Abstract
White adipose tissue (WAT) is a critical organ involved in regulating metabolic homeostasis under obese condition. Strategies that could positively affect WAT function would hold promise for fighting against obesity and its complications. The aim of the present study is to explore the effects of treadmill exercise training and rutin intervention on adipose tissue function from diet-induced obese (DIO) mice and whether fat depot-specific effects existed. In epididymal adipose tissue, high-fat diet (HFD) resulted in reduction in adiponectin mRNA expression, peroxisome proliferator-activated receptors (PPAR)-γ and DsbA-L protein expression, elevation in endoplasmic reticulum (ER) stress markers including 78 kDa glucose-regulated protein (GRP-78), C/EBP homologous protein (CHOP) and p-c-Jun N-terminal kinase (JNK). Isoproterenol-stimulated lipolysis and insulin stimulated Akt phosphorylation ex vivo were blunted from HFD group. The combination of rutin with exercise (HRE) completely restored GRP78 and p-JNK protein expression to normal levels, as well as blunted signaling ex vivo. In inguinal adipose tissue, HFD led to increased adiponectin mRNA expression, PPAR-γ, GRP78, and p-JNK protein expression, and reduction in DsbA-L. HRE is effective for restoring p-JNK, PPAR-γ, and DsbA-L. In conclusion, depot-specific effects may exist in regard to the effects of rutin and exercise on key molecules involved in regulating adipose tissue function (i.e., ER stress markers, PPAR-γ and DsbA-L, adiponectin expression, and secretion, ex vivo catecholamine stimulated lipolysis and insulin stimulated Akt phosphorylation) from DIO mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity remains one of the major public health problems reaching epidemic proportions not only in developed countries but also in several developing countries [15]. White adipose tissue (WAT) is a multifunctional organ and dysfunction of WAT is closely associated with impaired metabolic homeostasis under obese condition [36]. Therefore, strategies that could positively affect WAT function would hold promise for fighting against obesity and its complications.
The metabolic features of WAT depend on its anatomical location in the body [18]. Multiple theories have been put forth in the development of WAT dysfunction under obese condition such as inflammation, oxidative stress, and mitochondrial dysfunction [26]. Notably, endoplasmic reticulum stress (ER stress) [23, 34], reduction of adiponectin expression and secretion, and disturbances in catecholamine-induced lipolysis [9, 21] are emerging new players involved in WAT dysfunction. Specifically, Ozcan et al. [34] firstly demonstrated that obesity induced ER stress in the adipose tissue and liver of obese rodent models. Kawasaki et al. [23] further confirmed that ER stress is a key mediator in obesity-induced adipose tissue dysfunction such as chronic inflammation and insulin resistance [23]. Adiponectin is one of the most abundant adipokines secreted from adipose tissue exerting multiple biological functions in other organs such as liver and skeletal muscle [27]. Recent evidence suggest that adiponectin also plays an important role in regulating adipose tissue property, such as affecting adipogenesis [4], glucose uptake [43], and maintaining the metabolic flexibility of adipose tissue [3]. Adiponectin is initially synthesized as a single polypeptide of 30 kDa and is then assembled in the ER, primarily into three forms: trimer, hexamer, and high molecular weight multimer [35]; therefore, a close link between ER stress and adiponectin expression existed. Blunted catecholamine-induced lipolysis in adipose tissue would contribute to the development of increased fat storage, consequently leading to insulin resistance and inflammation [21].
Treadmill running is one of the most commonly utilized models for researching endurance exercise training. WAT is a key organ where exercise exerts its beneficial effects such as suppressing inflammation [22], inducing mitochondrial biogenesis [39], as well as improving ER stress conditions [8]. In regard to how exercise affects adiponectin mRNA expression and secretion, inconsistent findings existed with both induction [31] and reduction in subcutaneous and no alteration in visceral adipocytes [12] has been reported. Furthermore, whether treadmill training could improve impaired β-adrenergic signaling induced by high-fat diet (HFD) in adipose tissue [10] remains unexplored.
Rutin (quercetin-3-O-rutinoside) is a flavonol found in many plants such as buckwheat, apple, and tea [25]. Findings from different laboratories have demonstrated that rutin exerts multiple beneficial effects in obesity-related metabolic disorders [11, 16, 17, 32]. For example, rutin could suppress HFD-induced hyperlipidemia in rats [16] and ameliorate inflammation in the kidney of rats [17]. Rutin is also protective against Aβ-induced neurotoxicity in rats [32]. In regard to adipose tissue, Gao et al. [11] reported that supplementation of rutin could block palmitic acid-mediated inflammation in both macrophages and adipose tissue of diet-induced obese (DIO) mice. Naowaboot et al. [33] recently reported that in cultured 3T3-L1 adipocytes, rutin could induce adiponectin mRNA expression and secretion. Collectively, it is necessary to further explore how rutin will affect ER stress, adiponectin expression and secretion, and catecholamine-induced lipolysis under obese condition in vivo.
Consequently, the aim of the present study is to determine (1) the effects of treadmill exercise training and rutin intervention independently and in combination on in vivo ER stress markers, adiponectin expression, ex vivo catecholamine-induced lipolysis and insulin-stimulated signaling in adipose tissue from DIO mice and (2) whether fat depot-specific effects existed.
Methods
Materials
Medium 199 (cat. no. 31100-035) was from Life Technologies (NY, USA). Glycerol assay kit (cat. no. E1002) was from Applygen Technologies (Beijing, China). Reagents for SDS-PAGE were from Beyotime Institute of Technology (Jiangsu, China). Molecular weight marker and nitrocellulose membranes for SDS-PAGE, and iScript™cDNA synthesis kit were from Bio-Rad (CA, USA). Immobilon Western Chemiluminescent HRP substrate (cat. no. WBKLS0100), mouse adiponectin (cat. no. EZMADP-60 K), and insulin (cat. no. EZRMI-13 K) ELISA kit were purchased from Millipore (MA, USA). Antibodies against phospho-hormone-sensitive lipase (p-HSL) serine563, p-HSLserine660, total HSL, p-c-Jun N-terminal kinase (JNK), p-cAMP-response element binding protein (p-CREB), tubulin, beta-Actin, PPARγ, p-Akt serine 473, p-Akt threonine 308, Akt total, 78 kDa glucose-regulated protein (GRP78), and CHOP were purchased from Cell Signaling (MA, USA). An antibody against DsbA-L was from Abcam (Shanghai, China). Horseradish peroxidase-conjugated donkey anti-rabbit and goat anti-mouse IgG secondary antibodies were purchased from Jackson Immuno-Research Laboratories (PA, USA). Fatty acid-free bovine serum albumin (FA-free BSA) was from Bovogen (Melbourne, Australia).
Treatment of animals
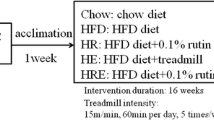
Twelve-week-old male C57BL/6J mice (N = 60) were purchased from Slac laboratory animal (Shanghai, China). All animals were treated in accordance with the Guidelines in the Care and Use of Animals and with the approval of the Animal Studies Committee of Soochow University, Suzhou, China. Mice were raised under standard specific pathogen-free conditions. Mice were housed together in groups of three animals in standard plastic mouse cages (30 × 20 × 13 cm3) and were acclimated to the animal housing facility for 1 week prior to the diet, exercise, and rutin intervention. At 13 weeks of age, mice were randomly divided into the 5 groups with 12 mice each as described previously [5, 6]. Briefly, the 5 groups are as follows:
-
1.
Chow group: The mice were allowed a chow diet ad libitum during the whole period of experimentation (i.e., 16 weeks). The chow diet was purchased from Research diets INC. (ca. no. D12450J, NJ, USA) and 10 % of total energy derived from fat, 70 % from carbohydrate, and 20 % from protein.
-
2.
HFD group: The mice were allowed a high-fat diet (HFD) ad libitum during the whole period of experimentation. The high-fat diet was purchased from Research diets INC. (cat. no. D12492) and 60 % of total energy derived from fat, 20 % from carbohydrate, and 20 % from protein.
-
3.
HR group: The mice were allowed an HFD ad libitum supplemented with 0.1 % rutin (i.e., 100 mg rutin dissolved in 100 g HFD) during the whole period of experimentation. The high-fat diet was the same as above (2) and rutin was added into the food strictly following the above recipe.
-
4.
HE group: The mice were allowed an HFD ad libitum as above (2) and also underwent treadmill training at an intensity of 15 m/min, 60 min per day, and 5 times/week during the whole period of experimentation.
-
5.
HRE group: The mice were allowed an HFD ad libitum supplemented with 0.1 % rutin as above (3) and also underwent treadmill training as above (4) during the whole period of experimentation.
All mice in the treadmill running group have completed the whole training program, and there are no observed harmful effects and no indication for exhaustion. Body weight and food intake were determined weekly for the duration of the study. The weekly body weight of the mice has been shown by Cheng et al. [6] from our research group. Forty-eight hours following the last bout of exercise and rutin intervention, mice were anesthetized with sodium pentobarbital (5 mg/100 g body weight, i.p.). Epididymal and inguinal adipose tissue depots were dissected for further analysis and final epididymal fat weights were shown by Chen et al. [5].
Adipose tissue explants
For the signaling experiment, adipose tissue minces (~50 mg cut into 10–20-mg fragments) from mice were incubated in 2.0 ml of M199 with 2.5 % fatty acid-free BSA at 37 °C in a shaking water bath. Explants were treated with isoproterenol (ISO, 1 μM) or insulin (0.25 mIU/mL) for 30 min. Tissue minces were collected for the determination of the lipolytic and insulin signaling pathway, respectively. For the lipolysis experiment, adipose tissue minces (~50 mg cut into 10–20 mg fragments) from mice were incubated in 2.0 ml of fresh M199 at 37 °C in a shaking water bath. Explants were treated with isoproterenol (ISO, 1 μM) for 2 h. Media were collected for the determination of glycerol release. Lipolytic rates are linear during this time period.
Serum glucose, insulin, and adiponectin measurement
Serum glucose was measured by the hexokinase method on a clinical chemistry analyzer (Hitachi7600, Tokyo, Japan). Serum insulin and adiponectin were measured by ELISA following the manufacturer’s protocol with absorbance read on a microplate reader. All samples were run in duplicate. The coefficient of variation for duplicate samples was <5 % in our laboratory.
Glycerol and adiponectin secretion measurement
Media was analyzed for glycerol concentrations using colorimetric assays according to the manufacturer’s instructions. Glycerol concentrations were corrected for tissue weight and reported as millimoles per liter released per gram tissue. Adiponectin levels in the cultured media were measured via ELISA and corrected for tissue weight and reported as nanograms per milliliter released per milligram tissue. All samples were run in duplicate. The coefficient of variation for duplicate samples was <5 % in our laboratory.
Glucose and insulin tolerance tests
Intraperitoneal glucose (GTT) and insulin tolerance tests (ITT) were performed at the end of the 16 weeks of intervention. For the GTT, mice were fasted for 6 h prior to an i.p. injection of glucose (2 g/kg body weight). For the ITT, mice had free access to food prior to an i.p. injection of insulin (0.75 IU/kg body weight). Blood glucose levels were determined by tail vein sampling at the indicated intervals (i.e., 0, 15, 30, 45, 60, 90, and 120 min) using a glucometer. Changes in glucose over time were plotted, and the area under the curve (AUC) was calculated for the GTT.
Western blot analysis
Protein was extracted from adipose tissue and the content and/or phosphorylation of Akt, HSL, CREB, GRP78, CHOP, PPAR-γ were determined by Western blotting according to Wan et al. [42]. Signals were visualized using Immobilon Western Chemiluminescent HRP substrate and captured using a Syngene chemi-imaging system (MD, USA). Subsequently, bands were quantified by densitometry via Gene Tool according to the manufacturer’s instructions (SynGene, ChemiGenius2, PerkinElmer). Beta-actin or tubulin was used as internal controls.
Real-time PCR
RNA was isolated from adipose tissue using an RNeasy kit according to the manufacturer’s instructions. One microgram of RNA was used for the synthesis of complementary DNA (cDNA) using SuperScript II Reverse Transcriptase, oligo (dT), and dNTP. Real-time PCR was performed using a 7500 Real-Time PCR system (Applied Biosystem). Taqman Gene Expression Assays for Eukaryotic 18S rRNA (cat. no. 4333760T), adiponectin (cat. no. Mm00456425_m1), and PPAR-γ (cat. no. Mm01184322_m1) were from Applied Biosytems (CA, USA). Results were normalized to the mRNA expression of 18S. Relative differences in gene expression between groups were determined using the 2−∆∆CT method [37]. The amplification efficiencies of the gene of interest and the housekeeping gene were equivalent.
Statistical analysis
Data are expressed as means ± SEM. For glucose AUC, serum metabolic characteristics, signaling pathways in vivo, comparisons among groups were made using a one-way ANOVA followed with Tukey’s post hoc test. For ex vivo experiment, comparison were made using Student’s t test within the same group. Statistical significance was established at a p < 0.05.
Results
Glucose and insulin tolerance test
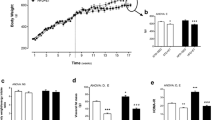
As seen in Fig. 1a, b, HFD led to impaired glucose tolerance compared to chow controls; rutin (HR) and exercise (HE) intervention independently could not reverse impaired glucose tolerance, while the combination of rutin with exercise (HRE) partially restored HFD-induced impaired glucose tolerance. As shown in Fig. 1c, HFD and HR groups have elevated blood glucose level at 15 min post-insulin injection, and the HRE group has reduced blood glucose levels post-insulin injection at 15, 30, 45, and 120 min compared to the HFD group. Meanwhile, the HE group has reduced blood glucose level post-insulin injection at 120 min compared to the HFD group. It is indicated that the combination of rutin with exercise could reverse impaired insulin tolerance post-HFD intervention. We also measured fasting serum glucose and insulin levels, the HFD and HR group have elevated fasting serum insulin levels compared to the chow group, while HE and HRE group have significant reduced insulin levels compared to the HFD group (i.e., chow 53.21 ± 10.66; HFD137.15 ± 7.23; HR 105.62 ± 17.28; HE 76.78 ± 9.99; and HRE 78.53 ± 14.32 mIU/L). The fasting glucose levels were significantly increased in the HFD and HE groups compared to the chow group, while the HRE group has decreased glucose level compared to the HFD group (i.e., chow 7.62 ± 0.46; HFD 9.48 ± 0.43; HR 8.53 ± 0.33; HE 8.88 ± 0.21; and HRE 8.36 ± 0.2 mM/L).
The glucose (a, b) and insulin (c) tolerance test of the mice at the end of the study. During the whole period of experimentation, chow means the mice were allowed a chow diet ad libitum; HFD means the mice were allowed high-fat diet (HFD) ad libitum; HR group means the mice were allowed an HFD ad libitum supplemented with 0.1 % rutin (i.e., 100 mg rutin dissolved in 100 g HFD); HE group means the mice were allowed an HFD ad libitum as above and also underwent treadmill training; HRE group means the mice were allowed an HFD ad libitum supplemented with 0.1 % rutin and underwent treadmill training as above. Data are presented as means + SEM for 12 mice per group. In panels a and b, *p < 0.05 versus chow group. # p < 0.05 versus HFD group. In panel c, the denotation of asterisk and number sign were shown in the figure
Serum adiponectin level and adiponectin mRNA in epididymal and inguinal adipose tissue
As shown in Fig. 2a, HFD reduced circulating total adiponectin level, and rutin or exercise intervention is unable to reverse the decreased adiponectin level. The adiponectin mRNA expression in epididymal adipose tissue was also significantly reduced from the HFD, HR, HE, and HRE group compared to the chow group (Fig. 2b). Surprisingly, the adiponectin mRNA expression in inguinal adipose tissue was significantly increased from the HFD, HR, HE, and HRE groups compared to the chow group (Fig. 2c). We also measured adiponectin secretion in cultured adipose tissue ex vivo via ELISA. As shown in Table 1, in cultured inguinal fat, the secretion of adiponectin level is significantly higher than that in epididymal fat, while we found no difference for adiponectin secretion in cultured inguinal and epididymal adipose among other groups.
Serum adiponectin (a) and adiponectin mRNA expression in epididymal (b) and inguinal adipose tissue (c), respectively. Serum adiponectin level was measured via ELISA and adiponectin mRNA expression was measured via real-time PCR. Data are presented as means + SEM for 12 mice per group in a and 10 mice per group in b and c. *p < 0.05 versus chow group. # p < 0.05 versus HFD group
PPAR-γ and DsbA-L protein expression in epididymal and inguinal adipose tissue
Because both circulating adiponectin level and adiponectin mRNA expression were altered in epididymal and inguinal fat depot, we sought to measure key molecules involved in the regulation of adiponectin expression and secretion. As an initial approach, we primarily focused on PPAR-γ and DsbA-L. PPARγ is a key molecule involved in both transcriptional and post-translational regulation of adiponectin expression [19, 29]. DsbA-L is critical for encoding proteins involved in adiponectin assembly and secretion in the ER [29]. As shown in Fig. 3a, HFD led to reduction in both PPAR-γ and DsbA-L protein expression from epididymal fat. Although rutin (HR) could not affect PPAR-γ and DsbA-L protein expression, exercise (HE) partially restored the decreased DsbA-L protein expression. In contrast, in inguinal adipose tissue, PPAR-γ protein expression was elevated, while DsbA-L was decreased by HFD. The combination of rutin with exercise (HRE) restored PPARγ and DsbA-L protein expression in inguinal fat (Fig. 3b). We also measured PPAR-γ mRNA expression from both epididymal and inguinal adipose tissue. Compared to the chow group, PPAR-γ mRNA expression was decreased in epididymal adipose tissue from the HFD, HR, HE, and HRE groups (i.e., chow 1.07 ± 0.15, HFD 0.7 ± 0.05; HR 0.19 ± 0.05; HE 0.58 ± 0.08; and HRE 0.31 ± 0.26). We found no difference for PPAR-γ mRNA expression among groups from inguinal adipose tissue (data not shown).
Protein expression of ER stress markers in epididymal and inguinal adipose tissue
As shown in Fig. 4a, in epididymal fat, GRP78, CHOP, and p-JNK, which are markers of ER stress [30], were significantly induced by HFD. The combination of rutin with exercise (HRE) completely restored GRP78 and p-JNK protein expression to normal levels. In inguinal fat, GRP78 and p-JNK were also significantly induced by the HFD, HE, and HRE groups have reduced p-JNK but not GRP78 protein expression compared to the HFD group (Fig. 4b).
The protein expression of ER stress markers including GRP78, CHOP, and p-JNK in epididymal (a) and inguinal (b) adipose tissue, respectively. Representative Western blot images are given below the quantified data in c. Data are presented as means + SEM for 12 mice per group. *p < 0.05 versus chow group. # p < 0.05 versus HFD group
Isoproterenol stimulated glycerol release in cultured epididymal and inguinal adipose tissue
In cultured epididymal adipose tissue, ISO significantly stimulated glycerol release at the chow, HE, and HRE groups, whereas ISO stimulated glycerol release was blunted at both the HFD and HR groups (Fig. 5a). In cultured inguinal adipose tissue, HFD resulted in blunted ISO-stimulated glycerol release and rutin and exercise (HR, HE, HRE) completely restored blunted ISO-stimulated glycerol release (Fig. 5b).
Isoproterenol stimulated glycerol release in cultured epididymal (a) and inguinal (b) adipose tissue ex vivo. Glycerol concentrations were corrected for tissue weight and reported as millimoles released per gram tissue weight in a and b. Data are presented as means + SE for ten animals per group.*p < 0.05 versus vehicle treatment within the same group
Isoproterenol-stimulated lipolytic signaling in cultured epididymal and inguinal adipose tissue
Consistently in cultured epididymal adipose tissue, ISO-stimulated phosphorylation of HSL at serine 563, serine 660, and CREB were blunted at both the HFD and HR groups. In the chow, HE, and HRE groups, ISO significantly stimulated the phosphorylation of HSL at serine 563, serine 660, and CREB (Fig. 6a–c). Similarly, in cultured inguinal adipose tissue, HFD resulted in blunted ISO-stimulated phosphorylation of HSL at serine 563, 660, and CREB, while rutin and exercise completely restored blunted ISO-stimulated lipolytic molecules (Fig. 6d–f). It should also be noted that we recently reported that HFD resulted in a reduction in p-HSL serine 660 in vivo from the same set of mice [5], while there is no difference for p-HSL serine 660 in cultured adipose tissue ex vivo in our present study. We interpreted this disconnection as p-HSL serine 660 would be altered if cultured ex vivo, and this also suggests that ex vivo culture could not totally mimic the in vivo situation.
Isoproterenol-stimulated phosphorylation of HSL at serine563, serine 660, and CREB in cultured epididymal (a, b, c) and inguinal (d, e, f) adipose tissue ex vivo. Representative Western blot images are given at the right of the quantified data in g. Data are presented as means + SEM for eight animals per group. *p < 0.05 versus vehicle treatment within the same group
Insulin stimulated Akt phosphorylation in cultured epididymal and inguinal adipose tissue
As shown in Fig. 7, in cultured epididymal adipose tissue, insulin-stimulated phosphorylation of Akt at serine 473 and threonine 308 were impaired from the HFD group, and insulin-stimulated phosphorylations of Akt were normal at the chow, HR, HE and HRE groups (Fig. 7a, b). In cultured inguinal adipose tissue, insulin significantly stimulated the phosphorylation of Akt at all groups (Fig. 7c, d).
Insulin-stimulated phosphorylation of Akt at serine 473 and threonine 308 in cultured epididymal (a, b) and inguinal (c, d) adipose tissue ex vivo. Representative Western blot images are given below the quantified data in e. Data are presented as means + SEM for eight mice per group. *p < 0.05 versus vehicle treatment within the same group
Discussion
In the present study, we found that systematically, the combination of rutin and exercise are efficacious in restoring impaired glucose and insulin tolerance induced by HFD. At the adipose tissue level, there might be depot-specific effects in regard to the effects of rutin and exercise on (1) in vivo ER stress markers, adiponectin mRNA expression and (2) ex vivo catecholamine-stimulated lipolysis and insulin-induced Akt phosphorylation from DIO mice. The combination of rutin with exercise could restore HFD-induced elevation in ER stress markers in epididymal fat, as well as altered PPARγ and DsbA-L protein expression under obese condition in inguinal fat.
The expression, assembly, and secretion of adiponectin are regulated at both transcriptional and post-translational level [28]. PPARγ and DsbA-L are two key molecules involved in these processes [19, 29]. The reduction in serum adiponectin levels post-HFD observed in our present study is consistent with findings by other research groups [2, 46]. This might be owing to the highly saturated fatty acids contained in HFD, which has been reported to reduce adiponectin expression both in humans [41] and in vitro [44]. In epididymal adipose tissue, we found that HFD resulted in reduction in adiponectin mRNA expression; this is consistent with reduced PPAR-γ mRNA and protein expression, as well as decreased DsbA-L protein expression. Neither rutin nor exercise is effective for inducing adiponectin mRNA expression although PPAR-γ protein expression has been restored to normal levels in the HE and HRE groups. In inguinal adipose tissue, a significant induction of adiponectin mRNA expression was observed in the HFD, HR, HE, and HRE groups, and this is explained at least partially by increased PPAR-γ protein expression from these groups. However, the adiponectin secretion in cultured adipose tissue is not consistent with the mRNA expression results, and this further supported that the post-translational regulation of adiponectin is important. It should also be realized that the study by Naowaboot et al. [33] demonstrated that rutin could positively affect adiponectin expression and secretion in 3T3-L1 adipocytes in vitro. Taken in concert, it is suggested that the effects of rutin on adiponectin expression might depend on the specific environment. Our results also suggest that there might be depot-specific responses in regard to adiponectin and PPAR-γ expression under HFD, rutin, and exercise intervention conditions. However, serum adiponectin levels were significantly decreased in the HFD, HR, HE, and HRE groups compared to the chow group. The inconsistency between adiponectin mRNA expression, adiponectin secretion ex vivo and serum adiponectin levels can be explained by multiple reasons. Firstly, owing to the limitation of adipose samples, we were unable to measure different isoforms of adiponectin from the fat depots; it is also likely that adiponectin assembly was disrupted in epididymal and/or inguinal adipose tissue. Secondly, it is possible that except epididymal and/or inguinal adipose tissue, other fat depots such as mesenteric or retroperitoneal adipose tissue are the major fat depots for affecting circulating adiponectin level. All these hypothesis require further clarification. Nevertheless, our present findings suggest that it is important to define fat depots when exploring how strategies affect adiponectin expression and secretion.
Accumulating evidence has suggested that ER stress is a critical factor for affecting adipose tissue function [23, 34]. Swimming exercise has been reported to improve ER stress markers in adipose tissue of obese rats [8]. In various cell lines including leukemic Jurkat cell [20], H9c2 cardiac muscle cell [24] and macrophages [11], and rutin and phytochemicals enriched with rutin could reduce key molecules involved in ER stress markers such as GRP78, ATF6, PERK, and IRE-1α. We also found that HFD induced reputed ER stress markers in both epididymal and inguinal adipose tissue of obese mice, which is in consistency with previous findings [8, 23]. We further found that the combination of rutin with exercise could improve HFD-induced elevation in ER stress markers (i.e., GRP78 and p-JNK) in epididymal fat; while rutin and exercise is ineffective for suppressing GRP78 protein expression in inguinal adipose tissue. GRP78 is a chaperone protein located in the lumen of ER and its synthesis is greatly induced under conditions that lead to the accumulation of unfolded proteins [13]. Our study might suggest that too much unfolded proteins are accumulated in the inguinal fat compared to visceral fat, thus exercise and rutin were ineffective for reversing elevated GRP78 expression in inguinal fat. Alternatively, inguinal fat might be less responsive to exercise and rutin intervention in regard to ER stress markers. Nevertheless, we concluded that the combination of exercise and rutin are effective strategies for reversing ER homeostasis disruption in adipose tissue of obese mice, while depot-specific effects might have existed.
An in vivo blunted catecholamine-induced lipolysis has been reported in obese subjects [9, 14]. Findings from Collins and his colleagues suggest that in virtually all animal models of obesity, catecholamine-stimulated lipolysis were blunted in adipose tissue [7]. We found that isoproterenol-stimulated glycerol release ex vivo was impaired at both epididymal and inguinal adipose tissue. In inguinal adipose tissue, both rutin and exercise restored impaired catecholamine-stimulated lipolysis, as evidenced by the significant induction of glycerol release, as well as isoproterenol-stimulated phosphorylation of HSL at serine 563, serine 660, and CREB, key molecules involved in catecholamine-induced lipolytic signaling pathways [1]. In contrast, in epididymal adipose tissue, only exercise or the combination of rutin with exercise is capable of reversing catecholamine resistance.
Akt is a key molecular player of insulin-stimulated metabolic signaling cascades. The phosphorylation of Akt at both serine-473 and threonine-308 is responsible for regulating most of the PI3K-mediated metabolic function of insulin [40]. Thus, Akt phosphorylation states are usually considered as markers for insulin stimulation status within a tissue. We found that insulin-induced phosphorylation of Akt ex vivo from HFD mice was impaired only in epididymal but not in inguinal adipose depot. In both rutin and exercise groups, insulin could significantly induce Akt phosphorylation in cultured adipose tissue ex vivo. It is interpreted that rutin and exercise are effective for restoring insulin signaling in adipose tissue of DIO mice. Systematically, similar to previous studies [11], we found that HFD caused glucose and insulin intolerance in C57BL/6J mice. The combination of rutin and exercise are efficacious in restoring impaired glucose and insulin tolerance induced by HFD. It should also be noted that in our present study, exercise per se could not reverse HFD-induced glucose tolerance, which is contradictory to findings by some [12, 45], but not all [38] from other research groups. This might be owing to exercise intensity or diet intervention duration etc.
Given the high and increasing prevalence of obesity and its complications, there is an urgent need to identify strategies that can cooperate to prevent and/or treat these conditions. As an approach for carrying out this notion, we also aimed to compare whether the combination of rutin with exercise could exert greater beneficial effects in DIO mice than intervention independently could. Although the combination were more effective for improving systemic glucose and insulin intolerance, at the adipose tissue level, we only found that the combination of rutin with exercise could restore HFD-induced elevation in ER stress markers in epididymal fat, as well as altered p-JNK, PPARγ, and DsbA-L protein expression under obese conditions in inguinal fat.
Finally, it should be noted that our study suffers from several limitations. First, we designed our exercise protocol based on published findings from other research group. We feel that no issue such as overloading training existed, and it would be better if we could have measured oxygen consumption during exercise. Second, different adipose depots would present distinct physiological responses, thus the observed differential effects post-interventions between epididymal and inguinal fat might be a depot-specific effect, but might also be owing to the different physiological responses in different fat depots. Last but not the least, it would be better if we could have measured the insulin level at 30-min post-glucose injection.
Conclusions
In conclusion, we demonstrated that there might be depot-specific effects in regards to the effects of rutin and exercise on in vivo ER stress markers, adiponectin mRNA expression, as well as ex vivo catecholamine-stimulated lipolysis and insulin-induced Akt phosphorylation from DIO mice and that (2) the combination of rutin with exercise could restore HFD-induced elevation in ER stress markers in epididymal fat, as well as altered p-JNK, PPARγ, and DsbA-L protein expression under obese conditions in inguinal fat.
References
Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sul HS (2007) Triacylglycerol metabolism in adipose tissue. Futur Lipidol 2:229–237
Akagiri S, Naito Y, Ichikawa H, Mizushima K, Takagi T, Handa O, Kokura S, Yoshikawa T (2008) A mouse model of metabolic syndrome; increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice. J Clin Biochem Nutr 42:150–157
Asterholm IW, Scherer PE (2010) Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol 176:1364–1376
Bauche IB, El Mkadem SA, Pottier AM, Senou M, Many MC, Rezsohazy R, Penicaud L, Maeda N, Funahashi T, Brichard SM (2007) Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology 148:1539–1549
Chen N, Cheng J, Zhou L, Lei T, Chen L, Shen Q, Qin L, Wan Z (2015) Effects of treadmill running and rutin on lipolytic signaling pathways and TRPV4 protein expression in the adipose tissue of diet-induced obese mice. J Physiol Biochem
Cheng J, Chen L, Han S, Qin L, Chen N, Wan Z (2015) Treadmill running and rutin reverse high fat diet induced cognitive impairment in diet induced obese mice. J Nutr Health Aging 1–6
Collins S, Martin TL, Surwit RS, Robidoux J (2004) Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav 81:243–248
da Luz G, Frederico MJ, da Silva S, Vitto MF, Cesconetto PA, de Pinho RA, Pauli JR, Silva AS, Cintra DE, Ropelle ER et al (2011) Endurance exercise training ameliorates insulin resistance and reticulum stress in adipose and hepatic tissue in obese rats. Eur J Appl Physiol 111:2015–2023
Ek I, Arner P, Bergqvist A, Carlstrom K, Wahrenberg H (1997) Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab 82:1147–1153
Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB (2010) Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol 298:C961–C971
Gao M, Ma Y, Liu D (2013) Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice. Pharm Res 30:2940–2950
Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, Goodyear LJ (2009) Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab 297:E495–E504
Gutierrez T, Simmen T (2014) Endoplasmic reticulum chaperones and oxidoreductases: critical regulators of tumor cell survival and immunorecognition. Front Oncol 4:291
Horowitz JF, Klein S (2000) Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am J Physiol Endocrinol Metab 278:E1144–E1152
Hruby A, Hu FB (2014) The epidemiology of obesity: a big picture. Pharmacoeconomics
Hsu CL, Wu CH, Huang SL, Yen GC (2009) Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J Agric Food Chem 57:425–431
Hu QH, Zhang X, Pan Y, Li YC, Kong LD (2012) Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem Pharmacol 84:113–125
Ibrahim MM (2010) Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11:11–18
Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I (2003) Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52:1655–1663
Jiang Y, Zhang Y, Wark L, Ortiz E, Lim S, He H, Wang W, Medeiros D, Lin D (2011) Wolfberry water soluble phytochemicals down-regulate ER stress biomarkers and modulate multiple signaling pathways leading to inhibition of proliferation and induction of apoptosis in Jurkat Cells. J Nutr Food Sci S2
Jocken JW, Blaak EE (2008) Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol Behav 94:219–230
Jun JK, Lee WL, Park HG, Lee SK, Jeong SH, Lee YR (2014) Moderate intensity exercise inhibits macrophage infiltration and attenuates adipocyte inflammation in ovariectomized rats. J Exerc Nutr Biochem 18:119–127
Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K (2012) Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep 2:799
Kim DS, Kwon DY, Kim MS, Kim HK, Lee YC, Park SJ, Yoo WH, Chae SW, Chung MJ, Kim HR et al (2010) The involvement of endoplasmic reticulum stress in flavonoid-induced protection on cardiac cell death caused by ischaemia/reperfusion. J Pharm Pharmacol 62:197–204
Kuntic V, Pejic N, Ivkovic B, Vujic Z, Ilic K, Micic S, Vukojevic V (2007) Isocratic RP-HPLC method for rutin determination in solid oral dosage forms. J Pharm Biomed Anal 43:718–721
Lafontan M (2014) Adipose tissue and adipocyte dysregulation. Diabetes Metab 40:16–28
Lee S, Kwak HB (2014) Role of adiponectin in metabolic and cardiovascular disease. J Exerc Rehabil 10:54–59
Liu M, Liu F (2010) Transcriptional and post-translational regulation of adiponectin. Biochem J 425:41–52
Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F (2008) A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci U S A 105:18302–18307
Malhi H, Kaufman RJ (2011) Endoplasmic reticulum stress in liver disease. J Hepatol 54:795–809
Miyazaki S, Izawa T, Ogasawara JE, Sakurai T, Nomura S, Kizaki T, Ohno H, Komabayashi T (2010) Effect of exercise training on adipocyte-size-dependent expression of leptin and adiponectin. Life Sci 86:691–698
Moghbelinejad S, Nassiri-Asl M, Farivar TN, Abbasi E, Sheikhi M, Taghiloo M, Farsad F, Samimi A, Hajiali F (2014) Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol Lett 224:108–113
Naowaboot J, Chung CH, Choi R (2015) Rutin stimulates adipocyte differentiation and adiponectin secretion in 3T3-L1 adipocytes. J Med Assoc Thail 98(Suppl 3):S1–S6
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461
Qiang L, Wang H, Farmer SR (2007) Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol 27:4698–4707
Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444:847–853
Rothstein DM, Livak MF, Kishimoto K, Ariyan C, Qian HY, Fecteau S, Sho M, Deng S, Zheng XX, Sayegh MH et al (2001) Targeting signal 1 through CD45RB synergizes with CD40 ligand blockade and promotes long term engraftment and tolerance in stringent transplant models. J Immunol 166:322–329
Straczkowski M, Kowalska I, Dzienis-Straczkowska S, Kinalski M, Gorski J, Kinalska I (2001) The effect of exercise training on glucose tolerance and skeletal muscle triacylglycerol content in rats fed with a high-fat diet. Diabetes Metab 27:19–23
Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC (2009) Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J Physiol 587:1607–1617
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96
van Dijk SJ, Feskens EJ, Bos MB, Hoelen DW, Heijligenberg R, Bromhaar MG, de Groot LC, de Vries JH, Muller M, Afman LA (2009) A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr 90:1656–1664
Wan Z, Thrush AB, Legare M, Frier BC, Sutherland LN, Williams DB, Wright DC (2010) Epinephrine-mediated regulation of PDK4 mRNA in rat adipose tissue. Am J Physiol Cell Physiol 299:C1162–C1170
Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ (2003) Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 52:1355–1363
Xi L, Qian Z, Xu G, Zhou C, Sun S (2007) Crocetin attenuates palmitate-induced insulin insensitivity and disordered tumor necrosis factor-alpha and adiponectin expression in rat adipocytes. Br J Pharmacol 151:610–617
Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G et al (2011) Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 300:R1115–R1125
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N et al (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946
Acknowledgments
This study is supported by the Natural Science Foundation of China (grant no. 81472975 to Wan Z; and grant no. 81273067 to Qin L).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Neng Chen and Ting Lei contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, N., Lei, T., Xin, L. et al. Depot-specific effects of treadmill running and rutin on white adipose tissue function in diet-induced obese mice. J Physiol Biochem 72, 453–467 (2016). https://doi.org/10.1007/s13105-016-0493-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0493-5