Abstract
Purpose of this study was to evaluate prognostic impact of rare variants of urothelial bladder cancer (BC) after treatment with combined radiochemotherapy (RCT). To this end tumour tissue of 238 patients with urothelial carcinoma (UC) treated with transurethral resection of the bladder (TUR-B) and RCT with curative intent was collected. Histomorphological analysis included re-evaluation and semi-quantitative assessment of rare UC subtypes. Additionally, human epidermal growth factor receptor 2 (HER2) chromogenic in situ hybridisation (CISH) was performed in tumours with a micropapillary component exceeding 30 %. Long-term follow-up was available for 200 patients (range 3–282 months). Variant UC histology was found in 45 of 238 tumours, most frequently micropapillary UC (N = 17) including cases with a small fraction of tumour with micropapillary morphology. The mere presence of micropapillary morphology did not affect prognosis. In tumours with extensive (≥30 %) micropapillary morphology (N = 8) Kaplan-Meier analysis revealed significantly worse cancer specific survival (CSS) (P = 0.002) compared to conventional UC (mean survival times 97 months and 229 months, respectively). Univariate Cox regression analysis of cases with ≥30 % micropapillary morphology revealed a hazard ratio of 4.726 (95 % CI 1.629–13.714) for CSS (P = 0.004). CISH revealed HER2 gene amplification in 3/10 tumours with ≥30 % micropapillary component. In conclusion, for BC treated with TUR-B and RCT, the presence of micropapillary morphology in more than 30 % of the tumour is an adverse prognostic factor. Further studies are needed to evaluate a potential benefit of different, especially multimodal treatment strategies for micropapillary UC and also other subtypes of UC. Her2 represents a promising therapeutic target in a subset of micropapillary UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer (BC) belongs to the most commonly diagnosed cancers worldwide, with 429,000 new cases and 165,000 deaths reported in 2012 [1]. The most frequently diagnosed histologic type of BC is urothelial carcinoma (UC), of which conventional UC represents about 90 % of BCs in Western European countries and the US. However, approximately 10 % of BCs show UC with aberrant morphology or are non-UC, as defined by the WHO classification of 2004 [2]. Among the latter, squamous and glandular differentiation are quite commonly found but there are also rare variants, e.g. the micropapillary variant of UC, which has a documented frequency of 0.6–8 % [2–6]. Consistent with micropapillary tumours in other organs, micropapillary UC is associated with advanced clinical and pathological stage and a high likelihood of lymph node metastasis at initial diagnosis, resulting in a high risk of understaging for those tumours [7–9]. However, data on outcome of micropapillary UC are controversial since some studies document aggressive behaviour and poor outcome, while others report no difference in overall and recurrence-free survival compared to conventional UC [5, 7, 10].
For a subset of BCs, i.e. pure squamous cell carcinoma (SCC), pure adenocarcinoma and small cell neuroendocrine carcinoma, treatment is adjusted according to the histologic type of tumour [11]. Recently, interest in rare UC subtypes has increased, the majority of studies focusing on their prognostic impact. However, the number of studies dealing with treatment modalities for micropapillary and other rare subtypes of UC is very limited. It seems that there is general consensus for aggressive treatment of micropapillary UC also in a non-muscle-invasive stage, for which early radical cystectomy with urinary diversion has been recommended as therapy of choice [12, 13]. Recommendations for neoadjuvant chemotherapy are controversial, especially due to studies investigating therapy response [7, 14, 15]. However, current therapy recommendations are based mostly on studies investigating outcome after cystectomy with or without (neo-) adjuvant chemotherapy. For micropapillary and other variant histology UCs, however, a very low number of studies on alternative treatment strategies has been conducted and this may be one of the reasons why therapy alternatives are lacking for subtypes of UC. We investigated the outcome of rare histologic variants of UC compared to that of conventional UC in a series of patients treated with transurethral resection of the bladder (TUR-B) combined with subsequent radiochemotherapy (RCT), as a bladder-sparing treatment option with curative intent.
This organ-sparing treatment approach has been shown to achieve in well-selected patients clinical outcome comparable to that of radical cystectomy [16]. We re-evaluated histomorphology of the tumours of patients treated with TUR-B and RCT according to the “Erlanger Schema” between 1982 and 2005, with emphasis on long-term follow-up in rare subtypes of UC. Furthermore we investigated amplification of the HER2 gene in micropapillary subtype of UC, as it has been reported in this subset of tumours before [17–19]. Moreover, several (pre-) clinical trials are in progress investigating the impact of Her2-targeted therapies in UC, as mentioned in a recent study [19].
Methods
Paraffin embedded TUR-B specimens of 238 patients (mean patient age at diagnosis 65.2 years, range 32.77–87.95 years, median 66.0 years) with UC of the bladder were obtained from the archives of the participating institutions. All patients had been treated with TUR-B and RCT with curative intent, according to the so-called “Erlanger Schema”, a treatment regimen established at the Department of Radiation Oncology, Erlangen, during the last 30 years [16]. In case of initial R1 resection, re-TUR-B was performed before initiation of RCT. More than 95 % of patients received chemotherapy based on cisplatin or carboplatin alone or in combination with 5-Fluorouracil (5-FU) or combined cisplatin and carboplatin. Complete clinical follow-up data of 200 patients (mean follow-up time 77.69 months, median 60 months, range 3 to 282 months) were collected between 1982 and 2014 by the Department of Radiation Oncology, Erlangen, and the Cancer Registry, Erlangen. Histopathological evaluation of haematoxylin and eosin stained tissue slides from each of the 238 cases was performed by two experienced uropathologists (S.B., A.H.) including staging according to the current TNM classification [20], documentation of the presence or absence of carcinoma in situ and grading and assessment of rare variants of UC, both according to the current WHO classification 2016 [2]. Additionally, grading according to the previous WHO classification 1973 was performed [21]. Analysis of rare histological subtypes of UC included semi-quantitative assessment of the amount of variant histology in four categories (<10 %, ≥10 %, ≥30 %, ≥50 %), according to a previous study by Compérat et al. [9], who found variable survival rates depending on the amount micropapillary morphology. Tumours with partial squamous and/or glandular differentiation were not considered in the variant histology group but were included into the conventional UC group. All other UC variants were completely excluded from survival analysis. Cases with pure SCC or adenocarcinoma were excluded from the study.
Additional HER2 CISH analysis using a ZytoDot 2C SPEC ERBB2/CEN 17 Probe and ZytoDot-2C-CISH-System (ZytoVision, Bremerhaven, Germany) was done on tumours with a micropapillary proportion of ≥30 % according to the recommendations of the manufacturer. Evaluation of the CISH results was performed on one tumour section on the tumour area identified as micropapillary. Cases were regarded as amplified if the HER2:CEN17 signal ratio was ≥2.2. When the HER2:CEN17 signal ratio was <2.2, HER2 was also regarded as amplified when the specific HER2 signals exceeded 6 signals per cell on average. In accordance to the current guidelines of HER2-testing in breast cancer [22], a total of at least 20 tumour cells were evaluated.
Statistical analyses
Cancer-specific survival (CSS) was estimated using the Kaplan-Meier product limit method. Survival rates for patients with or without micropapillary morphology were compared using the log-rank test. Univariate Cox proportional hazards models were used to define the influence of micropapillary morphology on CSS. For comparison of categorical variables, Fisher exact and Chi-square test statistics were used. All tests were performed as two-sided and p-values below 0.05 were considered as statistically significant. Statistical analyses were performed with SPSS Ver. 21.0 (IBM SPSS, Chicago, IL). Statistical analysis was restricted to cases with micropapillary variant of UC and conventional UC, due to small numbers and limited follow-up data of the remaining rare subtypes.
Results
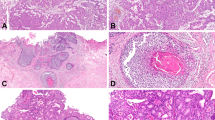
Rare UC histological subtypes detected in this patient cohort were invasive micropapillary, plasmacytoid, nested type, neuroendocrine, sarcomatoid, clear cell and lymphoepithelioma-like. Distribution of variant morphologies in our cohort is shown in Table 1. Patient and tumour characteristics are summarized in Table 2. Any variant morphology was found in 45 of 238 cases. The most frequent rare variant (17 cases) was micropapillary UC. In 10 of these tumours abundant (≥30 %) micropapillary morphology was found (Fig. 1). Complete follow-up data were available for 8 of 10 tumours with an abundant micropapillary component.
Statistical evaluation of CSS in both non-muscle-invasive and muscle-invasive tumours revealed a significant difference between tumours with ≥30 % of micropapillary component (N = 8) and conventional UC (P = 0.002). In muscle-invasive tumours, no significant differences in CSS were found between conventional UC and UC with a micropapillary component <30 % (Kaplan-Meier analysis; log Rank test: P = 0.124). However, a significant difference in CSS was found in the muscle-invasive subgroup for patients whose tumours exhibited a micropapillary component of ≥30 % (N = 8) compared with conventional UC (Kaplan-Meier analysis; log Rank test: P = 0.010) (Fig. 2). The mean survival time was 97 months for patients with ≥30 % micropapillary morphology and 229 months for patients with conventional UC if tumours for all tumour stages taken together. For the muscle-invasive subgroup, the mean survival time was 97 months for patients with ≥30 % micropapillary morphology compared to 221 months for patients with conventional UC. In univariate Cox proportional hazards regression analysis ≥30 % micropapillary morphology was associated with a hazard ratio (HR) of 4.726 (95 % CI 1.629–13.714) for CSS (P = 0.004).
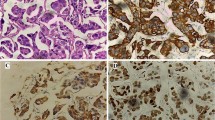
HER2 gene amplification by CISH was found in 3 of 10 (30 %) of tumours with ≥30 % micropapillary component (Fig. 3). The HER2:CEN17-ratio was >3 in each of the 3 cases. In one of the tumours, evaluation was not possible due to lack of residual tumour tissue on the paraffin block.
Discussion
According to the current guidelines for BC, a deviation from the standard treatment schemes of conventional UC is recommended for small cell neuroendocrine carcinoma, pure SCC, and pure adenocarcinoma of the bladder [11, 13]. Other subtypes e.g. plasmacytoid or micropapillary UC are associated with aggressive clinical behaviour and have been the subject of growing interest recently. However, there are no sufficient data yet to justify alternative treatment strategies for such cases. Evidence on how to treat rare variants of UC is scarce, not only because of the limited number of studies but also due to limited patient numbers in these studies. For micropapillary UC, most studies report an association with advanced clinical and pathologic stage, high nuclear grade and multifocal disease [10, 23]. This shaped the general opinion towards treating those tumours aggressively by rapid cystectomy with or without adjuvant chemotherapy. This opinion is based on studies, almost exclusively investigating patient outcome after cystectomy, whereas studies on alternative therapy schemes without cystectomy are very rare. Kamat et al. reported aggressive clinical behaviour of micropapillary UC and in addition report that micropapillary carcinoma is refractory to BCG instillations and recommended radical cystectomy in cases with surgically resectable disease [24]. Furthermore, for non-metastatic muscle invasive micropapillary UC a response rate to neoadjuvant chemotherapy of 45 % has been reported [15]. One also needs to take in account that UC patients are frequently of advanced age at diagnosis and commonly suffer from co-morbidities. Therefore, some patients might not qualify for aggressive treatment regimens like radical cystectomy with urinary diversion and aggressive (usually cisplatin-based) chemotherapy.
Recent studies on management of BC patients with multimodal therapy regimens combining radiotherapy with a bladder sparing approach have shown outcome comparable to that of radical cystectomy in selected patients [16, 25, 26]. However, to our knowledge, none of these included survival analysis of patients with rare variants of UC. Johansson et al. presented a large prospective cohort of rare variants of UC including a subgroup of 20 cases of micropapillary BC, treated with external beam irradiation [5] and concluded that radiotherapy with or without combined chemotherapy is ineffective in this subtype. In this study 4 patients received chemotherapy (3 platin-based), 7 patients radiotherapy alone after TUR-B and 9 cystectomy. In our patient cohort in contrast, a highly standardized tri-modal approach with TUR-B and RCT, including platin-based chemotherapy, was performed [16] which makes the results difficult to compare. In addition, Johansson et al. did not take into account the relative proportion of micropapillary morphology in the tumours and included cases with a relatively small amount (≤10 %).
Overall, histological variants of UC are poorly identified and even among experienced genitourinary pathologists only moderate interobserver concordance has been documented for micropapillary UC [27]. One of the most problematic issues in identifying small areas of micropapillary morphology is the existence of focal areas with a prominent retraction artefact, which mimicks micropapillary morphology. This phenomenon is frequently found in early invasive BC and may lead to overdiagnosis of micropapillary UC [28]. Studies investigating the impact of the proportion of micropapillary morphology report that a high proportion of micropapillary morphology is associated with advanced tumour stage and poor prognosis [4, 9, 29]. We found comparable results, micropapillary morphology only exceeding 30 % being associated with poor prognosis. Nevertheless, it has been recommended to report even a low percentage (<10 %) of micropapillary component in TUR-B specimen, in view of the risk of sampling error and subsequent upstaging after cystectomy [9]. Moreover, most existing studies did not distinguish between non-muscle invasive and muscle-invasive tumours or even included non-muscle-invasive tumours, without taking into account that the most important prognostic factor is tumour stage [2]. Our results show that, even after correcting for tumour stage by excluding pT1 tumours which were all conventional UC, patients with micropapillary UC have poorer CSS than those with conventional UC. However, survival rates of our patients with UC with an extensive micropapillary component were better than those in other studies [30].
The main limitation of our study is the small number of cases with extensive micropapillary differentiation. However, most studies investigating variant morphology in BC present a low number of cases due to the low frequency of rare subtypes of UC. Furthermore, histomorphological analysis was performed on TUR-B specimens, with well-known limitations in representativity and evaluation of tumour stage. The strength of our study is the large and unique cohort of patients treated with a highly standardized tri-modal treatment scheme with TUR-B and platin-based RCT within a bladder sparing approach, with long-term follow-up. In addition, we found in 3 tumours (30 %) with a micropapillary component of ≥30 % amplification of the HER2 gene, which is in agreement with other publications documenting HER2 amplification in 15–42 % of micropapillary UC. This suggests that Her2 is a potentially important therapy target in those patients [17, 18].
In summary, we show that a micropapillary component ≥30 % in UC represents a negative prognostic factor for CSS in UC patients treated with TUR-B and RCT. Micropapillary UC has a high propensity for lymphovascular invasion [31], which is responsible for progression through metastatic spread and poor survival. This calls for early aggressive treatment of micropapillary UC. For micropapillary UC treated with radical cystectomy and adjuvant chemotherapy reported overall survival rates are worse than for our patient cohort [30]. We contend that, in addition to recommendations to treat micropapillary UC aggressively with radical surgery and adjuvant chemotherapy [7, 10, 24], RCT should be considered an additional therapeutic approach for these tumours. This impact on therapeutic decision-making and prognosis underlines the importance of identifying rare variants of BC. Finally, Her2 protein may represent a most promising therapy target for the subset of about 30 % of micropapillary UC that harbour an amplification of the HER2 gene. Larger prospective studies are needed to confirm our results and to evaluate the prognostic impact not only of micropapillary but also of other variants of UC.
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Moch H, Humphrey P, Ulbright TM, Reuter VE (eds) (2016) WHO classification of tumours of the urinary system and male genital organs, 4th edn. IARC Press, Lyon
Lopez-Beltran A, Requena MJ, Alvarez-Kindelan J, Quintero A, Blanca A, Montironi R (2007) Squamous differentiation in primary urothelial carcinoma of the urinary tract as seen by MAC387 immunohistochemistry. J Clin Pathol 60:332–335
Alvarado-Cabrero I, Sierra-Santiesteban FI, Mantilla-Morales A, Hernández-Hernandez DM (2005) Micropapillary carcinoma of the urothelial tract. A clinicopathologic study of 38 cases. Ann Diagn Pathol 9:1–5
Johansson SL, Borghede G, Holmäng S (1999) Micropapillary bladder carcinoma: a clinicopathological study of 20 cases. J Urol 161:1798–1802
Alkibay T, Sözen S, Gürocak S, Işik Gönül I, Poyraz A, Ure I (2009) Micropapillary pattern in urothelial carcinoma: a clinicopathological analysis. Urol Int 83:300–305
Ghoneim IA, Miocinovic R, Stephenson AJ, et al. (2011) Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urology 77:867–870
Kamat AM, Gee JR, Dinney CP, et al. (2006) The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. J Urol 175:881–885
Compérat E, Roupret M, Yaxley J, et al. (2010) Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathology 42:650–654
Fairey AS, Daneshmand S, Wang L, et al. (2014) Impact of micropapillary urothelial carcinoma variant histology on survival after radical cystectomy. Urol Oncol 32:110–116
Willis DL, Porten SP, Kamat AM (2013) Should histologic variants alter definitive treatment of bladder cancer? Curr Opin Urol 23:435–443
Perepletchikov AM, Parwani AV (2009) Micropapillary urothelial carcinoma: Clinico-pathologic review. Pathol Res Pract 205:807–810
Witjes JA, Compérat E, Cowan NC, et al. (2014) EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 65:778–792
Willis DL, Flaig TW, Hansel DE, et al. (2014) Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol Oncol 32(6):826–832
Meeks JJ, Taylor JM, Matsushita K, Herr HW, Donat SM, Bochner BH, Dalbagni G (2013) Pathological response to neoadjuvant chemotherapy for muscle-invasive micropapillary bladder cancer. BJU Int 111:E325–E330
Krause FS, Walter B, Ott OJ, et al. (2011) 15-year survival rates after transurethral resection and radiochemotherapy or radiation in bladder cancer treatment. Anticancer Res 31:985–990
Ching CB, Amin MB, Tubbs RR, et al. (2011) HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: analysis by dual-color in situ hybridization. Mod Pathol 24:1111–1119
Schneider SA, Sukov WR, Frank I, et al. (2014) Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod Pathol 27:758–764
Tschui J, Vassella E, Bandi N, et al. (2015) Morphological and molecular characteristics of HER2 amplified urothelial bladder cancer. Virchows Arch 466:703–710
Wittekind C, Meyer HJ (eds) (2010) Klassifikation maligner Tumoren. 7th Edn. Wiley-VCH, Weinheim
Mostofi FK, Sobin LH, Torloni H (1973) Histological classification of urinary bladder tumours. In: Histological typing of urinary bladder tumours. International Classification of Tumours, No 10. Geneva: World Health Organisation;1973
Wolff AC, Hammond ME, Hicks DG, et al. (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Amin MB, Ro JY, el-Sharkawy T, et al. (1994) Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 18:1224–1232
Kamat AM, Dinney CP, Gee JR, et al. (2007) Micropapillary bladder cancer: a review of the university of Texas M. D. Anderson cancer center experience with 100 consecutive patients. Cancer 110:62–67
Mak RH, Hunt D, Shipley WU, et al. (2014) Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of radiation therapy oncology group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 32:3801–3809
James ND, Hussain SA, Hall E, et al. (2012) Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366:1477–1488
Sangoi AR, Beck AH, Amin MB, et al. (2010) Interobserver reproducibility in the diagnosis of invasive micropapillary carcinoma of the urinary tract among urologic pathologists. Am J Surg Pathol 34:1367–1376
Sangoi AR, Higgins JP, Rouse RV, Schneider AG, McKenney JK (2009) Immunohistochemical comparison of MUC1, CA125, and her2neu in invasive micropapillary carcinoma of the urinary tract and typical invasive urothelial carcinoma with retraction artifact. Mod Pathol 22:660–667
Samaratunga H, Khoo K (2004) Micropapillary variant of urothelial carcinoma of the urinary bladder; a clinicopathological and immunohistochemical study. Histopathology 45:55–64
Masson-Lecomte A, Xylinas E, Bouquot M, et al. (2015) Oncological outcomes of advanced muscle-invasive bladder cancer with a micropapillary variant after radical cystectomy and adjuvant platinum-based chemotherapy. World J Urol 33:1087–1093
McQuitty E, Ro JY, Truong LD, Shen SS, Zhai Q, Ayala AG (2012) Lymphovascular invasion in micropapillary urothelial carcinoma: a study of 22 cases. Arch Pathol Lab Med 136:635–639
Acknowledgments
The authors are grateful for the excellent technical support given by the laboratory staff of the Department of Pathology at the University Hospital Erlangen, in particular Rudolf Jung, Ute Zimmermann and Christa Winkelmann.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Prior institutional review board (University Hospital Erlangen, Germany) approval was obtained for the analysis on archival material (Reference-No. 4434).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bertz, S., Wach, S., Taubert, H. et al. Micropapillary morphology is an indicator of poor prognosis in patients with urothelial carcinoma treated with transurethral resection and radiochemotherapy. Virchows Arch 469, 339–344 (2016). https://doi.org/10.1007/s00428-016-1986-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1986-x