Abstract
Purpose of Review
This review will discuss micropapillary urothelial carcinoma with respect to biology, histopathologic characteristics, genetic and molecular features, diagnosis, clinical management, and future directions of research.
Recent Findings
Recent consensus opinion study showed only moderate interobserver reproducibility in the diagnostic criteria. The most reproducible criteria with the highest consensus were multiple nests in the same lacunar spaces. There are recent reports of high rates of intratumoral heterogeneity of ERBB2 amplification within tumor containing both micropapillary and classic urothelial components.
Summary
Micropapillary urothelial carcinoma is a well-documented highly aggressive variant of urothelial carcinoma with proven worse outcomes. Accurate recognition and reporting of this pattern is critical for optimal management. Newer therapeutic strategies related to the molecular and genetic findings seen in MPUC remain to be explored further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urothelial carcinomas, the most common histologic type of carcinoma in urinary tract, can arise in the upper urinary tract (pyelocaliceal cavities and ureter) or the lower urinary tract (bladder and urethra). The bladder is the most frequent site of malignancy in the urinary tract, constituting approximately 90–95% of all cancers of the urinary system and bladder cancer being the 10th most common malignancy in the world, with almost 550,000 new cases diagnosed worldwide in 2018 [1, 2, 3•, 4]. Urothelial carcinoma is mostly of the usual or conventional subtype but can also demonstrate a wide continuum of variant morphologies. One such distinct and clinically aggressive variant is micropapillary urothelial carcinoma (MPUC), which constitutes 0.6–2.2% of all urothelial carcinomas [5,6,7] and has histologic features resembling ovarian papillary serous carcinoma and micropapillary carcinomas arising in a wide range of other organs such as the breast, lung, colon, and stomach [8]. Tumor is characterized by slender filiform processes on the surface and/or multiple small infiltrating nests lacking fibrovascular cores, often surrounded by lacunar spaces, resembling vascular invasion in the invasive component [6, 9]. MPUC is almost always encountered alongside conventional urothelial carcinoma pattern and usually demonstrates high-grade histologic features and advanced clinical stage at diagnosis. These features include muscle invasive disease and nodal as well as distant metastases [10]. Unlike conventional urothelial carcinoma, it is difficult to detect by computed tomography (CT) scan, because it is often not apparent as a mass lesion on CT [11]. Much remains to be understood in terms of the poor prognosis and management of this aggressive variant. About half of the patients with micropapillary muscle invasive bladder carcinoma (MIBC) subsequently develop metastatic disease in spite of undergoing radical cystectomy [12•]. Similar to other cancer types, recent advances in bladder cancer therapeutics include agents that target specific molecules and pathways, including fibroblast growth factor receptor 3 (FGFR3) and immune checkpoint proteins such as programmed cell death-1 (PD-1) or programmed cell death ligand-1 (PD-L1) [13,14,15,16, 17••]. The aim of this review is to examine the unique aspects of micropapillary urothelial carcinoma with respect to biology, histopathologic characteristics, genetic and molecular features, diagnosis, clinical management, and future directions of research.
Clinical Features
Micropapillary urothelial carcinoma has a male predominance, with a male-to-female ratio of 5:1, and the peak incidence is in the 6th decade of life [6]. There is no agreement generally on the amount of micropapillary component required to make the diagnosis. Hence, the reported incidence varies in different reported series depending on the proportion of micropapillary component considered requisite for diagnosis. In early series, the reported incidence was 0.6–1.0% of urothelial carcinoma [6, 18]. Tumors in these series had at least 10% or 20% with most cases displaying greater than 50% of micropapillary carcinoma. In a more recent study, the incidence was found to be 6% [19]. Generally, in clinical practice, UC with any amount of micropapillary component is considered as MPUC. It is highly conceivable that the recent reported increase in incidence of MPUC could be due to variable percent cutoffs used for diagnosis in addition to the increased awareness of this entity among pathologists. The most common clinical presentation irrespective of upper or lower urinary tract location is hematuria.
Gross Pathology
The gross appearance of MPUC is highly variable, and there are no specific features to differentiate them from other variants of UC. The appearance may range from a noticeable ulcerated mass that may conspicuously appear malignant, to the other end of the spectrum, which is characterized by a barely visible tumor seen as granular mucosa with low suspicion of a neoplastic transformation [9, 20]. Also variable is the size of the tumor which can range from a few millimeters to several centimeters in greatest dimension. The appearance of the underlying bladder wall is determined by the extent of invasion, which is oftentimes extensive in MPUC [9]. Presence of hydronephrosis has been associated with poor prognosis and used as a risk stratifying factor in studies [21].
Histopathology
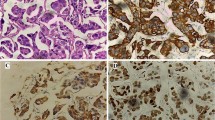
On histologic evaluation, MPUC is typically associated with conventional urothelial carcinoma [9]. Urothelial carcinoma in situ is demonstrable in more than 50% of the cases, and concurrent adenocarcinoma, carcinosarcoma, or small cell carcinoma has been known to occur [22]. MPUC demonstrates two recognizable morphologic patterns, with the more common being the invasive pattern, which shows clusters of atypical cells with high nuclear grade that form tight micropapillary structures lacking fibrovascular cores and surrounded by clear spaces or lacunae, mimicking lymphatic spaces (Fig. 1A). These retraction spaces are believed to be an artifact of fixation since they are usually not seen on frozen sections [23]. This pattern can be distinguished from retraction artifact around invasive carcinoma by the presence of multiple clusters within the same lacunar space (Fig. 1B). These empty spaces usually lack vascular features, such as an endothelial lining and cellular constituents of blood. The spaces may be lined focally by flattened spindled cells or may lack any lining [23]. Factor VIII-related antigen immunohistochemistry does not demonstrate the presence of endothelial cells, thus confirming retraction artifact rather than lymphovascular invasion [6, 11, 24]. True lymphovascular invasion is however present in most cases [25] (Fig. 1C). The second morphologic pattern is a non-invasive pattern and is made up of slender, delicate, filiform processes, rarely with a fibrovascular core (Fig. 1 D and E). On cross sections, these processes appear as glomeruloid bodies, sometimes with reversed polarity or palisading of cells. By convention, the term micropapillary carcinoma is limited to the invasive pattern only [5]. When micropapillary features are only seen as non-invasive component, the tumor must be designated as carcinoma in situ with micropapillary features or high-grade non-invasive papillary urothelial carcinoma with micropapillary features [26]. In addition, the absence of invasive carcinoma should be documented to prevent overtreatment. In the invasive component, the tumor cells are arranged in small tight nests or balls. Micropapillae are devoid of true fibrovascular cores, and this feature helps with their recognition. Micropapillary urothelial carcinoma always demonstrates a high nuclear grade [17••], although focal areas within a tumor may have cytologic features of low-grade urothelial carcinoma. Individual cells show vesicular nuclei with prominent nucleoli, moderate to marked nuclear pleomorphism, and markedly irregular chromatin with uneven distribution. The cytoplasm is voluminous and tends to be eosinophilic or clear, and there may be few to numerous mitotic figures [19]. The micropapillary clusters have cells with peripherally arranged nuclei, which is reminiscent of a rosette-like pattern. Mucin is usually absent. There may be variation of the nuclei in extent of anaplasia, but most are high grade. Psammomatous calcifications, typically present in ovarian papillary serous carcinoma, are usually absent in MPUC [9].
A Clusters of atypical cells forming tight micropapillary structures lacking fibrovascular cores and surrounded by clear spaces or lacunae, mimicking lymphatic spaces. B Multiple clusters within the same lacunar space. C True lymphovascular invasion. Vascular spaces lined by endothelial cells. D and E Non-invasive papillary urothelial carcinoma with micropapillary features showing slender, delicate, filiform processes. F Tumor growing locally within lamina propria under areas of normal appearing mucosa. G Tumor invading into muscularis propria. H Tumor cells demonstrate staining with GATA-3
MPUC grows by invasion and dissemination. The tumor can extend locally within lamina propria beneath areas of mucosa that may appear normal (Fig. 1F); hence, it may not be easy or possible to identify by routine follow-up cystoscopy or urine cytology [9]. Since MPUC is associated with muscle invasive disease in the vast majority of cases (Fig. 1G), a thorough search for muscularis propria invasion is essential [26]. A re-biopsy should be considered if the initial biopsy is superficial and does not contain muscularis propria. Deep muscle biopsies are recommended for MPUC detection because cold cup biopsy may miss tumor that infiltrates into the muscle layer beneath the benign surface epithelium. Non-invasive papillary urothelial carcinoma with micropapillary features is not usually associated with an adverse prognosis; hence, it must be distinguished from invasive micropapillary carcinoma. MPUC is almost always associated with lymphovascular invasion. Studies have shown that the percentage of micropapillary histology identified in transurethral resection specimens predicts stage- and disease-specific survival [27,28,29]. In a prior study, as little as 10% micropapillary component was deemed to be significant and recommended to be reported [27]. When micropapillary histology is present in metastatic lesions, the possibility of MPUC must be considered, especially if encountered in the abdominal lymph nodes or peritoneum of a male patient with an unknown primary or in a female patient without any evident abnormality of the gynecologic tract [6, 18, 30].
It has been hypothesized that the highly aggressive behavior of MPUC may be attributed to a reversed cell polarity in tumor nests, in which the stroma-facing (basal) surface of the cells acquires apical secretory properties [31]. This reversal of cell polarization enhances the release of molecules critical for tumor invasion directly into the stroma, leading to the tumor dissemination. Luna-More S et al. [32] used electron microscopy to demonstrate the presence of a large number of microvilli at the surface of the cells facing the stroma. Furthermore, Nassar et al. [33] used immunohistochemistry to demonstrate the presence of MUC1 on the basal surface of micropapillary carcinomas. MUC1 is usually expressed on the apical surface of the cells in normal glandular lining epithelium, so the presence of MUC1 on basal surface supports the reversed cell polarity or “inside out” growth pattern seen in micropapillary carcinoma. MUC1 has been known to play a key role in lymphovascular dissemination of the tumor cells due to induction of detachment of the neoplastic cells from the stroma [9, 33].
No specific threshold is currently used to classify a case as MPUC, but it is likely that any amount, even < 10%, is significant and should be reported [27, 29]. There is a need to further refine the diagnostic criteria, because a recent consensus opinion study showed only moderate interobserver reproducibility. The most reproducible criteria with the highest consensus were multiple nests in the same lacunar spaces [34].
The immunohistochemical profile of MPUC is similar to that demonstrated by typical urothelial carcinoma. Both the conventional and micropapillary urothelial carcinoma cells are usually positive for GATA-3 (Fig. 1H), cytokeratin 7 (CK7), cytokeratin 20 (CK20), CD15, epithelial membrane antigen (EMA), and carcinoembryonic antigen (CEA). The tumor cells express MUC1 (or EMA) in the stroma facing aspect of the cells, consistent with the reverse polarity characteristic of micropapillary carcinoma. The morphology and immunohistochemical profile suggest that MPUC is a form of glandular differentiation in urothelial carcinoma [9, 29]. High molecular weight cytokeratin (CK903) commonly demonstrates weak staining, consistent with glandular differentiation. This immunohistochemical profile does not discriminate between different variants of urothelial carcinoma, but it is helpful in establishing urothelial origin of the tumor and to reliably distinguish it from metastatic tumors from other sites. CA125 expression is more likely to be seen in micropapillary carcinoma than conventional urothelial carcinomas [9, 29, 35]. FOXA1 expression has been seen in MPUC as a marker of luminal phenotype [35].
Molecular Genetics
Urothelial carcinoma is a heterogenous disease in terms of the morphology and genomic characterization, and it demonstrates a broad spectrum of morphologic features and molecular alterations. Based on recent studies, bladder cancers have been divided into two molecular subtypes: the basal and luminal gene expression patterns [36,37,38]. A study on the gene expression profile of MPUC found that MPUC is characterized by widespread dysregulation of its expression profile, affecting 30% of the protein-coding genome. This expression signature is also seen in conventional urothelial carcinomas with progression into MPUC. MPUC is characterized by high mRNA expression levels of luminal markers such as KRT20, GATA3, uroplakins, ERBB2, ERBB3, CD24, FOXA1, and XBP1, confirming that it is almost exclusively of the luminal type [39]. The second subtype, basal or p53-like, typically shows infiltration with stromal cells [39, 40], and they are the most aggressive variant of the disease, as they are commonly associated with chemoresistance to cisplatin-based neoadjuvant chemotherapy. Higher rates of ERBB2 amplification have been reported in MPUC when compared with classic urothelial carcinoma, the presence of which has been associated with worse cancer-specific survival after radical cystectomy [41, 42]. There are recent reports of high rates of intratumoral heterogeneity of ERBB2 amplification within tumor containing both micropapillary and classic urothelial components. This heterogeneity is explained by ERBB2 being more commonly amplified in the micropapillary than the classic urothelial component [43••]. Furthermore, in these mixed tumors, the rate of ERBB2 amplification was much higher in the classic urothelial component than in pure classic urothelial carcinoma [44,45,46]. A recent study showed that downregulation of miR-296 with upregulation of its target genes and activation of the RUVBL1 pathway appears to drive the expression of signature of MPUC and contributes to its development [39]. Downregulation of miR-296 has been reported in many human cancers [47,48,49]. It typically occurs in the later phases of carcinogenesis and is associated with the progression to aggressive disease [49]. It acts as a global repressor of tumorigenicity, and the loss of function upregulates multiple oncogenic pathways involved in tumor progression including those controlled by Scrib, HMGA1, and Pin1 [47, 48].
Studies of miRNAs in bladder cancer indicate that their specific species can be associated with bladder cancer behavior and chemosensitivity [50]. Specifically, miRNA-296-5p modulation was shown to be associated with altered viability of cell lines exposed to cisplatin. In a similar way, activation of RUVBL1 has been reported in many cancers and is typically associated with clinically aggressive forms of disease [51, 52].
Differential Diagnosis
Carcinomas with micropapillary features have been described in organs other than the urinary tract, such as breast, lung, ovary, gastrointestinal tract, and salivary glands. Metastatic tumors from these sites can mimic MPUC and should be included in the differential diagnosis. The micropapillary architecture of tumor cell nests within lacunae is the distinct morphologic feature of this tumor type regardless of the site of origin. These tumors have an inevitably aggressive behavior irrespective of the original organ site, but it is important to distinguish between the tumors because treatment and prognosis are different for each. Similarities in morphologies, coupled with a high predisposition to metastasize, necessitate differentiation between primary and secondary micropapillary carcinoma. Apart from clinical and radiological correlation, detection of co-existing conventional urothelial carcinoma in the specimen and utilization of a broad panel of immunohistochemical stains can aid accurate diagnosis in respect to establishing the urothelial origin and differentiating from metastatic tumors. Applying an immunohistochemistry panel comprised of GATA-3, uroplakin, CK20, thyroid transcription factor-1 (TTF-1), WT-1, PAX-8, estrogen receptor (ER), and mammaglobin to a carcinoma with micropapillary features, whether in the bladder or a metastatic site, can accurately classify the most likely primary site of tumor [53]. CK20 expression excludes breast and ovarian primaries. Combined GATA-3, uroplakin, and CK20 positivity favors a diagnosis of MPUC. Ovarian serous carcinoma is almost always positive for WT-1 and PAX-8, whereas negative staining for WT-1 and immunoreactivity for ER and mammaglobin supports a diagnosis of micropapillary carcinoma of the breast. CA125 reveals a very strong cytoplasmic staining pattern in ovarian carcinomas and other tumors of Mullerian origin [29]. Positivity for TTF-1 is useful in differentiating a primary lung adenocarcinoma with micropapillary features from tumors of other sites.
Management
Management of MPUC remains controversial with no consensus guidelines at present. The variant histologies have also been known to respond differently to BCG, chemotherapy, and radiotherapy [54]. MPUC is one of the aggressive variant histologies which not only present with a higher stage disease, but are also more likely to be upstaged after radical cystectomy (RC) [40, 55, 56]. Gene expression profiling has shown that MPUC clusters with the luminal subtype of bladder carcinoma and has a high level of ERBB2 mutations [57], underlining a biologic basis for more aggressive treatment strategy for micropapillary non-muscle invasive bladder cancer (NMIBC) [40].
Conventional urothelial NMIBC is usually treated with surveillance and/or intravesical BCG therapy. In case of MPUC and NMIBC, the choice of treatment is between BCG therapy and early radical cystectomy (RC) [40]. Due to the aggressive nature of MPUC and shorter time intervals for progression of non-muscle invasive MPUC to muscle invasive and metastatic disease, this has prompted the recommendation for early radical cystectomy (RC) over intravesical BCG as a standard of care [3•, 10, 58, 59]. In a retrospective analysis performed at MD Anderson [7], most of the patients with MPUC did not respond to BCG (89%) and, in fact, progressed to metastatic disease in 22% cases within a median interval of 8 months compared to 9 months in conventional urothelial carcinoma. Five-year disease specific survival (DSS) in patients with upfront RC vs RC after BCG failure were 72% and 60%, respectively. Ghoneim et al. [58] showed similar results at Cleveland clinic. All the 10 patients with MPUC NMIBC, who received BCG therapy, subsequently were upstaged at RC to non-organ confined stage disease, including 6 with metastatic disease. A bigger cohort at MD Anderson Cancer Center [59] showed findings similar to their previous smaller study, statistically significant difference in 5-year disease-specific survival (DSS) in early cystectomy patients from patients first treated with BCG (100% vs 80%), thereby making a case for early RC as treatment of choice for MPUC NMIBC (contrasting with the bladder conserving strategy used in NMIBC with conventional morphology) because of statistically significant difference in 5-year DSS and short median time to progression of disease. On the other hand, it has been shown that when matched stage for stage, MPUC is not associated with worse outcome than conventional [60]. Therefore, some studies still suggest the role of bladder preservation therapies in selected patients of non-muscle invasive disease with limited micropapillary component [59, 60]. Studies at Memorial Sloan Kettering Cancer Center recommend re-resection after TUR and BCG therapy only for patients with negative re-TUR. Their study revealed no statistically significant difference in DSS between BCG cohort and primary cystectomy, and hence, they recommend either of these therapeutic approaches can be adopted [60].
In the case of muscle invasive MPUC, there is no consensus regarding the role of neoadjuvant chemotherapy (NAC), with some studies recommending immediate cystectomy and others advocating NAC and RC. As a result, there is no consensus among clinicians regarding the use of NAC in MPUC. If MPUC is not adequately responsive to NAC, then this may in fact delay time to definitive surgery and possibly compromise survival outcomes in a number of patients. Other studies found no significant difference in 5-year overall survival between the two groups [10]. There are studies that have reported significant downstaging in the NAC group after RC, although it still did not translate into significant difference in rates of recurrence or overall survival [61, 62].
Fernandez et al. [21] have tried to risk stratify cases of surgically resectable MPUC by creating 3 risk groups (low, high, and highest) based on overall survival (OS) and disease-specific survival (DSS). They observed a beneficial effect of NAC in the high-risk muscle invasive disease group without hydronephrosis in contrast to NMIBC low-risk group. No significant difference was observed in cases of highest risk group (muscle invasive disease with hydronephrosis). In terms of adjuvant chemotherapy, there is only one study which reported higher rates of recurrence in MPUC patients receiving chemotherapy compared to convention urothelial carcinoma patients [63].
Future Directions
MPUC is an aggressive variant histology that usually presents at an advanced stage at the time of diagnosis with nodal and distant metastases. Correct identification of the histology is essential for appropriate management. Up to 45–50% of urothelial carcinomas with variant histology are said to be missed by community pathologists, and variant histology is reported for the first time after RC in around half of the cases [40]. The reason is partly due to lack of strict diagnostic criteria or percent cutoff for diagnosis. One strategy that has been suggested is identification on the basis of surrogate markers for molecular profile.
Current recommendation is for the proportion of micropapillary pattern to be reported. Studies have shown the relation between proportion of micropapillary pattern and the prognosis [21, 29]. However, currently there is no clear well-defined classification scheme for correlating proportion of MPUC with prognosis. Well defined criteria may act as a risk stratifying aid in making management decisions.
Newer therapeutic strategies related to the molecular and genetic findings seen in MPUC remain to be explored further. The most frequent genetic finding in micropapillary UC has been noted to be ERBB2/HER2 gene amplification, seen in 15% to 74% of cases [10, 14, 17••, 34, 64•]. HER2 protein expression by immunohistochemistry (IHC) appears to correlate with ERBB2 amplification status suggesting the role of IHC-based diagnostic testing [10, 13, 65]. There is no evidence yet to support the role of targeted HER2 inhibitor therapy but needs to be explored as an additional therapy option. Similarly, the presence of unique gene expression patterns in p53-like MPUCs makes it likely for eventual development of objective biomarker panels for identification and management of these especially aggressive subtypes [40]. MPUC involves activation of miR-296 and RUVBL1 target genes which are also future therapeutic targets [39]. Another potential therapeutic agent could be the recently FDA-approved FGFR3 inhibitor, erdafitinib for patients with advanced urothelial carcinoma with alterations of FGFR2 or FGFR3 after progression on platinum-based chemotherapy [66•].
Immune checkpoint inhibitors have been approved in the second-line setting for patients with metastatic disease and locally advanced urothelial carcinoma and also as first-line therapy in cisplatin-ineligible patients [17••]. Role of these PD-L1 inhibitors needs to be further explored in these aggressive micropapillary carcinomas.
There is no consensus currently on the optimal management of NMIUC MPUC. More concrete guidelines for management of MPUC need to be established with the help of prospective studies involving various treatment modalities including immunotherapy. Identification of significant clinical as well as pathological risk factors can help in risk stratifying individual cases of micropapillary NMIBC for optimal management. A recent study using whole transcriptome RNA-sequencing has demonstrated the differential expression of over 3000 genes in MPUC as well as a 26-gene signature characteristic of MPUC as compared to conventional non-muscle invasive urothelial carcinoma [67]. High FABP3 and CD36 expression and low RAET1E expression were significantly associated with shorter time to progression, thereby helping classify these patients of NMIBC MPUC with high risk of early progression, with implications in terms of early radical cystectomy recommendation.
Conclusions
MPUC is a well-documented highly aggressive variant of urothelial carcinoma with proven worse outcomes. Accurate recognition and reporting of this pattern is critical for optimal management and cannot be overemphasized. Any amount of micropapillary differentiation in urothelial carcinoma appears to be prognostically significant and dictates treatment guidelines and therefore needs to be reported. Patients with micropapillary urothelial carcinoma might benefit from an aggressive treatment strategy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Teoh JY, Huang J, Ko WY, et al. Global trends of bladder cancer incidence and mortality, and their associations with tobacco use and gross domestic product per capita. Eur Urol. 2020;78(6):893–906.
Wigner P, Bijak M, Saluk-Bijak J. The green anti-cancer weapon The Role of Natural Compounds in Bladder Cancer Treatment. Int J Mol Sci. 2021;22(15):7787.
• Lobo N, Shariat SF, Guo CC, et al. What is the significance of variant histology in urothelial carcinoma? Eur Urol Focus. 2020;6(4):653–63. This paper provides an overview of the diagnostic, therapeutic, and prognostic significance of micropapillary urothelial carcinoma.
Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108.
Moch H, Humphrey PA, Ulbright TM et al. Eds. WHO classification of tumors of the urinary system and male genital organs. Lyon, France: International Agency for Research on Cancer, 2016
Amin MB, Ro JY, El-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol. 1994;18(12):1224–32.
Kamat AM, Gee JR, Dinney CPN, et al. The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. J Urol. 2006;175(3 Pt 1):881–5.
Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol. 2004;11(6):297–303.
Perepletchikov AM, Parwani AV. Micropapillary urothelial carcinoma: clinico-pathologic review. Pathol Res Pract. 2009;205(12):807–10.
Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas M D Anderson Cancer Center experience with 100 consecutive patients. Cancer. 2007;110(1):62–7.
Maranchie JK, Bouyounes BT, Zhang PL. Clinical and pathological characteristics of micropapillary transitional cell carcinoma: a highly aggressive variant. J Urol. 2000;163(3):748–51.
• Mitra AP, Fairey AS, Skinner EC, et al. Implications of micropapillary urothelial carcinoma variant on prognosis following radical cystectomy: a multi-institutional investigation. Urol Oncol. 2019;37(1):48–56. This study demonstrates that the presence of micropapillary urothelial carcinoma is associated with locally advanced disease at radical cystectomy.
Isharwal S, Huang H, Nanjangud G, et al. Intratumoral heterogeneity of ERBB2 amplification and HER2 expression in micropapillary urothelial carcinoma. Human Pathol. 2018;77:63–9.
Al-Ahmadie H, Iyer G. Updates on the genetics and molecular subtypes of urothelial carcinoma and select variants. Surg Pathol Clin. 2018;11(4):713–23.
• Butt SU, Malik L. Role of immunotherapy in bladder cancer: past, present and future. Cancer Chemother Pharmacol. 2018;81(4):629–45. This paper discusses advances in urothelial bladder cancer immunotherapy.
Aoun F, Rassy EE, Assi T, et al. Advances in urothelial bladder cancer immunotherapy, dawn of a new age of treatment. Immunotherapy. 2017;9(5):451–60.
•• Compérat E, Amin MB, Epstein JI, et al. The genitourinary pathology society update on classification of variant histologies, T1 substaging, molecular taxonomy, and immunotherapy and PD-L1 testing implications of urothelial cancers. Adv Anat Pathol. 2021;28(4):196–208. This paper demonstrates molecular evolution, emphasizing the aspects that impact the understanding concepts relevant to management of patients.
Johansson SL, Borghede G, Holmang S. Micropapillary bladder carcinoma: a clinicopathological study of 20 cases. J Urol. 1999;161(6):1798–802.
Alvarado-Cabrero I, Sierra-Santiesteban FI, Mantilla-Morales A, et al. Micropapillary carcinoma of the urothelial tract A clinicopathologic study of 38 cases. Ann Diagn Pathol. 2005;9(1):1–5.
Watts KE, Hansel DE. Emerging concepts in micropapillary urothelial carcinoma. Adv Anat Pathol. 2010;17(3):182–6.
Fernández MI, Williams SB, Willis DL, et al. Clinical risk stratification in patients with surgically resectable micropapillary bladder cancer. BJU Int. 2017;119(5):684–91.
Zhai QJ, Black J, Ayala AG, et al. Histologic variants of infiltrating urothelial carcinoma. Arch Pathol Lab Med. 2007;131(8):1244–56.
Guo CC, Tamboli P, Czerniak B. Micropapillary variant of urothelial carcinoma in the upper urinary tract: a clinicopathologic study of 11 cases. Arch Pathol Lab Med. 2009;133(1):62–6.
Larsen MP, Steinberg GD, Brendler CB, et al. Use of Ulex europaeus agglutinin I (UEAI) to distinguish vascular and “pseudovascular” invasion in transitional cell carcinoma of bladder with lamina propria invasion. Mod Pathol. 1990;3(1):83–8.
Domanowska E, Jozwicki W, Domaniewski J, et al. Muscle-invasive urothelial cell carcinoma of the human bladder: multidirectional differentiation and ability to metastasize. Hum Pathol. 2007;38(5):741–6.
Epstein JI, Amin MB, Reuter VE. Histologic variants of urothelial carcinoma. Bladder Biopsy Interpretation, (Biopsy interpretation series), Lippincott Williams & Wilkins, Philadelphia, PA (2017), 148–155.
Compérat E, Roupret M, Yaxley J, et al. Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathol. 2010;42(7):650–4.
Gaya JM, Palou J, Algaba F, et al. The case for conservative management in the treatment of patients with non-muscle-invasive micropapillary bladder carcinoma without carcinoma in situ. Can J Urol. 2010;17(5):5370–6.
Samaratunga H, Khoo K. Micropapillary variant of urothelial carcinoma of the urinary bladder; a clinicopathological and immunohistochemical study. Histopathol. 2004;45(1):55–64.
Vera J, Marigil M, García MD, Abascal M, Sanz JI. Micropapillary bladder carcinoma. Virchows Arch. 2002;441(4):412–3.
Peterson JL. Breast carcinomas with an unexpected inside-out growth pattern: rotation of polarization associated with angioinvasion. Path Res Pract. 1993;189:780A.
Luna-Moré S, Gonzalez B, Acedo C, Rodrigo I, Luna C. Invasive micropapillary carcinoma of the breast A new special type of invasive mammary carcinoma. Pathol Res Pract. 1994;190(7):668–74.
Nassar H, Pansare V, Zhang H, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol. 2004;17(9):1045–50.
Sangoi AR, Beck AH, Amin MB, et al. Interobserver reproducibility in the diagnosis of invasive micropapillary carcinoma of the urinary tract among urologic pathologists. Am J Surg Pathol. 2010;34(9):1367–76.
Lopez-Beltran A, Henriques V, Montironi R, et al. Variants and new entities of bladder cancer. Histopathology. 2019;74(1):77–96.
Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–65.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22.
Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111(8):3110–5.
Guo CC, Dadhania V, Zhang L, et al. Gene expression profile of the clinically aggressive micropapillary variant of bladder cancer. Eur Urol. 2016;70(4):611–20.
Burger M, Kamat AM, McConkey D. Does variant histology change management of non-muscle-invasive bladder cancer? Eur Urol Oncol. 2021;4(3):510–4.
Schneider SA, Sukov WR, Frank I, et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod Pathol. 2014;27(5):758–64.
Tschui J, Vassella E, Bandi N, et al. Morphological and molecular characteristics of HER2 amplified urothelial bladder cancer. Virchows Arch. 2015;466(6):703–10.
•• Isharwal S, Huang H, Nanjangud G, et al. Intratumoral heterogeneity of ERBB2 amplification and HER2 expression in micropapillary urothelial carcinoma. Hum Pathol. 2018;77:63–9. This study identifies the presence of intratumoral heterogeneity of ERBB2 amplification and HER2 expression in micropapillary urothelial carcinoma.
Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540-556.e25.
Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol. 2013;31(25):3133–40.
Fleischmann A, Rotzer D, Seiler R, et al. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011;60(2):350–7.
Lee KH, Lin FC, Hsu TI, et al. MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in prostate cancer by directly targeting Pin1. Biochim Biophys Acta. 2014;1843(9):2055–66.
Savi F, Forno I, Faversani A, et al. miR-296/Scribble axis is deregulated in human breast cancer and miR-296 restoration reduces tumour growth in vivo. Clin Sci (Lond). 2014;127(4):233–42.
Vaira V, Faversani A, Dohi T, et al. miR-296 regulation of a cell polarity-cell plasticity module controls tumor progression. Oncogene. 2012;31(1):27–38.
Nordentoft I, Birkenkamp-Demtroder K, Agerbæk M, et al. miRNAs associated with chemo-sensitivity in cell lines and in advanced bladder cancer. BMC Med Genomics. 2012;5:40.
Gentili C, Castor D, Kaden S, et al. Chromosome missegregation associated with RUVBL1 deficiency. PLoS One. 2015;10(7):e0133576.
Taniuchi K, Furihata M, Iwasaki S, et al. RUVBL1 directly binds actin filaments and induces formation of cell protrusions to promote pancreatic cancer cell invasion. Int J Oncol. 2014;44(6):1945–54.
Lotan TL, Ye H, Melamed J, et al. Immunohistochemical panel to identify the primary site of invasive micropapillary carcinoma. Am J Surg Pathol. 2009;33(7):1037–41.
Wasco MJ, Daignault S, Zhang Y, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urol. 2007;70(1):69–74.
Kassouf W, Agarwal PK, Grossman HB, et al. Outcome of patients with bladder cancer with pN+ disease after preoperative chemotherapy and radical cystectomy. Urol. 2009;73(1):147–52.
Turker P, Bostrom PJ, Wroclawski ML, et al. Upstaging of urothelial cancer at the time of radical cystectomy: factors associated with upstaging and its effect on outcome. BJU Int. 2012;110(6):804–11.
Willis DL, Flaig TW, Hansel DE, et al. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol Oncol. 2014;32(6):826–32.
Ghoneim IA, Miocinovic R, Stephenson AJ, et al. Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urol. 2011;77(4):867–70.
Willis DL, Fernandez MI, Dickstein RJ, et al. Clinical outcomes of cT1 micropapillary bladder cancer. J Urol. 2015;193(4):1129–34.
Spaliviero M, Dalbagni G, Bochner BH, et al. Clinical outcome of patients with T1 micropapillary urothelial carcinoma of the bladder. J Urol. 2014;192(3):702–7.
Meeks JJ, Taylor JM, Matsushita K, et al. Pathological response to neoadjuvant chemotherapy for muscle-invasive micropapillary bladder cancer. BJU Int. 2013;111(8):E325–30.
Abufaraj M, Foerster B, Schernhammer E, et al. Micropapillary urothelial carcinoma of the bladder: a systematic review and meta-analysis of disease characteristics and treatment outcomes. Eur Urol. 2019;75(4):649–58.
Masson-Lecomte A, Xylinas E, Bouquot M, et al. Oncological outcomes of advanced muscle-invasive bladder cancer with a micropapillary variant after radical cystectomy and adjuvant platinum-based chemotherapy. World J Urol. 2015;33(8):1087–93.
• Sangoi AR, Cox RM, Higgins JP, et al. Non-invasive papillary urothelial carcinoma with ‘micropapillary’ architecture: clinicopathological study of 18 patients emphasizing clinical outcomes. Histopathol. 2020;77(5):728–33. This study demonstrates the clinicopathological characteristics of non-invasive papillary urothelial carcinoma with emphasis on clinical outcomes.
Wang JK, Boorjian SA, Cheville JC, et al. Outcomes following radical cystectomy for micropapillary bladder cancer versus pure urothelial carcinoma: a matched cohort analysis. World J Urol. 2012;30(6):801–6.
• Casadei C, Dizman N, Schepisi G, et al. Targeted therapies for advanced bladder cancer: new strategies with FGFR inhibitors. Ther Adv Med Oncol. 2019;11:1758835919890285. This paper describes future therapeutic options of combination regimens with immune-checkpoint inhibitors for the treatment of urothelial carcinoma with FGFR2 and FGFR3 mutations.
Bowden M, Nadal R, Zhou CW, et al. Transcriptomic analysis of micropapillary high grade T1 urothelial bladder cancer. Sci Rep. 2020;10(1):20135.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Kumar, D., Adeniran, A.J. Clinicopathological Review of Micropapillary Urothelial Carcinoma. Curr Oncol Rep 24, 603–610 (2022). https://doi.org/10.1007/s11912-022-01219-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01219-x