 0.57 million new cases (3% of all cancers) and 0.21 million deaths annually (2.1% of all cancer-related deaths) [1]. It is mainly seen in the elderly and commonly presents as gross, painless haematuria with or without clots, increased frequency, and dysuria. Conventional urothelial carcinoma (transitional cell carcinoma) is the most common histological type of urinary bladder carcinoma, which accounts for about 90 and 80% of bladder cancers in developed countries and other parts of the world, respectively [2].
The latest edition of the WHO classification of tumours of the urothelial tract gives a review of the morphology of urothelial neoplasms, with special emphasis on their commendable ability to exhibit divergent differentiation, multiple morphologic variants, and a diverse genomic landscape [2]. Invasive micropapillary carcinoma (IMPC) of the bladder was initially described by Amin et al. in 1994 as an infrequent aggressive morphological variant of urothelial carcinoma [3, 4]. Since then, it has been increasingly recognised as a distinct entity from conventional urothelial carcinoma. IMPC is a rare variant of urothelial carcinoma, accounting for approximately 0.2–8.2% of cases [3]. The presence of a micropapillary component (MPC) in urothelial carcinoma was found to be associated with high-grade and high-stage disease, aggressive clinicopathologic features, including lymphovascular invasion, lymph node metastasis, and poor response to intravesical chemotherapy [3, 5]. Other forms of urothelial carcinoma, such as plasmacytoid, sarcomatoid, small cell, and other variants may also be associated with these unfavourable prognostic features. This high-grade variant morphology should be carefully reported during the examination of a bladder biopsy or cystectomy specimen, as it can modify prognosis and management strategies [3]. Various clinical trials have been conducted evaluating therapeutic strategies for micropapillary carcinoma. Radical cystectomy remains the treatment of choice for IMPC at all stages, with a limited and controversial role for neoadjuvant chemotherapy (with > cT2 disease) to potentially downstage disease before radical cystectomy [3, 6,7,8].
The exact immunohistochemical features and clinical behaviour of IMPCs are still not well-established due to the limited availability of information in the published literature, as most of the information is in the form of either a single case report or short series [4, 5, 9, 10]. The present study describes the histomorphological characteristics and clinicopathological correlation of twelve cases of invasive IMPCs/urothelial carcinomas with micropapillary differentiation (UCMD).
Methods
This was a retrospective study. Radical cystectomy specimens received in the department of pathology between January 2015 and August 2021 (total cases 144) were grossed according to standard grossing protocol. Haematoxylin & eosin (H&E)-stained sections from each case were reviewed independently by two pathologists for detailed morphological analysis including tumour type, tumour percentage, depth of invasion, lymphovascular and perineural invasion, lymph node metastasis and histology of tumour in lymph nodes involved by tumour. Any interobserver disagreement was resolved by mutual consensus by using a multiheaded microscope. A total of twelve cases out of 144 were identified as having IMPCs/UCMD. The clinical details including the survival data were also noted. Follow-up information was obtained by reviewing medical records from Hospital Information System. Individual follow-up was calculated as the number of months from the date of cystectomy to death or the most recent clinical follow-up at which time patients were found to be alive, with no evidence of disease.
Results
Out of twelve cases, eight were male and four were females (M:F 2:1) with ages ranging from 45 to 73 years (mean age: 61.9 years) (Table 1). All patients presented with a combination of symptoms with the most common presenting complaint being gross painless haematuria (n = 9) and dysuria (n = 8) followed by increased frequency (n = 5) and urgency. All male patients underwent radical cystoprostatectomy while one of them also received neoadjuvant chemotherapy. Two of the female patients underwent radical cystectomy with a total abdominal hysterectomy and bilateral salpingo-oophorectomy and two underwent radical cystectomy. Two patients received intravesical BCG therapy. A history of smoking was present in three patients, all were male.
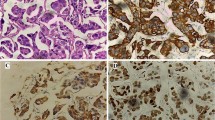
On gross examination, nine patients had tumours located at the lateral and posterolateral walls. The tumour was located at the anterior wall, dome (with extension to the anterior wall), and diverticula in one case each. Maximum tumour size ranged from 0.3 to 6.5 cm. The most common growth pattern grossly was proliferative (n = 7), whereas thickened whitish scar-like area was seen in two cases, and ulcerative, ulceroproliferative, and endophytic growth was seen in one case each. On histological examination, five cases had pure micropapillary components and did not show any component of conventional urothelial carcinoma. The characteristic micropapillary architecture was identified as delicate filiform projections on the mucosal surface and invasive small tight cell nests or balls contained in lacunae or stromal retraction spaces, mimicking lymphovascular invasion (LVI) (Fig. 1A). The tumour cells showed high-grade features with reversed polarity to the external surface of tumour nests. The tumour cells at places contained clear cytoplasm, imparting a signet-ring cell type appearance (Fig. 1B). Four cases had predominant components as conventional urothelial carcinoma with micropapillary carcinoma components ranging from 5 to 15%. One case had predominantly plasmacytoid components (~ 80%) (Fig. 1C) with conventional urothelial carcinoma (10%) and micropapillary carcinoma (10%) components. Of these four cases, one also had a minor neuroendocrine component (~ 10%) (Fig. 1D). In one case, the tumour was present at the mucosal surface as slender, delicate processes devoid of a fibrovascular core and appeared as glomeruloid bodies on cross-section (Fig. 2A). Two cases had tumour recurrences twice following transurethral resection of bladder tumour and Bacillus Calmette-Guerin therapy. Grossly, both of them showed irregular whitish scar-like areas, whereas histologically, both had chiefly micropapillary patterns in lymph node metastasis (Fig. 2B), whereas the primary specimen showed a minimal amount of residual tumour with areas of conventional urothelial carcinoma (and squamous differentiation in one of them). Lymphovascular invasion was identified in six cases (Fig. 2C), and perineural invasion was seen in three cases. Lymph node metastasis was seen in eight cases, of which six cases showed micropapillary patterns in the lymph nodes (Fig. 2D). The remaining two cases showed the morphology of conventional urothelial carcinoma in the lymph nodes. We also documented associated histological findings in the urinary bladder, prostate, and another organ (if simultaneously resected) and found two cases with granuloma in the bladder (cases 4 and 9), giant cell reaction (case 8), contiguous prostatic involvement by tumour (cases 1 and 3), prostatitis (case 12), and squamous cell carcinoma of the cervix in one patient (case 10), who underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy.
A Photomicrograph showing a micropapillary tumour composed of invasive small tight cell nests or balls contained in lacunae. The tumour cells exhibit high-grade features with reversed polarity to the external surface of tumour nests (H&E, × 10). B Areas with tumour cells containing clear cytoplasm, imparting a signet-ring cell type appearance (H&E, × 20). (C) Plasmacytoid areas with tumour cells containing eccentrically placed nuclei and a moderate amount of eosinophilic cytoplasm [case 6] (H&E, × 20). (D) Neuroendocrine component—tumour cells are arranged in a fused glandular pattern and rosettes [case 2] (H&E, × 10)
A Tiny tumour nodule present at the mucosal surface as small villiform papillary projections [case 9] (H&E, × 4). B Lymph node showing metastatic deposits by micropapillary carcinoma [case 10] (H&E, × 4). C Section showing lymphovascular emboli (H&E, × 40). D Focus of perineural invasion where tumour cells can be seen around the nerve bundles (H&E, × 10)
Higher stage (pT3 and pT4) was seen in five cases, of which four had > 95% of micropapillary carcinoma component. Two cases were in stage pT2, whereas five cases were in stage pT1, including one who had received neoadjuvant chemotherapy. Distant metastases occurred in five patients: the liver (two), rectum (one), rectus abdominis muscle (one), and iliac bone (one). Follow up period after cystectomy ranged from 15 days to 40 months. Follow-up data revealed that five patients died, of which two had distant metastasis, one patient died of another cause, three patients are alive with the disease with the presence of distant metastasis, and three patients are alive and well. Three patients with > 95% micropapillary component presented at a higher stage (III and IV). Two cases with another variant (neuroendocrine and plasmacytoid) morphologies also died of disease after 15 days and 11 months, respectively.
Discussion
Micropapillary urothelial carcinoma is often present at a higher stage and is recognised as an infrequent and aggressive variant of urothelial carcinoma [3,4,5]. Most of the urinary bladder carcinomas histologically reveal conventional papillary urothelial morphology. However, different variant morphologies are described in WHO 4th edition like urothelial carcinoma with squamoid differentiation, glandular differentiation, trophoblastic differentiation, nested variant, microcystic variant, micropapillary variant, plasmacytoid variant, sarcomatoid variant, some others [2]. Recognition of these variants is important as they are rare tumours and have prognostic significance. The first description of micropapillary urothelial carcinoma in the published literature was by Amin et al. in 1994 [4]. They reported a total of 18 cases, of which 14 had undergone radical cystectomy [4]. They described several morphological pointers for the recognition of micropapillary variant: (1) a filiform architecture usually seen in the surface component or small, tight aggregates of tumour cells usually seen in invasive and metastatic components, (2) the absence of psammoma bodies, a characteristic feature of serous papillary carcinoma of the ovary, and (3) presence of lacunae surrounding the tumour cells mimicking lymphovascular invasion. In this case series, all cases showed these characteristic pointers of pattern recognition. Retraction lacunae are carefully distinguished from lymphovascular invasion by the absence of a recognisable endothelial lining or lack of a cellular component of blood.
The micropapillary pattern can be present either focally or as the major pattern and is commonly accompanied by conventional urothelial carcinoma [5, 11, 12]. No cut-off criteria have been established to date to quantify the proportion of MPC. In a large series, Compérat et al. studied the proportion of MPC and clinicopathological correlation in 72 patients diagnosed with transurethral resections of the bladder and concluded that the presence of any quantity of MPC signifies a poor outcome [13]. Similarly, Samaratunga et al. in a clinicopathological and immunohistochemical study of 20 patients with MPC and found that cases with moderate or extensive MPC are at high risk of being advanced at presentation and cases with < 10% MPC and surface MPC have a high chance of detection at an early stage. They suggested that a higher proportion of MPC is related to further dismal clinical outcomes [5]. In this study, we also recorded > 95% micropapillary component in four out of five cases that presented at a higher stage. Therefore, it is recommended to report the presence and the proportion of the MP component in the histopathology report.
Micropapillary carcinoma may also be admixed with other patterns including squamoid differentiation, glandular differentiation, mucin production11, small cell carcinoma, sarcomatoid carcinoma, pleomorphic giant cell carcinoma, lipoid variant, or plasmacytoid variant of urothelial carcinoma [12]. Here, in five cases, micropapillary carcinoma was the predominant pattern (> 95%), whereas in the rest of the five, it was identified focally as a minor component, and in two cases, it chiefly presented in lymph node metastasis after BCG therapy. The latter two (cases 11 and 12) had to undergo repeat biopsies given tumour recurrence despite BCG therapy, as the morphology could not be confidently established in the biopsy specimens. Multiple representative sections were processed from the lesion in cystectomy specimens in both of these cases. Neuroendocrine components and plasmacytoid areas were identified in two cases (2 and 6), along with micropapillary components, and these cases were associated with poor clinical outcomes. The presence of associated histological findings (for example, granuloma and giant cells) usually indicate a prior BCG therapy, whereas the involvement of the prostate or other adjacent organ by bladder carcinoma portends a poor prognosis.
Limited information is available on immunohistochemical markers for urothelial carcinoma. Immunohistochemically, GATA3 and uroplakin have greater sensitivity and specificity over CK20 and P63 in distinguishing urothelial IMPC from other invasive micropapillary carcinomas [3, 14]. Levels of GATA3 in IMPC are similar to conventional urothelial carcinoma, it has been reported to be significantly lower in other variants of urothelial carcinomas, such as squamous and sarcomatoid [14]. P63 and P40 positivity are less in IMPC as compared to other variants, similar to the plasmacytoid variant [3]. No immunohistochemical marker can reliably distinguish IMPC from other histologic subtypes of urothelial carcinoma. However, the retention of GATA3 and loss of p63 and p40 are characteristic, though not essential, features of this entity.3
It is important to differentiate between an MPUC and a high-grade TCC with extensive retraction artefact. Hui et al. described distinct peripheral membranous staining of EMA and negative staining for E-cadherin at the periphery of tumour nests in IMPC, characteristic of “inverted-polarisation,” a hallmark of invasiveness. In contrast, urothelial carcinoma with retraction artefacts shows no distinct staining with EMA and E- cadherin at the periphery of tumour nests [15]. A simple immunostain for CD34 can also be used to highlight the vessels in such cases. Similarly, Sangoi et al. evaluated the utility of MUC1, CA125, and Her2Neu to distinguish IMPC from invasive urothelial carcinomas with retraction artefact and found that IMPC more often showed reactivity for these markers compared to conventional urothelial carcinoma and that MUC1 reached statistical significance, but the specificity is low (37%) [16]. Chatterjee et al. reported clinicopathological and immunohistochemical characteristics of seven cases of micropapillary carcinoma in a case series from the Indian population and found universal positivity for CK7 and EMA in all cases, CK 20 in five cases, and HER-2 neu in four cases with no clear prognostic significance [17].
Conclusion
IMPC is a rare and aggressive variant of urothelial carcinoma with unique histologic features. Although it is an increasingly recognised entity worldwide, only a few case reports and short series have been reported in the Indian population. This study will add to the existing Indian data on the clinicopathological features of IMPC. IMPC presents with metastasis and dismal overall survival. Cases with predominant/major micropapillary component or with coexisting another high-grade variant present with a higher stage. Hence, this variant should be reported in the histopathology report whenever identified.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol 70(1):106–119
Hsu J, Ro J (2019) Micropapillary carcinoma of the bladder: recent advances. Annals Urologic Oncol 7:1–10
Amin MB, Ro JY, el-Sharkawy T, Lee KM, Troncoso P, Silva EG, Ordóñez NG, Ayala AG (1994) Micropapillary variant of transitional cell carcinoma of the urinary bladder Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 18(12):1224–32
Samaratunga H, Khoo K (2004) Micropapillary variant of urothelial carcinoma of the urinary bladder; a clinicopathological and immunohistochemical study. Histopathology 45(1):55–64
Kamat AM, Dinney CP, Gee JR, Grossman HB, Siefker-Radtke AO, Tamboli P, Detry MA, Robinson TL, Pisters LL (2007) Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer 110(1):62–7
Meeks JJ, Taylor JM, Matsushita K, Herr HW, Donat SM, Bochner BH, Dalbagni G (2013) Pathological response to neoadjuvant chemotherapy for muscle-invasive micropapillary bladder cancer. BJU Int 111(8):E325-30
Sui W, Matulay JT, James MB, Onyeji IC, Theofanides MC, RoyChoudhury A, DeCastro GJ, Wenske S (2016) Micropapillary bladder cancer: insights from the National Cancer Database. Bladder Cancer 2(4):415–423
Cheng YT, Luo HL, Sung MT, Chiang PH (2010) Micropapillary variant of urothelial carcinoma: a report of 4 cases and literature review. Chang Gung Med J 33(4):461–5
Perepletchikov AM, Parwani AV (2009) Micropapillary urothelial carcinoma: clinico-pathologic review. Pathol Res Pract 205(12):807–10
Lopez-Beltran A, Montironi R, Blanca A, Cheng L (2010) Invasive micropapillary urothelial carcinoma of the bladder. Hum Pathol 41(8):1159–64
Kwon GY, Ro JY (2011) Micropapillary variant of urothelial carcinoma. Adv Urol 2011:217153
Compérat E, Roupret M, Yaxley J, Reynolds J, Varinot J, Ouzaïd I, Cussenot O, Samaratunga H (2010) Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathology 42(7):650–4
Monn M, Cheng L (2016) Evolving concepts of micropapillary variant urothelial carcinoma. Transl Cancer Res 5(S7):S1539–S1542
Hui Y, Lombardo KA, Quddus MR, Matoso A (2018) Cell polarity reversal distinguishes true micropapillary growth from retraction artifact in invasive urothelial carcinoma. Appl Immunohistochem Mol Morphol 26(1):e1–e6
Sangoi AR, Higgins JP, Rouse RV, Schneider AG, McKenney JK (2009) Immunohistochemical comparison of MUC1, CA125, and Her2Neu in invasive micropapillary carcinoma of the urinary tract and typical invasive urothelial carcinoma with retraction artifact. Mod Pathol 22(5):660–7
Chatterjee D, Das A, Radotra BD (2015) Invasive micropapillary carcinoma of urinary bladder: a clinicopathological study. Indian J Pathol Microbiol 58(1):2–6
Author information
Authors and Affiliations
Contributions
Pallavi Prasad: conceptualisation, methodology, software, reviewing, and editing. Harshita Baranwal: Data curation and writing—original draft preparation. Vinita Agrawal: supervision, reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prasad, P., Baranwal, H. & Agrawal, V. Invasive Micropapillary Urothelial Carcinoma: an Uncommon and Underreported Variant in Cystectomy Specimens. Indian J Surg Oncol 14, 222–227 (2023). https://doi.org/10.1007/s13193-022-01692-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-022-01692-7