Abstract
Nanostructured lipid carrier (NLC)-based gel was developed as a potential topical system for clobetasol propionate (CP) topical delivery for the treatment of eczema. The characterizations of the prepared NLC formulation for topical application on the skin were assessed by means of morphology (SEM), particle size distribution, zeta potential analysis, drug entrapment efficiency, and in vitro drug release studies to select the optimized NLC formulation. The optimized NLC formulation encompasses particle size of 137.9 nm with −20.5 mV zeta potential and 0.224 polydispersity index which indicates good stability of NLC dispersion. NLC formulation showed a good entrapment efficiency of 78.5 % ± 0.03 with cumulative in vitro release 85.42 % up to 24 h. The optimized NLC formulation was suitably gelled and characterized for rheology, drug content, ex vivo drug permeation studies, and drug release kinetics studies. The permeation study revealed that the permeability parameters like steady-state flux (Jss), permeability coefficient (Kp), and enhancement ratio were significantly higher for NLC-based gel formulation as compared to marketed formulation of clobetasol propionate. The value of r 2 (Korsmeyer–Peppas equation) indicated good linearity showing anomalous (non-Fickian) diffusion viz. drug release is controlled by more than one process, i.e., superposition of both phenomenon, the diffusion controlled as well as swelling controlled release. The anti-inflammatory activity of NLC gel via paw oedema technique showed a rapid onset of action, as well as a prolonged duration of action as compared with the marketed gel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology (Greek word “nano” means dwarf) can be defined as the science and engineering of constructing and assembling of objects on a scale smaller than one hundred nanometers [1]. Nanotechnology involves the fabrication of nanodevices which deliver drugs in a controlled and sustained manner. These nanodevices include colloidal carriers like lipid (solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), etc.) and polymeric nanoparticles (chitosan nanoparticles, PLGA nanoparticles) [2].
SLN are nanodevices with a solid lipid matrix. As compared to other lipoidal carriers like liposomes, emulsions, etc., lipid matrix of SLN enables the controlled release of drug due to slower degradation in vivo. SLN own some drawbacks like limited drug loading due to drug expulsion during storage which can be overcome by newer generation of lipid nanoparticles “NLC.” NLC comprises of a mixture of solid lipid and liquid lipid which creates a less perfect crystalline structure with many imperfections thus offering more space for drug accommodation [3]. Examples of solid lipids are triglycerides (tristearine, tripalmitine, trimiristine), fatty acids (stearic acid, palmitic acid), and waxes (carnauba, cetyl palmitate), whereas liquid lipids include, e.g., medium chain triglycerides, oleic acid, and isopropyl myristate. Epidermal targeting can be attained with SLN and NLC formulations [4, 5], which could help in averting distinctive side effects during topical corticosteroid treatment like skin thinning [6] since they are involved with deeper layers of the skin. Moreover, risk of systemic absorption could be eliminated. In fact, lipid nanoparticles (SLN, NLC) have been reported as suitable colloidal carrier systems to control the penetration/permeation of drugs throughout the skin layers [7].
Corticosteroid topical treatment of skin diseases requires assuring of adequate dose delivery of drug on desired area of the skin with less possibility of side effects of these potent drugs like skin atrophy, skin irritation, and photosensitivity. Furthermore, application of dosage form on the skin may be acquainted with a possibility of systemic uptake of the drug. For the elimination of these problems, advanced formulations are still on demand. NLC have been tested as effective carriers of a variety of drugs for topical therapy of skin diseases [8].
Their suitability for dermatological applications was also confirmed by successful formulations of drugs for skin disease treatment [9, 10]. Clobetasol propionate (CP), an analog of prednisolone, used in management of symptoms of asthma and skin disorders connected with inflammation and itching, such as atopic dermatitis [11] or psoriasis or eczema [12]. Being highly lipophilic, this corticosteroid is an excellent candidate for NLC encapsulation. CP is a useful drug with anti-inflammatory, anti-pruritic, and vasoconstrictive properties. CP occurs as a white, almost white or cream-colored, crystalline powder and is odorless.
The present investigation explored the possibility of NLC as a unique carrier system for the topical application of clobetasol propionate with regard to the modulation of release of CP. The in vitro permeation of the drug through rat skin and the anti-inflammatory activity of NLC gel were also evaluated.
Materials and methods
Materials
Clobetasol propionate was a kind gift from Pharmasynth Laboratories, New Delhi. Compritol ATO 888 was received as a gift sample from Gattefosse, France. Glyceryl monostearate, oleic acid, poloxamer 188, sodium deoxycholate, sodium taurocholate, carrageenan, carbopol 934, HPMC, glycerol, methyl paraben, and triethanolamine were purchased from S.D Fine-Chem Pvt. Ltd. (Mumbai, India). Dialysis membrane 70 was purchased from Sigma Aldrich Pvt. Ltd., Mumbai, India.
Statistical analysis of data
All the values of the experimental results are expressed as mean ± standard error of mean (SEM). The values were analyzed by ANOVA (Dunnett’s test) for the possible significant identification between various groups. P < 0.05 was considered statistically significant. Statistical analysis was carried out using the GraphPad Prism 5.04 (GraphPad Software Inc.).
Methods
Solid lipid screening
The lipids used for this study were glyceryl behenate (compritol 888 ATO) and glyceryl monostearate. This is performed by dissolving increasing amounts of drugs in various melted solid lipids and determining the maximum amount of the active that could be dissolved in each lipid. After dissolution, the lipid/drug mixtures were cooled down to room temperature for solidification [13]. The solid mixtures are visually observed for the presence or absence of crystalline active (when this ingredient is a solid substance at room temperature).
Partitioning behavior of CP in various lipids
Ten milligrams of both drugs was dispersed in a mixture of melted lipid (1 g) and hot distilled water (1 ml). The mixture was shaken for 30 min in a hot water bath. After cooling, the aqueous phase was separated by ultracentrifugation at 10,000 rpm for 20 min in refrigerated centrifuge (SIGMA F-18 K, Sartorious) [14], and the drug content was analyzed spectrophotometrically.
Preparation of NLC
CP-loaded NLC were formulated via hot high-pressure homogenization technique. Formulations were prepared after extensive literature survey based on hit and trial technique. Compritol® ATO 888 (solid lipid) and oleic acid (liquid lipid) were mixed and melted at 85 °C to obtain a clear lipid phase. Quantities of all excipients are given in Table 1. CP (drug) was then added to melted lipid mixture to obtain a clear solution. Meanwhile, an aqueous surfactant solution with poloxamer 188 (surfactant) and sodium taurocholate (co-surfactant) was prepared and heated at the same temperature. The hot surfactant solution was then dispersed drop wise via syringe in the hot lipid phase with continuous stirring on mechanical stirrer at 2000 rpm for 15 min [15]. The obtained emulsion was then homogenized at 85 °C, using an Ultra-Turrax T25 (IKA-werke GmBH, Germany) under a pressure of 500 bar and three homogenization cycles. NLC dispersion obtained was freeze dried to obtain a dry and free flowing powder.
Characterization of CP-loaded NLC
Particle size and particle size distribution measurement
The particle size of NLC systems was measured using photon correlation spectroscopy (PCS) on a Malvern Mastersizer 2000MU (Malvern, UK, detection limit 0.01–1000 μm). For the determination of particle size, the undiluted sample was transferred into a disposable plastic cuvette. The cuvette was then manually shaken for about 5–10 s and then kept on the sample holder. Average particle size and polydispersity index were measured by photon correlation spectroscopy at a 90o angle at 25 °C [16]. All measurements were carried out in triplicate.
Zeta potential analysis
The surface charge on the nanoparticles was quantified by measuring the zeta potential using Zetasizer (3000HS Malvern Instruments, UK). Prior to analysis, NLC suspensions were diluted with double distilled water (1:100) to obtain uniform dispersions and then conductivity was measured at 25 °C [16]. All reported values are the mean of three separate measurements.
Morphology
Shape and surface morphology of NLC was visualized by a scanning electron microscope (SEM) (LEO-430 Cambridge and UK). One drop of sample was placed on a slide, and excess water was left to dry at room temperature. The slide was attached on an aluminum stub using double-coated adhesive tape and then stubs were coated with gold to a thickness of 200 to 500 A under an argon atmosphere using a gold sputter module in a high vacuum evaporator [16]. Photomicrographs were taken at different magnifications.
Drug entrapment efficiency (DEE) and drug loading (DL)
A volume of 2.0 ml of drug-loaded sample was centrifuged (SIGMA F-18 K, Sartorious) at 12,000 rpm for 30 min at 20 °C to separate the lipid and aqueous phase. The supernatant was then filtered through 40 μm filter paper (Hi-media, Mumbai), and the absorbance of the sample was noted by the UV-VIS spectrophotometer (UV-1800, Shimadzu, Japan) at 236 nm [17]. The concentration of drug was calculated from the calibration curve (calibration curve range 0 to 16 ug/ml and linear equation y = 0.013 × −0.004 with r 2 = 0.997). The entrapment efficacy of NLC was calculated as follows:
Drug Entrapment Efficiency (%w/w) = Total amount of drug − Amount of drug in supernatant × 100/Total amount of drug
Drug Loading (%w/w) = Initial drug – free drug/Mixed lipid × 100
In vitro release study of CP-loaded NLC
The in vitro release studies were performed using Keshary-Chien (K-C) cell to evaluate the CP release profile from each formulation. The diffusion cells were thermoregulated with a water jacket at 37 ± 2 °C. Dialysis membrane 70 (Hi-Media, Mumbai, India) having a pore size of 2.4 nm and a molecular weight cutoff between 12,000–14,000 Da was used and mounted on K-C cells. The surface area of the release membrane was 3.14 cm2. Phosphate buffer saline (PBS; pH 7.4) was used as the receptor medium (10 ml) being stirred at 100 rpm. NLC dispersion (equivalent to 0.5 mg of CP) was placed in the donor compartment. During the experiments, the solution in receptor side was maintained at 37 ± 0.5 °C. At predetermined time intervals, 5 ml of the samples were withdrawn from the receiver compartment and replaced by the same volume of freshly prepared PBS (pH 7.4) [11]. The withdrawn samples were analyzed by the UV-Visible spectrophotometer (SYSTRONICS; Model: 2201) at 236 nm.
Preparation of CP-NLC-based gel
After optimization of clobetasol propionate-nanostructured lipid carriers (CP-NLC), CP-NLC-loaded topical gels were formulated by dispersion technique utilizing Carbopol 934 and HPMC K4M. All the accurately weighed ingredients were taken in quantity as shown in Table 2. Then, Carbopol 934 and HPMC K4M were dispersed in water and kept aside for 4 h for proper swelling of polymer. CP-loaded NLC powder (equivalent to 0.5 mg/g) was added to the gel with constant unidirectional mixing so as to avoid incorporation of air bubbles to it. The gel mixture was properly mixed and then sonicated to remove any air bubbles. Triethanolamine was then added to polymeric mixture of Carbopol 934 and HPMC K4M so as to enhance the cross linking between polymer which results in clear gel formation. Then, glycerol was added to the gel to balance its viscosity, and the pH was adjusted to skin pH to 7.4 ± 0.1 with the help of 0.1 N NaOH. [17].
Characterization of CP-NLC-loaded topical gel
The basic physicochemical parameters were studied for the developed formulation.
Physical appearance and formulation pH
The prepared gel formulation was inspected visually for their color, clarity, homogeneity, and appearance. The pH values of 1 % aqueous solution of the prepared gel was measured by a pH meter (Systronics, 361-micro pH meter). One gram per meter of gel was dissolved in 100 ml distilled water and stored for 2 h [18]. The measurement of pH of each formulation was done in triplicate and average values are calculated.
Rheological analysis
Viscosity
To analyze the rheological properties, all the formulated gels were taken in beakers and placed beneath the spindle, and the spindle (RV-7) was rotated at 10 rpm at room temperature (25–27 °C) in Brookfield viscometer.
Spreadability
The spreadability measurements of the CP-NLC gel was made in triplicate by using glass slide method. One gram per meter of gel was kept between the two slides. The preweighted plate was kept above the gel, and more weights were added on the plate until the gel stop spreading. Final cumulative weight and the total time taken by the gel to spread were measured and noted. Then total weight applied and mass of the gel were compared by the time [18].
Spreadibility = Mass × Length/Time
Drug content
To determine the drug content of gel, 10 mg of CP-NLC-loaded gel was weighed and mixed to the phosphate buffer saline (PBS pH 7.4), and the concentration of CP was observed spectrophotometrically (UV 1601, Shimadzu, Japan) measured at 236 nm. The NLC gel base containing the identical amount of ingredients without CP was used as a blank.
Ex vivo drug permeation studies
The ex vivo drug release studies were carried out with the help of K-C diffusion cell. Rat abdominal skin was used to study permeation. The subcutaneous tissue was removed surgically and the dermis side was wiped with isopropyl alcohol to remove adhering fat. The cleaned skin was washed with distilled water and stored at −18 °C until further use. The skin was mounted between the donor and receiver compartments of the K-C cell where the stratum corneum side was facing the donor compartment and the dermal side was facing the receiver compartment [13–15]. One gram (equivalent to 0.5 mg CP) of each gel (CP-NLCG1 and CP-NLCG2) and TEMOVATE gel (TG, marketed clobetasol propionate gel) was placed in a donor compartment. The receptor compartment was filled with 10 ml PBS (pH 7.4), thermoregulated at 37 °C and magnetically stirred at 400 rpm. Two milliliters of receptor fluid was withdrawn at an interval of 0.5, 1, 2, 3, 4, 8, 10, 12, 14, 18, and 24 h. An equal volume of PBS was simultaneously added to the receptor compartment after each sampling to maintain sink conditions. Each sample was filtered through a 0.45-um polyamide membrane filter (Sartorius AG, Germany) and then determined for CP content by UV spectrophotometer [19, 20]. The concentrations of all the formulations in withdrawn samples were calculated and then the percent drug release was determined.
Permeability data analysis
Percentage of drug permeated through the skin (mg cm−2) was plotted as a function of time for each formulation. Drug flux (permeation rate) at steady state (Jss) was calculated by dividing the slope of the graph linear portion with the diffusion cell area (mg cm−2 h−1). Permeability coefficient (Kp) was calculated by dividing Jss by the initial concentration of the drug in the donor cell (cm h−1). Enhancement ratio (Er) was calculated by dividing Jss of the respective formulation by Jss of the control formulation.
Drug release kinetics studies
The obtained data from in vitro drug release studies was plotted as percent drug released vs time (zero order equation), log percentage of drug remaining vs time (first order equation), percentage of drug released v/s square root of time (Higuchi’s model equation), and log percentage drug released vs log time (Korsmeyer’s equation) to evaluate the drug release mechanism. Standard values of release mechanism with variation in n values are tabulated in Table 3.
Stability studies
The optimized formulation was subjected for the stability studies. The samples were stored at 5 ± 1 °C, 25 ± 2 °C, 40 ± 2 °C, 60 ± 2 °C, and 75 ± 5 % RH for 6 months to access their stability. Samples were analyzed for drug content vs time and log percent drug content vs time graph was plotted in order to evaluate shelf life of the formulation [21]. The protocols of stability studies were in compliance with WHO guidelines for stability testing intended for the global market.
In vivo anti-inflammatory studies: carrageenan-induced paw edema technique
The anti-inflammatory study was conducted on adult male Wistar albino rats having weight range between 150–250 g. The study was reviewed and approved by the Institutional Animal Ethical Committee with registration number 837/ac/O4/CPCSEA. The animals were divided into three groups, each group comprises of six animals. Group I received topical saline application (control group), group II received marketed gel (standard group), and group III received NLC-based gel formulation (test group). The volume of paw edema (milliliter) was measured for each animal using a digital plethysmometer (Orchid Scientfics, India). The left hind paws of rats were marked just beyond the tibia-tarsal junction, and to ensure constant paw volume, every time the paw was dipped in an electrolyte fluid column up to a fixed mark. The test preparation was applied to the left hind paws of rats for 30 min before the induction of inflammation by carrageenan. The initial paw volume of the rats was measured just before carrageenan injection, and the increase in volume due to fluid displacement was noted from a digital display, followed by the injection of 0.1 ml of 1 % (w/v) carrageenan solution in saline in the sub plantar region of left hind paws of the rats. Measurement of paw volumes was done after 1, 2, 4, 8, 12, and 24 h. The edema rate and inhibition rate of each group were calculated as follows:
Edema Rate (E%) = (Vt − Vo)/Vo × 100
Inhibition Rate (I%) = (Ec − Et)/Ec × 100
Where Vo is the mean paw volume before carrageenan injection (ml), Vt is the mean paw volume after carrageenan injection (ml), Ec is the edema rate of control group, and Et is the edema rate of the treated group.
Result and discussion
Screening of components for CP solubility
To develop a NLC system of poorly water-soluble drug CP, a selection of suitable solid lipid, liquid lipid, surfactant, and co-surfactant is very critical. All excipients under the generally regarded as safe (GRAS) category were selected for the formulation of NLC.
Solid lipid screening
Lipophilic drugs mostly show high solubility in liquid lipids, but exhibited limited solubility in solid lipids. In order to develop stable NLC, two solid lipids have been studied, i.e., Compritol 888 ATO and glyceryl monostearate. Additionally, the higher solubility of the drug in the solid lipid is important for the NLC formulation to maintain the drug in solubilized form. CP was found to have maximum solubility in Compritol 888 ATO compared to glyceryl monostearate in this analysis as it shows no solid crystalline structures on visual inspection. Thus, Compritol 888 ATO was preferred to make CP-loaded NLC as solid lipid matrix. In fact, the crystalline structure of glyceryl behenate comprises of very minute quantity of unstable α polymorphic form of triacylglycerol, which vanishes after thermal stress of bulk lipid. Thus, drug gets easily solubilized in this lipid [22].
Partitioning behavior of CP in various lipids
The estimation of the partitioning behavior of the drug is a significant decisive factor, as it has an effect on the entrapment efficiency as well as the drug release from the formulation. CP revealed higher partitioning in Compritol 888 ATO, i.e., 3.561 as compared to Glyceryl Mono Stearate, i.e., 1.597. Thus, Compritol 888 ATO was selected to formulate CP-loaded NLC system.
Selection of formulation technique
For the NLC formulation, two techniques were tried, i.e., hot high-pressure homogenization and solvent emulsification evaporation technique. On the basis of particle size and drug entrapment efficiency, selection of formulation techniques was done. Remarkably, hot high-pressure homogenization technique exhibited the smallest particle size (419.50 nm ± 0.654) and highest entrapment efficiency (50.19 % w/w ± 0.134) in comparison to solvent emulsification evaporation technique with particle size of 1510 nm ± 0.765 and entrapment efficiency of 47.08 % w/w ± 0.234. Additionally, this technique is very simple compared to others and less use of organic solvents revealed its green and eco-friendly nature.
Optimization of process parameters
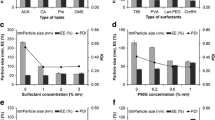
Prior to formulation of NLC, several process parameters viz. solid lipid to liquid lipid ratio, surfactant, and co-surfactant concentration, stirring time, stirring speed, and homogenization cycles were optimized at a broad range to enable the selection of best formulation. Solid lipid (SL) to liquid lipid (LL) ratio was optimized on the basis of particle size and entrapment efficiency. Different ratios of SL to LL were taken as 60:40, 70:30, 80:20, and 90:10, respectively. Out of which, 70:30 SL to LL ratio turned out to give minimum particle size, i.e., 307.78 nm ± 0.321, and optimum entrapment efficiency of 56.18 % w/w ± 0.743 as shown in Fig. 1. Surfactant properties and concentrations greatly affect the quality and efficacy of lipid nanoparticles. Surfactants possess surface activity, meaning they preferentially locate in interfacial regions Therefore; the concentration of surfactant (Poloxamer 188) was optimized. The various ratios used for the formulation were 1, 1.5, 2, and 2.5 % w/v. The surfactant was added at the time of emulsification, and the concentration of surfactant was optimized regarding the drug entrapment, particle size, and aggregation after 24 h. Surfactant concentration of 1.5 % w/v revealed clear transparent dispersion having the lowest particle size of 216 nm ± 0.176 with 45.38 % w/w ± 0.386 entrapment efficiency, while 2.0 and 2.5 % w/v gave translucent and opaque dispersion which may be due to particle aggregation. Thus, 1.5 % w/v of surfactant concentration was selected [23]. Surfactant mixtures often reduce interfacial tension more than single surfactant formulations. This phenomenon is largely due to an increased surfactant concentration at the interface, or surface excess, made possible by the minimization of repulsion forces of closely packed, like surfactant molecules. The types of non ionic co-surfactant (sodium taurocholate and sodium cholate) were optimized for a 1 % w/v surfactant. The co-surfactant was added in aqueous phase at the time of emulsification, and the type co-surfactant was optimized regarding the drug entrapment and particle size. Due to smaller particle size of 254.9 nm ± 0.187 and high drug entrapment of 53.35 % w/w ± 0.245, sodium taurocholate was selected as co-surfactant. Different stirring speeds, i.e., 1000, 1500, 2000, 2500 rpm, for the formulation of NLC were taken, out of which 1500 rpm was selected since it results in the lowest particle size 217.8 nm ± 0.234 and optimum entrapment efficiency 56.68 % w/w ± 0.294 as given in Fig. 2. Additionally, stirring time was also optimized for different time intervals, i.e., 10, 15, 20, and 35 min. Fifteen min was selected as optimum stirring time since it gives the lowest particle size. Homogenization cycles were optimized on the basis of particle size and entrapment efficiency for 3, 5, and 7 cycles. It was observed that as the cycle number increases, particle size first decreases followed by slight increase. This might be due to decrease in temperature of the system with increase on homogenization cycle which results in increase in kinetic energy, thus particles reassemble when prepared [24]. Three cycles give the smallest particle size of 228.76 nm ± 0.187 and optimum drug entrapment of 54.84 % w/w ± 0.126 as shown in Fig. 3.
On the basis of evaluation parameters, formulation table was developed taking in consideration of optimized parameters, i.e., 0, 1 (optimized parameter), and +1 level was taken as given in Table 1. Clobetasol propionate-loaded NLC were successfully formulated using hot pressure homogenization technique and characterized for several parameters like particle size, entrapment efficiency, zeta potential, and in vitro drug release.
CP-loaded NLC showed particle size in the range of 137.9 nm ± 0.45 to 196.8 nm ± 0.36. Formulation CPF2 displayed minimum particle size of 137.9 nm ± 0.45 which is the prime condition for the topical delivery of NLC. This might be due to optimum concentration of combination of surfactant and co-surfactant which decrease the particle size because of its synergism. It is observed that particle size decreases with increase in surfactant concentration, as examined for CPF1 and CPF2 as given in Table 4. But it eventually increases which may be attributed to the increase in homogenization cycle and decrease in liquid lipid content. As homogenization cycle increases, kinetic energy of the system increases which results in particle aggregation during particle–particle collision [24]. Smaller size helps in targeting and increases penetration of drug through biological membranes. The foremost criterion for nanoparticle-loaded topical drug delivery system is its permeation through the skin; especially in the case of NLC, particle size should be small enough to permeate the skin. Therefore, entrapment efficiency can be overruled or is considered less significant than particle size for the development of NLC formulation. The zeta potential values for all three formulations were in the range of −16.3 to −17.5 mV. A high zeta potential will confer storage stability, i.e., the dispersion will resist aggregation. It is investigated that zeta potential increases (CPF1 < CPF2) first and then decreases. This may be credited to the combination of surfactant (Poloxamer 188) which is non ionic in nature and co-surfactant (sodium taurocholate) which is ionic in nature. Ionic emulsifier leads to the highest zeta potential because after ionization in water, it gets adsorbed on particle/water interface and forms electric double layer. Formulation CPF2 has high co-surfactant concentration, thus high zeta potential as given in Table 4. Polydispersity index is the measure of particle size distribution of nanoparticles. Samples with broad size distribution have polydispersity values less than 0.7 which clearly indicates a uniform dispersion. The values of polydispersity index (PDI) lies in the range of 0.224 to 0.462. Formulation CPF2 revealed the smallest value of PDI viz. 0224. It is observed that value of PDI decreases with increase in oleic acid content.
Shape and surface morphology of the CP-loaded NLC were visualized by scanning electron microscopy (SEM). They were found to be spherical and with a smooth appearance as shown in Fig. 4. NLC revealed uniform size distribution as shown in Fig. 5. This could be due to optimum solid lipid to liquid lipid ratio along with other optimized process parameters.
Entrapment efficiency of CP-loaded NLC was found to be in the range of 63.24 % w/w ± 0.71 to 85.42 % w/w ± 0.03. It is investigated that as the concentration of liquid lipid (oleic acid) increases, entrapment also increases. Encapsulation efficiency is always correlated with the crystallinity degree of lipid nanoparticles. The more oleic acid in the mixture, the higher the encapsulation efficiency. Incorporation of oleic acid increases the amorphous proportion in the solid lipid matrix, and as a result decreases the overall particle crystallinity, thereby improving the encapsulation efficiency. Drug loading of CP-loaded NLC were found to be in the range of 12.38 % w/w ± 0.24 to 17.43 % w/w ± 0.17. CP-loaded NLC (CPF2) displayed maximum in vitro drug release 85.42 % up to 24 h as compared to CPF1 (69.24 % ± 0.19) and CPF3 (75.28 % ± 0.15) as shown in Fig. 6. The in vitro release of the CP from the NLC dispersion was found to be biphasic, with the initial burst effect followed by gradual release of the CP. The initial burst release might be due to either the presence of unentrapped drug in the NLC dispersion or due to localization of liquid lipid in outer shell which contains lipophilic drug in dissolved form and leads to initial burst release at initial stage. Release may occur by matrix erosion or diffusion [11]. Initial burst release provides the drug for immediate therapeutic action and improves the drug penetration while sustained release deliver the drug over a prolonged period of time and maintain therapeutic drug concentration at the site of action. This clearly indicates that sustained release could be obtained by using this formula with single dosing frequency.
Characterization of CP-NLC-loaded topical gel
Formulations were transparent, clear, and homogeneous in texture. pH ranges between 7.1–7.4 range for both formulations, which could easily be tolerated on skin without irritation. CP-NLG1 has low viscosity 56,000–63,000 cps, i.e., because of low concentration of HPMC as compared to CP-NLG2 (65,000–72,000 cps). CP-NLCG1 is quite easy spreadable on application as compared to CP-NLCG2 since its viscosity is slightly low. Results for NLC-loaded gel characterization are tabulated in Table 5. Values for percent in vitro release for CP-NLCG1, CP-NLCG2, and TG are 86.28 % (up to 24 h), 84.98 % (up to 18 h), and 85.29 % (up to 12 h), respectively, as given in Fig. 7.
The permeation study revealed that the permeability parameters like steady-state flux (Jss), permeability coefficient (Kp), and enhancement ratio were significantly higher in both of the CP-NLCG1 and CP-NLCG2 formulations, compared to TG. The cumulative amount of the permeated drug at the end of 24 h was 0.4314, 0.4281, and 0.4114 mg/cm2 with a steady-state flux (Jss) of 0.137, 0.1362, and 0.131 mg/cm2/h for CP-NLCG1, CP-NLCG2, and TG, respectively. Value of permeability coefficient is high for CP-NLCG1 (2.66 cm/h) > CP-NLCG2 (2.1 cm/h) > TG (1.84 cm/h). Enhancement ratio of CP-NLCG1 and CP-NLCG2 is 1.045 and 1.040, respectively, as compared to TG gel formulation. It was found that the in vitro drug release from the optimized CP-NLCG1 was best explained by Higuchi’s equation, as the plots showed the highest linearity (R 2 = 0.9836), followed by first order (R 2 = 0.9637) and zero order (R 2 = 0.892). The corresponding plot of (log % cumulative drug release vs log time) for the Korsmeyer–Peppas equation indicated good linearity (R 2 = 0.9819). The release exponent “n” was found to be 0.6048, which appears to indicate the anomalous (non-Fickian) diffusion indicating the drug release is controlled by more than one process, i.e., superposition of both phenomenon, the diffusion controlled as well as swelling controlled release as given in Table 6.
Stability studies
Data gathered from the stability studies is compiled in Table 7. CP-loaded NLC gel formulations show the shelf life in range of 2.79 to 2.81 years. Thus an arbitrary shelf life of 2 years can be assigned to the CP-NLCG formulation.
In vivo anti-inflammatory studies
The anti-inflammatory activity of the optimized formulation was evaluated by the carrageenan-induced hind paw inflammation method on Wistar albino rats. The percentage inhibition value of CP-NLC gel (test) was compared to marketed TEMOVATE gel (standard) as stated in Table 8. Test formulation not only decreased the inflammation by a greater magnitude but also sustained the effect for a prolonged period.
Conclusion
The potential of NLC as carriers for topical administration was confirmed by the results obtained, demonstrating drug penetration. CP-loaded NLC have shown excellent characterization parameters with regard to particle size, PI, zeta potential, and in vitro cum in vivo release profiles. Even, the phenomenon by which these mechanisms occur are not completely recognized, but active pharmaceutical ingredient embedded nanoparticles extends the anti-inflammatory effect presenting prolonged release. This research discloses sufficient facts that targeting and prolonged release effect can be achieved with immense prospective in dermal delivery.
References
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–84.
Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Del Rev. 2001;47:165–96.
Müller RH, Petersen RD, Hommoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Del Rev. 2007;59:522–30.
Proksch E, Fölster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006;43:159–69.
Chowdhury MMU. Dermatological pharmacology: topical agents. Medicine. 2008;37:232–4.
Simpson EL. Atopic dermatitis: a review of topical treatment options. Curr Med Res Opin. 2010;26(3):633–40.
Keshri L, Pathak K. Development of thermodynamically stable nanostructured lipid carrier system using central composite design for zero order permeation of econazole nitrate through epidermis. Pharm Dev Technol. 2013;18(3):634–44.
Nanjwade BK, Kadam VT, Manvi FV. Formulation and characterization of nanostructured lipid carrier of ubiquinone (Coenzyme Q10). J Biomed Nanotechnol. 2013;9(3):450–60.
Seyfoddin A, Al-Kassas R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev Ind Pharm. 2013;39(4):508–19.
Shah M, Agrawal Y. Development of ciprofloxacin HCl-based solid lipid nanoparticles using ouzo effect: an experimental optimization and comparative study. J Dispers Sci Technol. 2013;34(1):37–46.
Li B, Ge ZQ. Nanostructured lipid carriers improve skin permeation and chemical stability of idebenone. AAPS PharmSciTech. 2012;13(1):276–83.
Khurana S, Bedi PMS, Jain NK. Development of nanostructured lipid carriers for controlled delivery of mefenamic acid. Int J Biomed Nanosci Nanotechnol. 2012;2(3/4):232–50.
Thatipamula RP, Palem CR, Gannu R, Mudragada S, Yamsani MR. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. DARU. 2011;19(1):23–32.
Bhalekar MR, Pokharkar V, Madgulkar A, Patil N, Patil N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS PharmSciTech. 2009;10:289–96.
Mira EG, Egeaa MA, Garciaa ML, Souto EB. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf B: Biointerfaces. 2010;81:412–21.
Sanad AR, Abdelmalak NS, Elbayoomy TS, Badawi AA. Formulation of a novel oxybenzone-loaded nanostructured lipid carrier (NLCs). AAPS PharmSciTech. 2010;11:1684–94.
Misal G, Dixit G, Gulkari V. Formulation and evaluation of herbal gel. Indian J Nat Prod Resour. 2012;3(4):501–5.
Das MK, Ahmed AB. Formulation and ex-vivo evaluation of rofecoxib gel for topical application. Acta Pol Pharma Drug Res. 2007;63(5):461–7.
Pople PV, Singh KK. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus. Eur J Pharm Biopharm. 2011;79:82–94.
Araújo J, Nikolic S, Egea MA, Souto EB, Garcia ML. Nanostructured lipid carriers for triamcinolone acetonide delivery to the posterior segment of the eye. Colloids Surf B: Biointerfaces. 2011;88:150–7.
Sharma P, Dube B, Sawant K. Development and evaluation of nanostructured lipid carriers of cytarabine for treatment of meningeal leukemia. J Nanosci Nanotechnol. 2011;11(8):6676–82.
Chawla V, Saraf SA. Glyceryl behenate and its suitability for production of aceclofenac solid lipid nanoparticles. J Am Oil Chem Soc. 2011;88:119–26.
Jain NK, Ram A. Development and characterization of nanostructured lipid carriers of oral hypoglycemic agents: selection of surfactants. Int J Pharm Sci Rev Res. 2011;7(2):125–30.
Shi F, Yang G, Ren J, Guo T, Du T, Feng N. Formulation design, preparation and in vitro and in vivo characterization of ß-Elemene loaded Nanostructured lipid carriers. Int J Nanomed 2013; 8:2533–2541
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was reviewed and approved by the Institutional Animal Ethical Committee with registration number 837/ac/O4/CPCSEA.
Conflict of interest
The authors declare that they have no conflict of interest.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Nagaich, U., Gulati, N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: design and in vivo characterization. Drug Deliv. and Transl. Res. 6, 289–298 (2016). https://doi.org/10.1007/s13346-016-0291-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0291-1