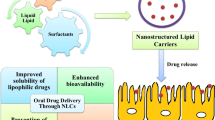
Abstract
For the delivery of the drug, the oral route is the most preferable due to ease of administration. However, the effective delivery of poorly aqueous soluble drugs is very challenging for the pharmaceutical scientist which leads to a low drug permeation profile across the biological membrane, poor drug bioavailability, and ultimately low therapeutic profile with less patient comfort. The inclusion of a therapeutic agent into the nanostructured-based lipid carrier can improve the limitation associated with the poorly soluble drug and it includes better drug therapeutic, pharmacokinetic profile, and controlled drug release up to a longer duration of time which causes patient compliance. Nanostructure lipid carriers (NLCs) are nanosized-based carrier systems which comprise solid lipid matrix combined with liquid lipids and surfactants. The aim of the paper is to explore the various advantages of formulation technology along with the characterization parameter of the NLCs and also report the clinical finding of the investigated NLCs for oral drug delivery system. This paper also highlighted the various patents on the NLCs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
An oral drug delivery system is a technique for giving medication via mouth. This can refer to a variety of pharmaceuticals including tablets, capsules, liquids, and powders. Also due to ease in the administration of drugs through the oral route, this route has been preferred over the other route, but the major limitation associated with the administration of the oral route is the low drug bioavailability, and it may be due to poor drug solubility profile, poor drug permeation across the biological membrane, and hepatic metabolism of the active drug. Drug bioavailability is defined as the amount of the drug that reaches the systemic circulation and is accessible to the body for therapeutic activity. Additionally, it can be significantly influenced by the route of administration of drug delivery method. However, they might not be appropriate for all medications, as some drugs get quickly metabolized which leads to poor absorption of the drug [1]. Various oral drug delivery systems have been formulated to overcome these problems with enhanced drug pharmacokinetics profile, targeted and sustained releases are among them. Sustained and controlled release formulations are developed with the objective to release the drug gradually over a longer duration of time, which can increase patient compliance and lower the frequency of dosing [2]. In this paper, we have emphasized the development and characterization of nanostructured lipid carrier (NLC) as a novel nanocarrier for the oral delivery along with their clinical finding. And also to enhance a drug’s pharmacokinetics and target body regions, novel oral drug delivery systems have been developed [4, 5]. We have also reported the various patents for the oral delivery of NLCs.
1.1 Nanostructured Lipid Carrier (NLC)
Nanostructured lipid carriers (NLCs) are advanced versions of solid lipid nanoparticles (SLN) [6]. The term “nanoparticle” refers to colloidal particulate systems with sizes between 10 and 1000 nm [7]. The second-generation lipid nanocarriers known as NLCs are made of a solid lipid matrix combined with liquid lipids [8]. The structure of NLCs is depicted in Fig. 1.
As a result of the structural issues caused by the integration of liquid lipids (oil), solid lipids have a less ordered crystalline structure, which prevents drug leakage [9]. Researchers are very much focused on NLCs in recent years as a potential replacement for SLNs, polymeric nanoparticles, emulsions, microparticles, liposomes, etc. These nanocarriers have the usefulness in medication distribution for both lipophilic and hydrophilic drugs. For the administration of drugs via oral, parenteral, ophthalmic, pulmonary, topical, and transdermal routes, NLCs have emerged as an efficient carrier system. Recently, NLCs have also been used in the administration of cosmeceuticals and nutraceuticals as well as chemotherapy, gene therapy, and brain targeting [10]. Various benefits and limitations of NLCs are listed in Table 1.
1.2 Types of NLC
NLC can be divided into three types according to the variations in lipid and oil mixture composition and the varied fabrication techniques. The different types of NLCs are presented in Fig. 2.
1.2.1 Imperfect Types
These kinds of NLC include the mixing of lipids that are spatially dissimilar, including glycerides, which are made up of many fatty acids and induce defects in the crystal order. By combining a combination of different glycerides with differing levels of saturation and carbon chain length, the drug loading can be further improved by increasing defects [10, 24].
1.2.2 Amorphous Type
Special lipids like hydroxyoctacosanyl hydroxystearate or isopropyl myristate are mixed with the solid lipid to create an amorphous, structure less matrix. Because of this, the NLC is not in an ordered state but is amorphous, which avoids drug ejection brought on β-modification during storage [7, 10].
1.2.3 Multiple Types
These kinds of NLC have a lot of tiny liquid oil compartments spread throughout the solid matrix. Due to the improved drug solubility in these nanosized compartments, the drug loading is improved. Additionally, solid lipid matrix surrounds the compartments; therefore, the drug release is delayed [10, 26].
1.3 Composition of NLC
NLC is composed of lipid phase and non-aqueous phase. NLCs contain lipids (solid and liquid), surfactants, organic solvents, and other components such as surface-modifying agents. Additionally, the final behavior of the developed formulation depends on the components and their ratios [27].
1.3.1 Lipids
Various types of lipids can be used for the formulation of NLC, and it includes glyceryl dibehenate (Compritol® 888 ATO), oleic acid, etc. However, the selection of lipids depends upon various characteristics such as physiological tolerance, physiochemical structure, drug solubility, and solid lipid/liquid miscibility. The lipids are considered generally recognized as safe (GRAS), meaning that they are unlikely to have harmful effects at the used concentration. The second factor that will affect the condition of lipid at room temperature is its physicochemical structure. Additionally, it is important to check the drug’s solubility in lipid before fabricating NLCs. If the drug is not preferentially dissolved in the lipid core, it will adhere to the surface of the particles resulting in relatively little drug entrapment and loading. Also, solid lipids and liquids can coexist, which requires a study of miscibility by examining the homogeneity and separation of the macroscopic lipid phase below the melting point of fat. The molten lipid phase should be a single phase [26, 28].
1.3.2 Surfactants
Surfactant type and their concentration give directly impact on the effectiveness and quality of NLC like toxicity, physical stability, and crystallinity of NLC [7]. Likewise, drug permeability and the drug dissolution are also affected by surfactant. Due to their amphipathic nature, surface active agents (emulsifiers) are adsorbed on the interface where they lower the tension between the lipid and aqueous phases [29]. Various surfactants and lipids used for the development of NLCs are depicted in Table 2.
1.4 Methods of Preparation of NLCs
There are three different ways to prepare NLCs, which include using high-energy, low-energy, and organic solvents. The various methods of preparation of NLCs are represented in Fig. 3.
1.4.1 Micro Emulsion Method
In this method, drug is added to melted lipids (fatty acids or glycosides, like stearic acid). The melted lipid is added to a mixture of water, co-surfactant(s), and surfactant that has been heated to the same temperature as the lipids. When the compounds are combined in the ideal ratios for micro emulsion development, a transparent, thermodynamically stable system forms. Thus, the micro emulsion serves as the starting point for the development of nanoparticles with a nanosize range. The hot micro emulsion is then gently mechanically mixed with water in a ratio of 1:25–2:50 to disperse it in a cold aqueous media. Then the oil droplets quickly recrystallize [12, 33, 39, 40].
1.4.2 High-Pressure Homogenization
This technique is very crucial for the development of NLCs and SLN due to employing a high pressure of 100–2000 bar onto the liquid lipid via a narrow gap which ultimately generates a high speed of 1000 km/h or more. Additionally, high speed in the form of shear stress and cavitation force minimized the particle size of the liquid upto the nanosize range. Also, when high-pressure homogenization is performed at elevated temperature, then it is called hot homogenization, while at low temperature 2 to 6 °C, it is called cold homogenization. However, the hot method is not good for heat-sensitive drugs, so cold homogenization technique can be used. This method of high-pressure homogenization has been widely used for the preparation of cosmetics and topical formulations. The methods of preparation are shown in Fig. 4 [41, 42].
1.4.3 Hot Homogenization
In this technique, homogenization is carried out under this method at a high temperature that is 5 to 10 °C above the melting point in which the solid lipids melted. Liquid lipid and the drug to be encapsulated are combined to create dispersion. The mixture is disseminated in an aqueous surfactant solution that has been heated to the same temperature using a high-shear mixing apparatus, which results in the production of a pre-emulsion. A regulated temperature pre-emulsion is added to a high-pressure homogenizer. Homogenization typically requires 3 to 5 cycles at 500 to 1500 bar. The lipid recrystallizes as the nano emulsion is gradually cooled, resulting in the creation of nanoparticles. High temperatures used throughout the procedure may cause heat-sensitive components to degrade. Another issue that can develop is a decrease in the surfactants’ ability to emulsify as a result of the high temperature as surfactants have cloud points below 85 °C, but this might make nanocarriers unstable [9, 43,44,45].
1.4.4 Cold Homogenization
In this method, overall temperature is lower than that of a hot homogenization process, effectively eliminating any potential heat-related problem. Also, liquid nitrogen allows for a quick cooling of the drug and lipid mixture. The resulting lipid matrixes are ground, the particles are disseminated in the emulsifier solution, and finally, the mixture is homogenized to produce fine particles. This method has a number of benefits over hot homogenization, including minimized thermal deterioration, increased effectiveness of drug entrapment, and medication diffusion throughout the lipid. Larger particle sizes and a wider size dispersion are seen when using the cold homogenization approach as opposed to the hot homogenization method [46, 47].
1.4.5 Double Emulsion Technique
This technique is basically used for the development of hydrophilic drug-loaded lipid nanoparticle. Additionally, the drug is initially dissolved in an aqueous solvent (inner aqueous phase), then dispersed into a lipid phase (composed of molten solid lipid, liquid lipid, lipophilic surfactant, and lipophilic active components) to form a primary emulsion (water-in-oil). Thereafter, both the lipid and aqueous phases are kept at the same temperature. And the primary emulsion is dispersed into a large volume of aqueous surfactant solution followed by sonication to create a double emulsion (water-in-oil-in-water). The use of stabilizers in this technique is to prevent the drug loss from the external phase during solvent evaporation. Then the lipid nanoparticles are subjected to purification by ultrafiltration or solvent evaporation. This drug delivery technology also facilitates for surface modification of the nanoparticles. Furthermore, for the preparation of NLCs, various factors are to be considered and they include optimized drug concentration, optimized lipid blend, little toxicity, optimized process, nano dimension size, and controlled drug release profile along with improved stability profile [48,49,50]. The preparation of NLCs by this method is shown in Fig. 5.
1.4.6 High Shear Homogenization Followed by Sonication
High shear homogenization followed by ultra-sonication is a preferable method for making NLCs. Under high shear homogenization followed by ultra-sonication, solid and liquid lipid is melted and dispersed in an aqueous surfactant solution, for the development of nano dispersion [49, 50]. Ultrasonic cavitation, which creates violently and asymmetrically imploding vacuum bubbles and breaks apart particles down to the nanoscale, delivers the intense shear forces required for the nano emulsification. The intended outcomes of homogenization, dispersion, de-agglomeration, milling, and emulsification are produced via probe-type ultra-sonication [51]. Strong shear forces are required to create small-size nanocarriers using a process that is repeatable, various factors to be considered, including the type of lipid and surfactant concentration, their ratio, the length of sonication or agitation, and speed. High shear homogenization and ultra-sonication have the limitation of having poor dispersion quality. These techniques frequently result in micro particles, which degrade the lipid nanoparticles dispersion quality and thus lead to physical instability when stored. The other limitation with ultra-sonication is metal contamination from the apparatus [52, 53].
1.4.7 Solvent Emulsification Evaporation Method
This approach involves dissolving the drug and the lipids (solid lipid + liquid lipid) in an organic solvent that is insoluble in water (cyclohexane, chloroform, etc.). The resultant mixture is mixed with emulsifiers in water to prepare an o/w emulsion, followed by vaporization at low pressure. Utilizing evaporation to remove causes the solvent to dissolve nanoparticles in the aqueous phase (by lipid precipitation in the aqueous medium). This technique prevents heat stress; however, using an organic solvent has drawbacks. The solid lipid and surfactant have an impact on particle size, which can range from 30 to 100 nm [46, 47, 54,55,56]. A schematic representation of preparation of NLCs via solvent emulsification evaporation method has been given in Fig. 6.
1.4.8 Solvent Emulsification-Diffusion Method
The solvent employed in the solvent emulsification-diffusion technique must be partially miscible with water, and it includes benzyl alcohol, butyl lactate, and ethyl acetate and can be utilized in either an aqueous phase or an oil. In this phase, water and the solvent were first mutually saturated in order to maintain the two liquids’ original thermodynamic equilibrium. The saturation process is carried out at the temperature that is needed to solubilize the lipid. The drug and lipid were then dissolved in a solvent that was saturated with water, and the resulting organic phase (internal phase) was then emulsified with water and introduced to the system after the development of the o/w emulsion to allow solvent diffusion into the continuous phase and develop aggregation of the lipid in the nanoparticles. The diffusion stage was carried out at either room temperature. Both phases were kept at the same high temperature. The mixture was constantly stirred throughout the procedure. Finally, vaccum distillation or lyophilization was utilized to get rid of the diffused solvent [57,58,59].
1.4.9 Solvent Injection Technique
In the solvent injection approach, lipids are dissolved in a water-miscible solvent like acetone, methanol, or water-miscible solvent combination then rapidly injected into an aqueous solution of surfactants by an injection needle. Additionally in this technique, less technological advanced equipment with simple handling and speed is used [6, 24, 60].
1.4.10 Phase Inversion Technique
This novel and cost-efficient technique for formulating lipid nanocarriers adopts a solvent-free method by transitioning from an oil-in-water (o/w) to a water-in-oil (w/o) emulsion through phase inversion. The process unfolds in two steps.
During Step 1, a mixture of lipid, surfactant, and water in optimized proportions is stirred as the temperature gradually rises at a rate of 4 °C, reaching 85 °C from room temperature. The system undergoes three temperature cycles (85–60–85–60–85 °C) to enter the phase inversion zone.
In Step 2, an irreversible shock is introduced to the system by swiftly diluting it with cold water (0 °C). This rapid addition of cold water prompts the formation of nano structured-based carrier. To prevent particle aggregation, a gentle magnetic stirring is applied for 5 min. The use of low energy results in the formation of stable transparent dispersions with particle sizes smaller than 25 nm, suitable for encapsulating various types of therapeutic agents [61, 62].
1.4.11 Membrane Contactor Technique
This technique is novel for the development of NLCs. In this technique, the development of nano droplets is made possible by the lipid phase being forced through the membrane pores at a temperature above the lipid’s melting point. The droplets that form at the pore exits are removed by the membrane module’s internal aqueous phase, which is constantly circulating. Thereafter, cooling the preparation at room temperature for NLC development. The aqueous phase and lipid phase temperatures, aqueous phase cross-flow velocity, lipid phase pressure, and membrane pore size are process variables that affect NLC particle size. This technique has several advantages and it includes simplicity, ability to manage the NLC size using the proper process parameters, and its scaling-up capabilities [63, 64].
1.4.12 Micro Fluidization Method
This method uses a micro fluidizer, a high-shear fluid device, and the liquid is accelerated to 400 m/s and driven through micro channels at high operating pressures to an impingement region. The method is applicable to both production and laboratory-scale applications [65]. Various drugs used for preparation of NLC are listed in Table 3.
2 Characterization of NLC
To assure their performance, product quality, and stability, appropriate approaches must be used to characterize the physicochemical parameters of NLC. The feasibility of NLCs as a drug delivery system is investigated by a number of evaluation parameters, including particle morphology, interfacial characteristics, drug entrapment efficiency, crystallinity, and polymorphism.
2.1 Particle Size
The most effective techniques for routinely measuring particle size are photon correlation spectroscopy (PCS) and laser diffraction. Dynamic light scattering (DLS) is another name for PCS. It monitors the variations in scattered light intensity brought on by particle motion. This method is limited to a size range of a few nanometers (nm) to 3 µm [75]. Laser diffraction allows for the detection of the greater size. The relationship between the diffraction angle and particle radius provides the foundation for the conclusion. The type and ratio of lipid and emulsifier employed in NLCs show a significant impact on particle size. High emulsifiers are always helpful for better thorough emulsification and a better rigid structure, which allows for a smaller particle size [76].
2.2 Polydispersity Index (PDI)
The polydispersity index (PDI), which is provided by the sample particle size distribution, can also be measured using PCS. Low PDI (0.1–0.25) denotes a uniform and nano size distribution, while PDI above 0.5 denotes a broad size distribution and higher levels of polydispersity. In terms of Brownian motion, PCS demonstrates NLC differs from a nano emulsion due to the non-spherical nature of the particles. Higher PDI in NLC than in nano emulsions is a result of the particle asymmetry [77, 78].
2.3 Zeta Potential
Zeta potential (ZP), which is connected to electrophoretic mobility in the liquid and is known as the sliding or shear plane, is the electric potential of a particle that is not on its surface but rather distant from it in a diffuse layer. It is strongly connected to the stability of the suspension and particle surface shape. In contrast to particle size or molecular weight, ZP depends not only on the particles but also on their surrounding conditions, including pH, ionic strength, and the kinds of ions that are present [79]. ZP provides crucial details regarding nanoparticle stability over time and their susceptibility to aggregate. Excellent stability is generally indicated by a ZP of 60 mV. However, a minimum ZP of 20 mV is preferred for circumstances where stability is the result of both steric and electrostatic stabilization, and a minimum ZP of 30 mV is preferred for cases where stability is the consequence of only electrostatic stabilization. High negative or positive potential on all the particles will cause them to repel one another, reducing the propensity to assemble. Additionally, high zeta potential causes repulsion of charge particles thus leads to the prevention of aggregation and coagulation, while on the other hand, low zeta potential attracts the surrounding particles and tends to coagulate. Analytical tools based on the electrophoretic/electroacoustic mobility principle can be used to measure ZP with ease. A ZP unit and DLS instrument are combined in commercial devices to measure particle charge and particle size with the same device [7, 80, 81].
2.4 NLC Morphology
Morphology describes particle’s surface features, such as its form and surface integrity. In comparison to spherical particles, isometric particles have a higher surface area and shorter diffusion paths [78]. Non-spherical particles need more surfactants to stabilize them because of their larger surface area. In addition, particle size has a big impact on NLC targeted delivery, pharmacokinetics profile, drug loading, encapsulation efficiency, and drug release properties. Additionally, it is crucial for cell interaction, receptor binding, and cellular uptake [83, 84].
For the determination of various features of NLCs like particle size, state of aggregation, shape, surface topography, and internal structure of the NLC can be done by using electron microscopy techniques including scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) [54]. SEM provides details on the particle’s three-dimensional shape and surface properties. The nano dispersion is often transformed into a powder for SEM, primarily through freeze drying. Prior to measurement under high vacuum, these dried particles are then sputtered with a conductor, such as gold, coating the surface. In comparison to TEM, the SEM has a relatively low resolving power of 3–4 nm, and it does not offer any information for the internal structure of the particles [85,86,87].
With a resolving capability of about 0.4 nm, TEM typically produces a two-dimensional image of the interior structure of the nanoparticles. Since the sample electron density affects the fraction of electrons transmitted, components with different electron densities show up as areas of different intensities in the picture. To improve contrast, the sample is typically dyed with heavy metals before being dried and preserved for examination. Compared to SEM, the sample preparation process is relatively complicated because the sample must have an ideal thickness of a few hundred nanometers [38, 88].
Additionally, sample preparation is relatively easy, no vacuum is required to work, and the nanocarriers may be measured in their hydrated condition without causing any morphological distortions or artifact generation; AFM has been widely employed for NLC characterization. A probe is used to scan the sample, which produces an extremely high-resolution image of the sample surface. AFM typically has a lateral resolution of up to 30 nm and a vertical resolution of up to 0.1 nm [12, 49, 59, 89].
In this context, Chun-Yang Zhuang et al. developed vinpocetine-loaded nanostructured lipid carrier. And the formulations were subjected to morphological evaluation with the help of SEM and TEM (JEM-1200EX, JEOL). SEM allowed for the visualization of the surface morphology of VIN-loaded NLC (SSX-550, Shimadzu, Kyoto, Japan). The TEM study showed the particles nearly spherical, homogeneous morphologies and the fact that they did not adhere to one another. A 100–150-nm range covered the average diameter. The TEM and SEM morphology images of VIN-NLC are depicted in Figs. 7 and 8, respectively, with permission [90].
2.5 Entrapment Efficiency
Entrapment efficiency identifies how much of the drug is contained in NLCs, indicating the efficiency of the nanocarriers. High entrapment efficiency of lipophilic drugs was reported due to the nature of NLCs (i.e., high lipid content), which is attributed to the homogeneous solubilization of the drug inside the lipid and development of a stiff solid core during cooling which prolongs the release of the entrapped drug [91]. Equation (1) can be used to determine the quantity of drug trapped in NLCs and measure it spectrophotometrically using UV spectrophotometer or with HPLC at the appropriate λmax after adding enough organic solvent (such as methanol) to dissolve the NLCs and release the drug [30, 92].
The amount of free drug in the supernatant after centrifuging the loaded NLCs suspension can also be determined indirectly using Eq. (2) by taking spectrophotometric measurements at the relevant λmax [93]. In this context, Mara Ferreira et al. developed methotrexate-loaded NLC for the treatment of cancer. They prepared the formulation by using Witepsol1 E85 as the solid lipid and Mygliol1 812 as liquid lipid along with hot ultra-sonication technique and characterized for various parameter like morphology, particle size, zeta potential, entrapment efficiency, stability profile, and in vitro drug release along with cytotoxicity studies. The prepared formulation of NLCs showed spherical shape along with 252 nm of particle size; the PDI and zeta potential was found to be 0.06 ± 0.02 and 14 mv; additionally, the entrapment efficiency was found to be 87%; also, the in vitro drug release was found to be initially rapid and thereafter prolonged release upto 24 h with negative cytotoxicity effect on the fibroblast cell model. these results advocate that NLCs are the portential angent for the drug delivery [94].
2.6 In Vitro Release
The dialysis approach is frequently used to study the in vitro release of drugs from NLCs [92, 95]. Basically, drug-loaded NLCs are put in a dialysis bag that has been soaked in distilled water overnight, secured at both ends, and then placed in a dissolving media while being maintained at 37 °C in a thermostatic shaker. To maintain sink conditions, samples are taken out and replaced at regular intervals with an equal volume of new release medium. Drug concentration can be assessed spectrophotometrically at a suitable λmax or can be calculated using a high-performance liquid chromatography (HPLC) method that has been validated in comparison to standards with known concentrations. The mean cumulative amount of drug release is plotted against time in triplicate. Under the same experimental systems, it is crucial to employ a free drug solution as a control. Thereafter, release data is examined to check the kinetics of drug release from the NLCs [95, 96]. The release profile of NLC is affected by the composition of particle, type and ratio of stabilizing agent employed, difference in dissolution kinetics, the partition coefficient, and the electrostatic behavior of the active molecules [26, 97, 98]. Additionally, Tuan Hiep Tran et al. developed fenofibrate-loaded NLC. They performed in vitro release studies on NLC dispersion within 24 h of preparation by using a dialysis bag. According to their study, the result was found to be approximately 60% of the drug was released from the formulation within 4 h which is showed in Fig. 9 [99].
3 Beneficial Role of NLCs in Oral Drug Delivery
The oral route is the most recommended method of administration due to a number of benefits including patient compliance, precise dose, convenience of administration, and lack of pain. However, these routes have various drawbacks including physical, chemical, and enzymatic degradation in the digestive tract, along with solubility problem therefore very low bioavailability.
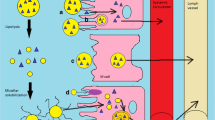
In this regard, it is very urgent to explore the nanoparticulate-based carrier system for the effective delivery of the drug as this novel system has several advantages like improve therapeutic effectiveness, reduce dosage, and low side effects. Additionally, when compared to other nanocarriers, lipid nanoparticles like SLN and NLC also have a number of benefits, including improved permeability, bioavailability, stability by protecting against pH and enzymatic degradation, longer circulation times, less clearance, and longer mean residence times (MRT). NLC has been utilized specifically for oral delivery with the goal of increasing oral bioavailability by improving the lymphatic system uptake of the drug via microfold cells (M cells) in the intestinal membrane and avoiding first-pass metabolism [100, 101]. The lipids in the NLC are partially digested inside the GIT, first in the stomach and subsequently in the small intestines, where they are converted into diglycerides and free fatty acids. Also, the presence of lipids shortens the transit time and lengthens the time spent in the stomach and upper small intestine, therefore leads to increasing absorption. Additionally, the nanoscale significantly increases surface area, which improves NLC’s adherence to gut walls. Also, NLC promotes bile production, which aids in the formation of micelles and the solubilization of drugs. Additionally, it enhances carrier movement through the stagnant barrier between intestinal bulk fluid and enterocytes brush border membrane and improves drug absorption [102].
3.1 Enhancement of Oral Bioavailability
The US Food and Drug Administration (FDA) define bioavailability as the rate at which the active ingredient is absorbed from a drug product and the extent to which it is available at the site of action. The extent refers to how much of the drug concentration enters into the systemic circulation. Poor bioavailability of drugs taken orally is still a problem. However, it is primarily attributed to either physiologically related problems, such as a significant first-pass effect, enterocyte efflux transportation, instability of the drug moiety in gastric fluids, rapid gastric emptying, and intestinal barrier restriction, or physicochemical-related problems, such as poor solubility, an incorrect drug partition coefficient, and a large drug molecular size. Various strategies for improving oral bioavailability have been identified in the literatures which are listed along with drug disposition from NLC and are represented in Fig. 10 [103].
-
a.
Increasing drug solubility is thought to be one method of increasing bioavailability, especially for biopharmaceutical classification system class II (BCS class II) drugs which have low solubility and high permeability (such as atorvastatin [104], olanzapine [71], raloxifene [105], lovastatin [106], fluvastatin [107], nintedanib [95], and vinpocetine [90] and class IV drugs, as low drug solubility is one of the barriers that causes low bioavailability (such as saquinavir [108] and etoposide [109].
-
b.
Due to the fatty composition of NLCs, lipase and co-lipase break them down into micelles, which are composed of drug and lipid monoglycerides and encourage bile flow to generate mixed micelles. By avoiding the first-pass effect and absorbing mixed micelles via chylomicron production into lymphatic capillaries, drugs are more effectively absorbed. This layer is present between the bulk fluid and brush border membrane of enterocytes [102]. When a drug is embedded into a chylomicron, the process of absorbing fat also causes the drug to be absorbed (Trojan horse effect) [110].
-
c.
NLCs and other nano particulate systems have been shown to increase the intracellular uptake of drugs by Peyer’s patch M cells, increasing oral drug bioavailability [111].
-
d.
With the action of various surfactants utilized in the preparation, such as Tween 80, efflux transporter (P-gp) is inhibited.
-
e.
The hydrophilic surfactant pluronic utilized during preparation has a steric hindrance effect that causes the particles to degrade slowly.
-
f.
Drug diffusion through the gastrointestinal barrier will be increased because passive diffusion will continue as long as the concentration gradient is maintained since drug release from nanoparticles is efficient (large surface area).
-
g.
Due to the effect of highly lipophilic surfactants on enhancing paracellular absorption, tight junctions (gaps between two adjacent intestinal epithelial cells) briefly opened [112].
-
h.
Improved retention and uptake caused by nanoparticle adhesion to the intestinal underlying epithelium [113].
4 Patent Status of NLCs
Various patents on NLCs are listed in Table 4.
5 Conclusion and Disscussion
The oral route of drug administration is most preferable due to ease in application. The loading of drug into the lipid-based carrier is termed as nanostructred lipid carriers (NLC), and these are lipid nanoparticles made of a solid and a liquid lipid, as well as a stabilizing surfactant. The special feature of NLCs includ the prevention of drug molecules from degradation and increase the bioavailability of poorly water-soluble drugs along with enhance permeability across biological membrane. Also, due to their capacity to encapsulate both hydrophilic and lipophilic drug, nanostructured lipid carriers (NLCs) have demonstrated significant potential agents as drug delivery. Various methods for the development of NLCs have been discussed in this paper including microemulsion method, high-pressure homogenization method, hot and cold homogenization, double emulsion technique, high shear homogenization followed by sonication, solvent emulsification evaporation, solvent emulsification diffusion, solvent injection technique, phase inversion technique, membrane contactor technique, and micro fluidization method. Additionally, further study is required to perfect the formulation and development of NLCs, as they offer a promising means of enhancing the effectiveness and safety of oral drug administration, including chemotherapy drugs and in gene delivery.
Data Availability
No datasets were generated or analysed during the current study.
References
Homayun, B., Lin, X., & Choi, H.-J. (2019). Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics, 11(3), 129.
Hua, S. (2020). Advances in oral drug delivery for regional targeting in the gastrointestinal tract - Influence of physiological, pathophysiological and pharmaceutical factors. Frontiers in Pharmacology, 11, 524. https://doi.org/10.3389/fphar.2020.00524
Sah, A. K., Vyas, A., Suresh, P. K., & Gidwani, B. (2018). Application of nanocarrier-based drug delivery system in treatment of oral cancer. Artificial Cells, Nanomedicine, and Biotechnology, 46(4), 650–657. https://doi.org/10.1080/21691401.2017.137328
Sahoo, D., Bandaru, R., Samal, S. K., Naik, R., Kumar, P., Kesharwani, P., et al. (2021). Oral drug delivery of nanomedicine. In S. Taurin & K. Greish (Eds.), Kesharwani P (pp. 181–207). Academic Press.
Rahman, M. A., Harwansh, R., Mirza, M. A., Hussain, S., & Hussain, A. (2011). Oral Lipid Based Drug Delivery System (LBDDS): Formulation, characterization and application: A review. Current Drug Delivery, 8(4), 330–345.
Khosa, A., Reddi, S., & Saha, R. N. (2018). Nanostructured lipid carriers for site-specific drug delivery. Biomedicine & Pharmacotherapy, 103, 598–613.
Chauhan, I., Yasir, M., Verma, M., & Singh, A. P. (2020). Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Advanced Pharmaceutical Bulletin, 10(2), 150–165.
Zauner, W., Farrow, N. A., & Haines, A. M. (2001). In vitro uptake of polystyrene microspheres: Effect of particle size, cell line and cell density. Journal of Controlled Release, 71(1), 39–51.
Jain, P., Rahi, P., Pandey, V., Asati, S., & Soni, V. (2017). Nanostructure lipid carriers: A modish contrivance to overcome the ultraviolet effects. Egyptian Journal of Basic and Applied Sciences., 4(2), 89–100.
Jaiswal, P., Gidwani, B., & Vyas, A. (2016). Nanostructured lipid carriers and their current application in targeted drug delivery. Artif Cells Nanomed Biotechnol, 44(1), 27–40.
Müller, R. H., Mäder, K., & Gohla, S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery - A review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics, 50(1), 161–177.
Beloqui, A., Solinís, M. Á., Rodríguez-Gascón, A., Almeida, A. J., Préat, V. (2016). Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine: Nanotechnology, Biology and Medicine, 12(1), 143–61.
Agrawal, Y., Patil, K., Mahajan, H., Potdar, M., Joshi, P., Nakhate, K., et al. (2022). In vitro and in vivo characterization of entacapone-loaded nanostructured lipid carriers developed by quality-by-design approach. Drug Delivery, 29(1), 1112–1121.
Pandita, D., Kumar, S., Poonia, N., & Lather, V. (2014). Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Research International, 62, 1165–1174.
Anwar, W., Dawaba, H. M., Afouna, M. I., Samy, A. M., Rashed, M. H., & Abdelaziz, A. E. (2020). Enhancing the oral bioavailability of candesartan cilexetil loaded nanostructured lipid carriers: In vitro characterization and absorption in rats after oral administration. Pharmaceutics, 12(11), 1047. https://doi.org/10.3390/pharmaceutics12111047
Rao, S., & Prestidge, C. A. (2016). Polymer-lipid hybrid systems: Merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opinion on Drug Delivery, 13(5), 691–707.
Alavi, S. E., Bakht, U., Koohi, Moftakhari, E.M., Adelnia, H., Abdollahi, S.H., Ebrahimi, S.H., & Raza, A. (2022). A PEGylated nanostructured lipid carrier for enhanced oral delivery of antibiotics. Pharmaceutics, 11, 14(8):1668. https://doi.org/10.3390/pharmaceutics14081668
Schäfer-Korting, M., Mehnert, W., & Korting, H. C. (2007). Lipid nanoparticles for improved topical application of drugs for skin diseases. Advanced Drug Delivery Reviews, 59(6), 427–443.
Sartaj, A., Annu, B. L., Verma, A. K., Sahoo, P. K., Baboota, S., & Ali, J. (2022). Ribociclib nanostructured lipid carrier aimed for breast cancer: Formulation optimization, attenuating in vitro specification, and in vivo scrutinization. BioMed Research International, 3, 6009309. https://doi.org/10.1155/2022/6009309
Sanad, R. A., Abdelmalak, N. S., Elbayoomy, T. S., & Badawi, A. A. (2010). Formulation of a novel oxybenzone-loaded nanostructured lipid carriers (NLCs). AAPS PharmSciTech, 11(4), 1684–1694.
Müller, R. H. R. M., & Wissing, S. A. (2002). Nanostructured lipid matrices for improved microencapsulation of drugs. International Journal of Pharmaceutics, 242, 121–128.
Cirri, M., Maestrini, L., Maestrelli, F., Mennini, N., Mura, P., Ghelardini, C., et al. (2018). Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Delivery, 25(1), 1910–1921.
López-García, R. G-RAx-dj. (2015). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Occlusive effect and penetration enhancement ability. Journal of Cosmetics, Dermatological Sciences and Applications, 5(2). https://doi.org/10.4236/jcdsa.2015.52008
Subramaniam, B., Siddik, Z. H., & Nagoor, N. H. (2020). Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. Journal of Nanoparticle Research, 22(6), 141.
Sangsen, Y., Wiwattanawongsa, K., Likhitwitayawuid, K., Sritularak, B., & Wiwattanapatapee, R. (2015). Modification of oral absorption of oxyresveratrol using lipid based nanoparticles. Colloids and Surfaces. B, Biointerfaces, 131, 182–190.
Shah, R. M., Eldridge, D. S., Palombo, E. A., & Harding, I. H. (2017). Microwave-assisted microemulsion technique for production of miconazole nitrate- and econazole nitrate-loaded solid lipid nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics, 117, 141–150.
Syed Azhar, S. N. A., Ashari, S. E., Zainuddin, N., & Hassan, M. (2022). Nanostructured lipid carriers-hydrogels system for drug delivery: Nanohybrid technology perspective. Molecules, 27(1), 289.
Noor, N. M., Sheikh, K., Somavarapu, S., & Taylor, K. M. G. (2017). Preparation and characterization of dutasteride-loaded nanostructured lipid carriers coated with stearic acid-chitosan oligomer for topical delivery. European Journal of Pharmaceutics and Biopharmaceutics, 117, 372–384.
Karn-orachai, K., Smith, S. M., Phunpee, S., Treethong, A., Puttipipatkhachorn, S., Pratontep, S., et al. (2014). The effect of surfactant composition on the chemical and structural properties of nanostructured lipid carriers. Journal of Microencapsulation, 31(6), 609–618.
Fang, C. L., Al-Suwayeh, S. A., & Fang, J. Y. (2013). Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Patents on Nanotechnology, 7(1), 41–55.
Farboud, E. S., Nasrollahi, S. A., & Tabbakhi, Z. (2011). Novel formulation and evaluation of a Q10-loaded solid lipid nanoparticle cream: In vitro and in vivo studies. International Journal of Nanomedicine, 6, 611–617.
Brugè, F., Damiani, E., Marcheggiani, F., Offerta, A., Puglia, C., & Tiano, L. (2015). A comparative study on the possible cytotoxic effects of different nanostructured lipid carrier (NLC) compositions in human dermal fibroblasts. International Journal of Pharmaceutics, 495(2), 879–885.
Ghate, V. M., Lewis, S. A., Prabhu, P., Dubey, A., & Patel, N. (2016). Nanostructured lipid carriers for the topical delivery of tretinoin. European Journal of Pharmaceutics and Biopharmaceutics, 108, 253–261.
Kheradmandnia, S., Vasheghani-Farahani, E., Nosrati, M., & Atyabi, F. (2010). Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomedicine, 6(6), 753–759.
Sarabjot, K., Ujjwal, N., Ramandeep, S., Satvinder, S., & Anita, D. (2015). Nanostructure lipid carrier (NLC): The new generation of lipid nanoparticles. Asian Pacific Journal of Health Sciences., 2(2), 76–93.
Nagaich, U., & Gulati, N. (2016). Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: Design and in vivo characterization. Drug Delivery and Translational Research, 6(3), 289–298.
Liu, C.-H., & Wu, C.-T. (2010). Optimization of nanostructured lipid carriers for lutein delivery. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 353(2–3), 149–156.
Khan, S., Shaharyar, M., Fazil, M., Baboota, S., & Ali, J. (2016). Tacrolimus-loaded nanostructured lipid carriers for oral delivery – Optimization of production and characterization. European Journal of Pharmaceutics and Biopharmaceutics, 108, 277–288.
Moulik, S., & Paul, B. (1998). Structure, dynamics and transport properties of microemulsions. Advances in Colloid and Interface Science, 78, 99–195.
Joshi, M. D., Prabhu, R. H., & Patravale, V. B. (2019). Fabrication of nanostructured lipid carriers (NLC)-based gels from microemulsion template for delivery through skin. Methods in Molecular Biology, 2000, 279–292. https://doi.org/10.1007/978-1-4939-9516-5_19
Leonida, M. D. K. I. (2016). Bionanomaterials for skin regeneration (pp. 55–56). Springer International Publishing.
Sharma, A. K., & Baldi, A. (2018). Nanostructured lipid carriers: A review. Journal of Developing Drugs, 7, 0–0.
Naseri, N., Valizadeh, H., & Zakeri-Milani, P. (2015). Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Advanced Pharmaceutical Bulletin, 5(3), 305–313.
zur Mühlen, A., Schwarz, C., Mehnert, W. (1998) Solid lipid nanoparticles (SLN) for controlled drug delivery--Drug release and release mechanism. European Journal of Pharmaceutics and Biopharmaceutics, 45(2), 149-55.
Severino, P., Santana, M. H., & Souto, E. B. (2012). Optimizing SLN and NLC by 2(2) full factorial design: Effect of homogenization technique. Materials Science & Engineering, C: Materials for Biological Applications, 32(6), 1375–1379.
Wong, H. L., Bendayan, R., Rauth, A. M., Li, Y., & Wu, X. Y. (2007). Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Advanced Drug Delivery Reviews, 59(6), 491–504.
del Pozo-Rodríguez, A., Solinís, M. A., Gascón, A. R., & Pedraz, J. L. (2009). Short- and long-term stability study of lyophilized solid lipid nanoparticles for gene therapy. European Journal of Pharmaceutics and Biopharmaceutics, 71(2), 181–189.
Date, A. A., Joshi, M. D., & Patravale, V. B. (2007). Parasitic diseases: Liposomes and polymeric nanoparticles versus lipid nanoparticles. Advanced Drug Delivery Reviews, 59(6), 505–521.
Uner, M. (2006). Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Die Pharmazie, 61(5), 375–386.
Pinheiro, M., Ribeiro, R., Vieira, A., Andrade, F., & Reis, S. (2016). Design of a nanostructured lipid carrier intended to improve the treatment of tuberculosis. Drug Design, Development and Therapy, 10, 2467–2475.
Keservani, R. K., Sharma, A. K., Kesharwani, R. K. (Eds.). (2019). Nanocarriers for brain targeting: Principles and applications. Apple Academic Press.
Wissing, S. A., Kayser, O., & Müller, R. H. (2004). Solid lipid nanoparticles for parenteral drug delivery. Advanced Drug Delivery Reviews, 56(9), 1257–1272.
Müller, R. H., Petersen, R. D., Hommoss, A., & Pardeike. (2007). Advanced Drug Delivery Reviews, 59(null), 522.
Iqbal, M. A., Md, S., Sahni, J. K., Baboota, S., Dang, S., & Ali, J. (2012). Nanostructured lipid carriers system: Recent advances in drug delivery. Journal of Drug Targeting, 20(10), 813–830.
Varshosaz, J., Minayian, M., & Moazen, E. (2010). Enhancement of oral bioavailability of pentoxifylline by solid lipid nanoparticles. Journal of Liposome Research, 20(2), 115–123.
Shahgaldian, P., Da Silva, E., Coleman, A. W., Rather, B., & Zaworotko, M. J. (2003). Para-acyl-calix-arene based solid lipid nanoparticles (SLNs): A detailed study of preparation and stability parameters. International Journal of Pharmaceutics, 253(1–2), 23–38.
Moinard-Chécot, D., Chevalier, Y., Briançon, S., Beney, L., & Fessi, H. (2008). Mechanism of nanocapsules formation by the emulsion-diffusion process. Journal of Colloid and Interface Science, 317(2), 458–468.
Trotta, M., Cavalli, R., Carlotti, M. E., Battaglia, L., & Debernardi, F. (2005). Solid lipid micro-particles carrying insulin formed by solvent-in-water emulsion-diffusion technique. International Journal of Pharmaceutics, 288(2), 281–288.
Hu, F. Q., Jiang, S. P., Du, Y. Z., Yuan, H., Ye, Y. Q., & Zeng, S. (2005). Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids and Surfaces. B, Biointerfaces, 45(3–4), 167–173.
Schubert, M. A., & Müller-Goymann, C. C. (2003). Solvent injection as a new approach for manufacturing lipid nanoparticles–Evaluation of the method and process parameters. European Journal of Pharmaceutics and Biopharmaceutics, 55(1), 125–131.
Heurtault, B., Saulnier, P., Pech, B., Proust, J. E., & Benoit, J. P. (2002). A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharmaceutical Research, 19(6), 875–880.
Jacob, S., Nair, A. B., Shah, J., Gupta, S., Boddu, S. H. S., Sreeharsha, N., et al. (2022). Lipid nanoparticles as a promising drug delivery carrier for topical ocular therapy-An overview on recent advances. Pharmaceutics., 14(3), 533.
Charcosset, C., El-Harati, A., & Fessi, H. (2005). Preparation of solid lipid nanoparticles using a membrane contactor. Journal of Controlled Release, 108(1), 112–120.
Shidhaye, S., Vaidya, R., Sutar, S., Patwardhan, A., & Kadam, V. (2008). Solid lipid nanoparticles and nanostructured lipid carriers-innovative generations of solid lipid carriers. Current Drug Delivery, 5(4), 324–331.
Karakucuk, A., Celebi, N., & Teksin, Z. S. (2016). Preparation of ritonavir nanosuspensions by microfluidization using polymeric stabilizers: I. A Design of Experiment approach. European Journal of Pharmaceutical Sciences, 95, 111–121.
Nimtrakul, P., Sermsappasuk, P., & Tiyaboonchai, W. (2020). Strategies to enhance oral delivery of amphotericin B: A comparison of uncoated and enteric-coated nanostructured lipid carriers. Drug Delivery, 27(1), 1054–1062.
Khan, N., Shah, F. A., Rana, I., Ansari, M. M., Din, Fu., Rizvi, S. Z. H., et al. (2020). Nanostructured lipid carriers-mediated brain delivery of carbamazepine for improved in vivo anticonvulsant and anxiolytic activity. International Journal of Pharmaceutics, 577, 119033.
Shadambikar, G., Marathe, S., Ji, N., Almutairi, M., Bandari, S., Zhang, F., et al. (2021). Formulation development of itraconazole PEGylated nano-lipid carriers for pulmonary aspergillosis using hot-melt extrusion technology. Int J Pharm X, 3, 100074.
Eid, R. K., Ashour, D. S., Essa, E. A., El Maghraby, G. M., & Arafa, M. F. (2020). Chitosan coated nanostructured lipid carriers for enhanced in vivo efficacy of albendazole against Trichinella spiralis. Carbohydrate Polymers, 232, 115826.
Khan, A. A., Jahanzeb, M., Akhtar, S., Vikneswaran, M., & Yusrida, D. (2019). Freeze-dried lopinavir-loaded nanostructured lipid carriers for enhanced cellular uptake and bioavailability: Statistical optimization, in vitro and in vivo evaluations. Pharmaceutics, 11(2), 97. https://doi.org/10.3390/pharmaceutics11020097
Jawahar, N., Hingarh, P. K., Arun, R., Selvaraj, J., Anbarasan, A., S S., et al. (2018). Enhanced oral bioavailability of an antipsychotic drug through nanostructured lipid carriers. International Journal of Biological Macromolecules, 110, 269-75.
Shah, N. V., Seth, A. K., Balaraman, R., Aundhia, C. J., Maheshwari, R. A., & Parmar, G. R. (2016). Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: Design and in vivo study. Journal of Advanced Research, 7(3), 423–434.
Li, J., Yang, M., & Xu, W. (2018). Development of novel rosuvastatin nanostructured lipid carriers for oral delivery in an animal model. Drug Des Devel Ther, 12, 2241–2248.
Zhang, Q., Yang, H., Sahito, B., Li, X., Peng, L., Gao, X., et al. (2020). Nanostructured lipid carriers with exceptional gastrointestinal stability and inhibition of P-gp efflux for improved oral delivery of tilmicosin. Colloids and Surfaces B: Biointerfaces, 187, 110649.
Mehnert, W., & Mäder, K. (2001). Solid lipid nanoparticles: Production, characterization and applications. Advanced Drug Delivery Reviews, 47(2–3), 165–196.
Li, H., Chen, M., Su, Z., Sun, M., & Ping, Q. (2016). Size-exclusive effect of nanostructured lipid carriers on oral drug delivery. International Journal of Pharmaceutics, 511(1), 524–537.
Jores, K., Mehnert, W., Drechsler, M., Bunjes, H., Johann, C., & Mäder, K. (2004). Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. Journal of Controlled Release, 95(2), 217–227.
Tamjidi, F., Shahedi, M., Varshosaz, J., & Nasirpour, A. (2013). Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innovative Food Science & Emerging Technologies, 19, 29–43.
Xu, R. (2008). Progress in nanoparticles characterization: Sizing and zeta potential measurement. Particuology, 6(2), 112–115.
Thatipamula, R., Palem, C., Gannu, R., Mudragada, S., & Yamsani, M. (2011). Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. Daru, 19(1), 23–32.
Ullah, F., Ali Khan, M. F., Khan, N. H., Rehman, M. F., Shah, S. S., Mustaqeem, M., et al. (2022). Simvastatin-loaded lipid emulsion nanoparticles: Characterizations and applications. ACS Omega, 7(27), 23643–23652.
Chaudhari, V. S., Murty, U. S., & Banerjee, S. (2021). Nanostructured lipid carriers as a strategy for encapsulation of active plant constituents: Formulation and in vitro physicochemical characterizations. Chemistry and Physics of Lipids, 235, 105037.
Moghimi, S. M., Hunter, A. C., & Andresen, T. L. (2012). Factors controlling nanoparticle pharmacokinetics: An integrated analysis and perspective. Annual Review of Pharmacology and Toxicology, 52, 481–503.
Truong, N. P., Whittaker, M. R., Mak, C. W., & Davis, T. P. (2015). The importance of nanoparticle shape in cancer drug delivery. Expert Opinion on Drug Delivery, 12(1), 129–142.
Teeranachaideekul, V., Souto, E. B., Junyaprasert, V. B., & Müller, R. H. (2007). Cetyl palmitate-based NLC for topical delivery of Coenzyme Q(10) - Development, physicochemical characterization and in vitro release studies. European Journal of Pharmaceutics and Biopharmaceutics, 67(1), 141–148.
Das, S., Ng, W. K., & Tan, R. B. (2012). Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? European Journal of Pharmaceutical Sciences, 47(1), 139–151.
Barbosa, J. P., Neves, A. R., Silva, A. M., Barbosa, M. A., Reis, M. S., & Santos, S. G. (2016). Nanostructured lipid carriers loaded with resveratrol modulate human dendritic cells. International Journal of Nanomedicine, 11, 3501–3516.
Klang, V., Valenta, C., & Matsko, N. B. (2013). Electron microscopy of pharmaceutical systems. Micron, 44, 45–74.
Carbone, C., Campisi, A., Musumeci, T., Raciti, G., Bonfanti, R., & Puglisi, G. (2014). FA-loaded lipid drug delivery systems: Preparation, characterization and biological studies. European Journal of Pharmaceutical Sciences, 52, 12–20.
Zhuang, C.-Y., Li, N., Wang, M., Zhang, X.-N., Pan, W.-S., Peng, J.-J., et al. (2010). Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. International Journal of Pharmaceutics, 394(1), 179–185.
Haider, M., Shifaa, M. A., Kamal, L., & Orive, G. (2020). Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics, 12(3), 288. https://doi.org/10.3390/pharmaceutics12030288
Fathi, H. A., Allam, A., Elsabahy, M., Fetih, G., & El-Badry, M. (2018). Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids and Surfaces. B, Biointerfaces, 162, 236–245.
Soni, N. K., Sonali, L. J., Singh, A., Mangla, B., Neupane, Y. R., & Kohli, K. (2020). Nanostructured lipid carrier potentiated oral delivery of raloxifene for breast cancer treatment. Nanotechnology, 31(47), 475101.
Ferreira, M., Chaves, L. L., Lima, S. A. C., & Reis, S. (2015). Optimization of nanostructured lipid carriers loaded with methotrexate: A tool for inflammatory and cancer therapy. International Journal of Pharmaceutics, 492(1), 65–72.
Zhu, Y., Liang, X., Lu, C., Kong, Y., Tang, X., Zhang, Y., et al. (2020). Nanostructured lipid carriers as oral delivery systems for improving oral bioavailability of nintedanib by promoting intestinal absorption. International Journal of Pharmaceutics, 586, 119569.
Makoni, P. A., Khamanga, S. M., & Walker, R. B. (2021). Muco-adhesive clarithromycin-loaded nanostructured lipid carriers for ocular delivery: Formulation, characterization, cytotoxicity and stability. Journal of Drug Delivery Science and Technology, 61, 102171.
Nahak, P., Karmakar, G., Chettri, P., Roy, B., Guha, P., Besra, S. E., et al. (2016). Influence of lipid core material on physicochemical characteristics of an ursolic acid-loaded nanostructured lipid carrier: An attempt to enhance anticancer activity. Langmuir, 32(38), 9816–9825.
Zhao, S., Yang, X., Garamus, V. M., Handge, U. A., Bérengère, L., Zhao, L., et al. (2014). Mixture of nonionic/ionic surfactants for the formulation of nanostructured lipid carriers: Effects on physical properties. Langmuir, 30(23), 6920–6928.
Tran, T. H., Ramasamy, T., Truong, D. H., Choi, H.-G., Yong, C. S., & Kim, J. O. (2014). Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. An Official Journal of the American Association of Pharmaceutical Scientists, 15(6), 1509–1515.
Lin, C. H., Chen, C. H., Lin, Z. C., & Fang, J. Y. (2017). Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. Journal of Food and Drug Analysis, 25(2), 219–234.
(2012). Overcoming poor oral bioavailability using nanoparticle formulations - Opportunities and limitations. Drug Discovery Today: Technologies, 9(2), e71-e174.
Zhou, X., Zhang, X., Ye, Y., Zhang, T., Wang, H., Ma, Z., et al. (2015). Nanostructured lipid carriers used for oral delivery of oridonin: An effect of ligand modification on absorption. International Journal of Pharmaceutics, 479(2), 391–398.
Nguyen, V. H., Thuy, V. N., Van, T. V., Dao, A. H., & Lee, B.-J. (2022). Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano, 8, 100064.
Elmowafy, M., Ibrahim, H. M., Ahmed, M. A., Shalaby, K., Salama, A., & Hefesha, H. (2017). Atorvastatin-loaded nanostructured lipid carriers (NLCs): Strategy to overcome oral delivery drawbacks. Drug Delivery, 24(1), 932–941.
Murthy, A., Ravi, P. R., Kathuria, H., & Malekar, S. (2020). Oral bioavailability enhancement of raloxifene with nanostructured lipid carriers. Nanomaterials (Basel), 10(6), 1085. https://doi.org/10.3390/nano10061085
Zhou, J., & Zhou, D. (2015). Improvement of oral bioavailability of lovastatin by using nanostructured lipid carriers. Drug Design, Development and Therapy, 9, 5269–5275.
El-Helw, A. R., & Fahmy, U. A. (2015). Improvement of fluvastatin bioavailability by loading on nanostructured lipid carriers. International Journal of Nanomedicine, 10, 5797–5804.
Beloqui, A., Solinís, M. Á., Gascón, A. R., del Pozo-Rodríguez, A., des Rieux, A., & Préat, V. (2013) Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. Journal of Controlled Release, 166(2), 115–123.
Zhang, T., Chen, J., Zhang, Y., Shen, Q., & Pan, W. (2011). Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. European Journal of Pharmaceutical Sciences, 43(3), 174–179.
Müller, R. H., Radtke, M., & Wissing, S. A. (2002). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews, 54, S131–S155.
Harde, H., Das, M., & Jain, S. (2011). Solid lipid nanoparticles: An oral bioavailability enhancer vehicle. Expert Opinion on Drug Delivery, 8(11), 1407–1424.
Pathak, K., & Raghuvanshi, S. (2015). Oral bioavailability: Issues and solutions via nanoformulations. Clinical Pharmacokinetics, 54, 325–357.
Beloqui, A., Coco, R., Alhouayek, M., Solinís, M. Á., Rodríguez-Gascón, A., Muccioli, G. G., et al. (2013). Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. International Journal of Pharmaceutics, 454(2), 775–783.
Thapa, C., Ahad, A., Aqil, M., Imam, S. S., & Sultana, Y. (2018). Formulation and optimization of nanostructured lipid carriers to enhance oral bioavailability of telmisartan using Box-Behnken design. Journal of Drug Delivery Science and Technology, 44, 431–439.
Fox, C. B., Khandhar, A. P., Van Hoeven, N., Erasmus, J. H., & Lin, S. S. (2018). Inventors; Infectious disease res inst, assignee. Nanostructured lipid carriers and stable emulsions and uses thereof. WO2018232257A1, 20 December.
Voigt rmkag. (2022). inventor; Advanced Health Institute, assignee. Co-lyophilized rna and nanostructured lipid carrier. Canada patent CA3174411A1. 10/03/2022.
Natalia Niezgoda, A. G. (2021). inventor; patent Office of the Republic of Poland, assignee. Nanostructured lipid carriers with conjugated linoleic acid isomer and method of their preparation. Poland patent PL239568B1. 21/06/2019.
Lígia Nunes De Morais Ribeiro, E. D. P., Michelle, F.-M., Ramos De Castro, S., Rodrigues Da Silva, G. H., Aparecida Guilherme Damasio, V. (2018). Inventor method of obtaining nanostructured lipid-biopolymer films, nanostructured lipid-biopolymer films and their use. Brazil patent BR102017011378A2. 18/12/2018.
Munhoz, F., De Vecchi, R., Caballero, N. E. D., Dini, A. X. P., De Jesus, M. B. (2017) inventor Nanostructured lipid carriers and methods for making them and using them. patent WO 2017/185155 A1.
Geun, K. B. J. P., Heaven, J. J. J. (2017) inventor; Korean patent assignee. Nano-structured lipid carrier comprising α-tocopherol and preparing method thereof. patent KR101777616B. Sep 13.
Lal, R. L. P., Mo, A. (2017). inventor; The Regents of the University of California, assignee. Nanostructured carriers for guided and targeted on-demand substance delivery. United States Patent Application 20170119891. May 4.
Ismail, R. Q. L., Linan, H. L., Hassan, H. A., Yunmei, Z., Basili, M. H., et al. (2016). inventor; Malaysian Palm Oil Council, assignee. Preparation of nanostructured lipid carriers (NLC) method and products made. patent CN102283809B. 16/12/2016.
Acknowledgements
The author Shruti Soni (SS) is grateful to the research supervisor Dr. R. K. Maheshwari and co-supervisor Dr. Abhishek K. Sah, Department of Pharmacy, Shri G. S. Institute of Technology and Science, Indore (M.P.) for their support and guidance for successful completion of this research. Authors SS also thanks the Head of Department, Department of Pharmacy and the Director SGSITS Indore (M.P.) for providing facilities for gathering essential data for my research. Authors SS also acknowledge AICTE, New Delhi, for providing financial assistance during M. Pharm. Research.
Author information
Authors and Affiliations
Contributions
A-wrote the manuscript, B-supervised the research, C-Proof reading, correction and reviewed the manuscript, C-rewriting the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Funding
None.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soni, S., Maheshwari, R.K. & Sah, A.K. Nanostructured Lipid Carrier: Beneficial Role in Oral Drug Delivery System. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01416-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01416-x