Abstract
The Kharaqan hot springs are located in the historic city of Abe-Garm, famous for its hot springs, in Qazvin province, in northwestern Iran. Thermal waters with temperatures ranging from 28.7 to 52 °C vary in chemical composition and TDS contents. Those waters generally are enriched in Na–Cl–HCO3 and suggest deep water circulation. Chemistry of all of the water samples are graphed in the Cl–SO4–HCO3 ternary diagram. There is a trend of mixing along a line of constituent proportions between recently recharged water and older water. The trend toward the chloride corner is mainly the result of contact in the subsurface with evaporite-bearing formations and/or mixing with brines. Relatively high concentrations of Na, Ca, K, Cl, and SO4 resulted from rock/water interactions. These hot spring waters show high concentrations of arsenic (0.14–0.95 mg L−1). The diffusion of As-bearing spring waters into shallow aquifers could contaminate the groundwater which is used for drinking purposes. Also discharges of this As-enriched water into streams and rivers could affect irrigated crops in downstream fields. In both cases, the health of local residents could be at risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Abe-Garm area is located in the northwestern part of Iran which is related to the volcanic belt of the Orumieh-Dokhtar zone (Bolourchi et al. 1979; Aghanabati 2004) (Fig. 1). It is a well-known geothermal area in Iran (Yousefi et al. 2010). These hot springs are used for swimming, bathing, and medical purposes. Visitors and local people use these resources and there is substantial room for expansion of use of this water as international and domestic tourism becomes established in Iran (Erfurt-Cooper and Cooper 2009; Navi et al. 2012). The natural contamination of local rivers and groundwater resources as a result of hot spring discharges is a global occurrence, yet the characteristics of toxic elements in hot spring waters have rarely been addressed. The purpose of this study was to establish the geochemical characteristics and possible adverse environmental impacts of the hot spring waters in the Abe-Garm geothermal field and surrounding areas.
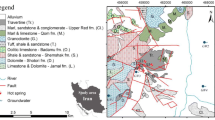
Location map of the Abe-Garm area (a, b). Geological map of Abe-Garm area (c) (Bolourchi et al. 1979)
The host rocks of the area are Cambrian to Recent age, but are interrupted by several unconformities and gaps. Lithologic aspects of different rock units reveal the presence of several sedimentary environments. The oldest exposed rocks are Carboniferous sediments (Sink Formation) consisting of sandstone and dolomite. The early Permian sandstone, conglomerates and shales of Dorud Formation are underlain by the older Paleozoic sediments and overlain by the Ruteh Formation and Nesen Formation. Triassic sediments (Elika Formation), consisting of limestone and dolomite, also rest on an erosional surface of Permian sediments. Plant-bearing sandstone and shales of Rhaeto-Liassic Shemshak Formation transgressively overlie the Triassic dolomite. The Shemshak Formation is overlain by ammonite-rich marly limestone of Middle Jurassic Dalichay Formation which transitions into the Late Jurassic Lar Formation, consisting of dolomite, limestone, marl, gypsum and volcanic rocks. Lower Cretaceous rocks unconformably overlie the Jurassic sediments consisting of conglomerate, sandstone, Orbitolina limestone and volcanic rocks. A clear disconformity is seen between the Upper Cretaceous marly limestones and limestones, and the Lower Cretaceous rocks. The Eocene is represented mainly by thick volcanic rocks and tuffaceous sediments of the “Karaj Formation”, locally having a basal limestone (Ziarat Formation) and conglomerate (Fajan Formation) and resting with distinct angular unconformity on various older formations. The Oligocene clastic deposit (Lower red formation) and Oligo-Miocene marine deposit (Qom Formation) unconformably overlie the older formations. The Qom Formation is overlain by the Miocene upper red formation consisting of sandstone, marl and conglomerate, locally with salt dome and gypsum. The young deposits are Quaternary terraces, travertine and alluvium, which unconformably overlie the older rocks (Bolourchi et al. 1979).
The Abe-Garm range is the southeastern continuation of the Soltanieh Mountains. It has a NW–SE strike and is separated into two longitudinal ranges by Hassanabad fault (Bolourchi et al. 1979). The continuation of the Hassanabad fault extends to the Ipak active fault (Berberian 1971). In addition to the longitudinal faults and thrusts, the range displays a great number of transverse faults in various directions that disrupt the pre-Tertiary formations into a complicated mosaic-like fault block pattern (Bolourchi et al. 1979; Taheri et al. 2012b; Yazdi et al. 2013).
Sampling and analytical methods
A total of five thermal and one cold water samples were collected from the Abe-Garm area. To investigate the seasonal changes of field and chemical parameters and to trace element concentrations in dry and wet seasons, springs were sampled twice in May and November. The locations of the water samples are shown in Fig. 1. Temperature, pH and electrical conductivity (EC) of the water samples were measured on-site. Water samples were collected into 250-ml polyethylene containers. All water samples were collected as two filtered batches. 2.5-ml ultrapure Merck HNO3 was added into one of the batches for cation analyses. The other batch taken for anion analyses was untreated. Water analyses were performed using standard methods in the “Geological Survey of Iran” Laboratories. Bicarbonate and chloride analyses were measured by titration methods, sulfate concentration by spectrophotometry and cations by flame photometry. Acidified samples were analyzed for major and trace elements with an ICP-OES method.
Results and discussion
Main chemical characteristics
The main physical and chemical characteristics of the hot springs and cold spring waters are shown in Table 1. The arbitrary temperature for considering a spring as a thermal spring is 36.7 °C according to Pentecost et al. (2003). Temperatures of the thermal springs range from 28.7 °C (sample HS-3) to 52 °C (sample HS-1). pH values in the samples were between 6.30 and 7.35 (Table 1).
Total dissolved solids (TDS) is the term used to describe the inorganic salts and small amounts of organic matter present in solution in water. The principal constituents are usually calcium, magnesium, sodium, and potassium cations and carbonate, bicarbonate, chloride, sulfate, and nitrate anions (WHO 2003). Excluding the sample CS-6 (drinking water), TDS contents of the thermal waters range from 5,060 to 7,760 mg L−1. Reliable data on possible health effects associated with the ingestion of TDS in drinking water are not available. Water containing TDS concentrations below 1,000 mg/liter is usually acceptable to consumers, although acceptability may vary according to circumstances. However, the presence of high levels of TDS in water may be objectionable to consumers owing to the resulting taste and to excessive scaling in water pipes, heaters, boilers, and household appliances (WHO 2003). The spring waters have apparently high EC values and high Ca, K, Mg, Na, Cl, HCO3, and SO4 concentrations. Cations show the following order of abundance: Na > Ca > K > Mg and anions follow the order of Cl > HCO3 > SO4.
The values in the WHO guidelines for drinking water quality can be used to screen for potential risks arising from swimming pools and similar environments, while making appropriate allowance for the much lower quantities of water ingested, shorter exposure periods and non-ingestion exposure (WHO 2006).
Since thermal springs emanate from a hydrothermal reservoir beneath their surface manifestation, geochemical investigation of water samples can reveal the processes occurring or occurred recently in the hydrothermal reservoir and give indications on the source of elements (Modabberi and Jahromi Yekta 2014). Information on the geochemistry of thermal waters is scarce or limited to major ions (Chudaev et al. 2006). The Cl–SO4–HCO3 triangular plot is used for an initial classification of geothermal water samples (Giggenbach 1988; Marini 2000). All of the samples listed in Table 1 are plotted in the Cl–SO4–HCO3 ternary diagram (Fig. 2). The waters of Abe-Garm plot between HCO3 and Cl fields yielding a mixing along the line between peripheral and mature water fields, but they never attain maturity. The trend toward the chloride corner is mainly the result of contact in the subsurface with evaporite-bearing formations and/or mixing with brines (Karimi and Moore 2008).
Relative Cl, SO4 and HCO3 contents of the Abe-Garm spring waters (Giggenbach 1988) (Wet season)
Figure 3 shows that none of the Kharaqan waters attains a water–rock chemical equilibrium. The model is based on the following geothermometers assuming that activities of minerals are close to unity.
Graphical evaluation of the water–rock equilibration temperatures (Giggenbach 1988) using relative Na, K and Mg concentrations of the Kharaqan thermal waters (Dry season)
The plot of 10 Mg/(10 Mg + Ca) versus 10 K/(10 K + Na) (Giggenbach 1988) for waters of the Abe-Garm area is presented in Fig. 4. Like the model given in Fig. 3, the positions of the samples do not indicate equilibration between the rock and waters. Another result deduced from Fig. 4 is that the waters of the Abe-Garm area have not been produced through the dissolution of average crustal rock or they have gained their salinity by simple rock leaching or mixing.
Plot of 10 K/(10 K + Na) vs. 10 Mg/(10 Mg + Ca) (Giggenbach 1988) of the Kharaqan thermal waters (Dry season)
Assuming equilibrium with calcite, CO2 partial pressures (\( P_{{CO_{2} }} \)) of the Kharaqan thermal waters can be evaluated in the K–Mg–Ca geoindicator diagram (Fig. 5). In the diagram, all of the values for the hot waters are below the full equilibrium line.
K–Mg–Ca geoindicator diagram (Giggenbach 1988) for the Kharaqan thermal waters (Dry season)
The presence of many active fault systems, the widespread occurrence of highly fractured carbonate rocks, and large hydraulic head differences allow a deep, large-scale (regional) circulation of waters before their emergence at the surface as springs (Minissale 1991). Based on chemical analyses of the thermal waters, data interpretations, and lithologic aspects in the study area, earlier investigators concluded that the waters discharged from the hot springs are of meteoric origin (Ghafouri 2003). The waters are heated as they circulate in the system through joints, fractures and the Hassanabad fault. During their circulation, the waters come into contact with evaporite-bearing formations and brines, resulting in an increase in dissolved ion concentrations. High surface heat flow is perhaps due to the Orumieh-Dokhtar volcanic belt, with an attendant high-temperature gradient with depth (Taheri et al. 2012b).
The diagnostic chemical character of water solutions in hydrologic systems has been determined with the application of the concept of hydrochemical facies (Back 1966), which enables a convenient subdivision of water compositions by identifiable categories and reflects the effect of chemical processes occurring between the minerals within the subsurface rock units and the groundwater. Statistical distribution diagrams such as Piper trilinear (Piper 1944) are used to gain better insight into the hydrochemical processes operating in the groundwater system. The Piper trilinear diagram was used for the purpose of characterizing the water type present in the area. It permits the cation and anion compositions of many samples to be represented on a single graph in which major groupings or trends in the data can be discerned visually (Freeze and Cherry 1979). Water types are often used in the characterization of waters as a diagnostic tool (Leybourne et al. 1998; Pitkanen et al. 2002). The Piper trilinear diagram (Fig. 6) for the study area shows that hot spring waters are of the Na–Cl type.
Travertine currently precipitates all around the Abe-Garm area. A sudden drop in the pressure and the decreasing temperature are accompanied by CO2 loss cause calcite to precipitate mostly at shallow depths (Mutlu 1998).
Trace elements in spring waters
As the usable water availability is rapidly decreasing globally, the quality of the drinkable water is also becoming a major concern. Specifically, the presence of geogenic, non-point source, natural contaminants like arsenic (As) and other oxyanion-forming metals and metalloids (e.g., Mo, V, W, Se, Sb), can limit the availability of suitable potable water sources in some of the most densely populated parts of the world (Welch et al. 2000; Charlet and Polya 2006; Nicolli et al. 2012; Mukherjee et al. 2008, 2011; Kim et al. 2011; Thakur et al. 2011; Raychowdhury et al. 2013). Trace element concentrations in the hot and cold spring waters are given in Table 2. The hot spring waters show high concentrations of arsenic and other trace elements (Taheri et al. 2012a). In natural environments, As is present in four oxidation states: −III, 0, +III, +V. Of these, in hydrologic systems, it exists most commonly as arsenite [As(III), e.g., H3AsO3 and H2AsO3 −] and arsenate [As(V), e.g., H2AsO4 − and HAsO4 2−] (Smedley and Kinniburgh 2002; Choong et al. 2007; Raychowdhury et al. 2013). In neutral oxygenated waters, As(V) is the thermodynamically favored form, whereas As(III) is stable under reducing conditions (Caporale et al. 2013). The presence of these inorganic, oxyanions of As in ground water is dependent on climatic conditions, geomorphology/geology, tectonic setting, hydrogeochemical characteristics like pH, redox potential, ionic strength, ionic concentrations, organic matter content and microbial activities, among others (Scanlon et al. 2009). The level of arsenic in natural waters, including open ocean seawater, generally ranges between 1 and 2 μg L−1 (Hindmarshand McCurdy 1986; USNRC 1999). Concentrations may be elevated, however, in areas with volcanic rock and sulfide mineral deposits (Hindmarsh and McCurdy 1986); in areas containing natural sources, where levels as high as 12 mg L−1 have been reported (WHO 2011); near anthropogenic sources, such as mining and agrochemical manufacture; and in geothermal waters (mean 500 μg L−1, maximum 25 mg L−1). Mean arsenic concentrations in sediment range from 5 to 3,000 mg kg−1; the higher levels occur in areas of contamination (USNRC 1999) but are generally unrelated to arsenic concentrations in water.
In the Abe-Garm geothermal field all of the spring water samples contained arsenic concentrations that were significantly higher than the 10 μg L−1 guideline set by the World Health Organization. The actual source of groundwater arsenic contamination, in the Abe-Garm area, is yet to be established. The sources of arsenic are geothermal or may be derived from sedimentation and mining. There is no proof regarding the volcanic emission of As in the Abe-Garm area so far. However, the release of As, by the natural processes in groundwater has been recognized, from the Miocene sediments comprising sandstone, marl and conglomerate, locally with salt dome and gypsum (Upper red formation) and plant-bearing sandstones and shales of the Rhaeto-Liassic Shemshak Formation.
Arsenic adsorbs to and reacts with hydrous iron and aluminum oxides, and is, therefore, preferentially adsorbed in soils with high clay content (Woolson 1983). Arsenic can leach out if reactive concentrations of iron, aluminum, and exchangeable calcium are low (WHO 2011). The clay fraction can apparently behave either as a source or as a sink of arsenic (Carmen Blanco et al. 2012).
Smedley and Kinniburgh (2002) identified two distinct ‘triggers’ that can lead to the release of As on a large scale. The first is the development of high pH (>8.5) conditions in semiarid or arid environments usually as a result of the combined effects of mineral weathering and high evaporation rates. This pH change leads either to the desorption of adsorbed As [especially As(V) species] and a range of other anion-forming elements (V, B, F, Mo, Se and U) from mineral oxides, especially Fe oxides, or it prevents them from being adsorbed (see also Nicolli et al. 1989; Pearcy et al. 2011). Because arid regions are typically characterized by low biomass compared to more humid regions, dissolved organic C concentrations in groundwaters and aquifer sediments in these settings tend to be low, and groundwaters are commonly oxic (Pearcy et al. 2011).
The second trigger for large-scale arsenic release is the development of strongly reducing conditions at near-neutral pH values, leading to the desorption of As from mineral oxides and to the reductive dissolution of Fe and Mn oxides, also leading to As release. Iron(II) and As(III) are relatively abundant in these groundwaters and SO4 concentrations are small (typically 1 mg L−1 or less). Large concentrations of phosphate, bicarbonate, silicate and possibly organic matter can enhance the desorption of As because of competition for adsorption sites (Smedley and Kinniburgh 2002; Ravenscroft et al. 2009). The second environment where naturally occurring, high-As groundwaters appear common are geologically young (Holocene) fluvial sedimentary deposits associated with modern deltas that have strongly reducing conditions owing to abundant sedimentary organic matter (i.e., peat) (McArthur et al. 2004; Polya et al. 2005).
Trace elements in geothermal rock samples
The concentrations of As in sinter deposits and rock samples around the Kharaqan hot springs are much higher than the average earth crust and limestone values (Table 3; Krauskopf and logue 2002). The sinter precipitated around the hot springs is able to entrap As from the discharged waters and act as a sink for this toxic element. However, As minerals such as orpiment, realgar, and stibnite were not identified in these deposits.
Potential environmental impact of hot spring effluents
Hot spring waters tend to have high contents of total dissolved solids (TDS) and may contain toxic elements such as arsenic, uranium and other trace elements (Smedley and Kinniburgh 2002; Vaughan 2006; Zhang et al. 2008; Yoshizuka et al. 2010; Bundschuh et al. 2013). The release of these waters into the surrounding environment may not only raise concern about the aquatic ecology (Mroczek 2005), but could also pose a health risk to local residents (Webster 1999; Pehlivan 2002; Robinson et al. 2003).
Hot springs water from the Abe-Garm geothermal field flow into the Khareh-roud River between the Kharaqan hot springs (upstream) and Chehel-Cheshme cold spring (downstream) sampling sites (Fig. 1). The influence of the thermal springs on the chemistry of the cold spring water is obvious. Downstream from the springs the water in the cold spring contained considerably higher concentrations of Ca, K, Na, SO4, Cl, As, B, Mo, Pb, Se and other trace elements (see Tables 1, 2). Downstream from the point where the hot spring water flows into a small stream, the water is used to irrigate crops. The potential health threat posed by the hot spring water would be related to (1) the drinking of ground and surface waters that may have mixed with the hot spring discharge and/or (2) field irrigation with As-contaminated water, leading to an accumulation of As in the crops. Consumption of the crops by humans, cattle and poultry could lead to serious health problems that deserve further investigation. Heavy metal toxicity which is frequently the result of long-term, low-level exposure to pollutants has often been investigated in air, water, food and numerous consumer products (Eleni et al. 2006).
The presence of arsenic in groundwater in concentrations sufficient to affect human health constitutes a worldwide high-priority groundwater quality problem (Duker et al. 2005). Long-time exposure to arsenic may cause various diseases including skin disorders (Tondel et al. 1999; Ahmad et al. 1997; Rahman et al. 2001), circulatory system problems (Chen et al. 1996; KarimiNezhad et al. 2010), cardiovascular disease (Wang et al. 2007), neurological complications (Mukherjee et al. 2003), reproductive disorders (Ahmad et al. 2001), respiratory effects (Milton et al. 2001; Guha Mazumder et al. 2000), diabetes mellitus (Rahman et al. 1998) and an increased cancer risk, especially of the skin, bladder, lungs and kidney (KarimiNezhad et al. 2010; Chen et al. 1992; Smith et al. 1998; Bates et al. 1992; Chiou et al. 1995; Rahman et al. 2009). Increased risks of lung and bladder cancer and of arsenic-associated skin lesions have been reported to be associated with ingestion of drinking water at concentrations below 50 μg of arsenic per liter (WHO 2011). An interim water quality guideline for total arsenic of 100 µg L−1 in irrigation water is recommended for the protection of agricultural crop species (CCME 1999). Data on the toxicity of arsenic were available for 25 crop species. Beans, peas, and spinach seem to be the most sensitive, while cabbage was found to be the least sensitive. The recommended water quality guideline for total arsenic for the protection of livestock is 25 µg L−1 (CCME 1999).
Conclusions
Relatively high concentrations of Na, Ca, K, Cl, and SO4 resulting from rock/water interactions have been observed in the hot springs water of the Abe-Gram area. The presence of As and related trace elements in these hot spring waters is of considerable concern, especially the high level of total As. The problem becomes even greater if we consider the high fraction of As(III) in some of these thermal water samples. As is trapped in the sinter precipitating at the spring outlets, but most As enters the surrounding environment and contaminates soils, as well as surface and ground water sources. In the Abe-Garm area the diffusion of As spring waters into shallow aquifers could contaminate the groundwater used for drinking purposes. Also, the thermal spring discharges into the streams and rivers could affect irrigated crops in downstream fields. In both cases, the health of local residents could be at risk.
References
Aghanabati A (2004) Geology of Iran. GSI Publication, Tehran
Ahmad SA, Bandarnayake D, Khan AW, Hadi SA, Uddin G, Halim MA (1997) Arsenic in ground water and arsenicosis in Bangladesh. Int J Environ HealthRes 7:271–276
Ahmad SA, Sayed MHSU, Barua S, Khan MH, Faruquee MH, Jalil A, Hadi SA, Talukder HK (2001) Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect 109:629–631
Back W (1966) Hydro chemical facies and groundwater flow patterns in northern part of Atlantic Coastal Plain. US Geological Survey Professional Paper 498–A:42
Bates MN, Smith AH, Hopenhayn-Rich C (1992) Arsenic ingestion and internal cancers: a review. Am J Epidemiol 135:462–476
Berberian M (1971) Preliminary report on structural analysis of Ipak Active Fault, internal report. Geological Survey of Iran, Tehran
Bolourchi MH, Hajian J, Ohanian T, Vahdati F (1979) Explanatory text of Kabudar Ahang Quadrangle map 1:250,000 (D5). Geological and Mineral Survey of Iran, Tehran
Bowen HJM (1979) Environmental Geochemistry of the Elements. Academic Press, London
Bundschuh J, Maity JP, Nath B, Baba A, Gunduz O, Kulp TR, Jean JS, Kar S, Yang HJ, Tseng YJ, Bhattacharya P, Chen CY (2013) Naturally occurring arsenic in terrestrial geothermal systems of western Anatolia, Turkey: potential role in contamination of freshwater resources. J Hazard Mater 262:951–959
Canadian Council of Ministers of the Environment (CCME) (1999) Canadian Water Quality Guidelines for the Protection of agricultural water uses/Arsenic. http://ceqg-rcqe.ccme.ca/
Caporale AG, Pigna M, Azam SMGG, Sommella A, Rao MA, Violante A (2013) Effect of competing ligands on the sorption/desorption of arsenite on/from Mg–Fe layered double hydroxides (Mg–Fe-LDH). Chem Eng J 225:704–709
Carmen Blanco M, Paoloni J, Morrás H, Fiorentino C, Sequeira M, Amiotti N, Bravo O, Diaz S, Espósito M (2012) Partition of arsenic in soils sediments and the origin of naturally elevated concentrations in groundwater of the southern pampa region (Argentina). Environ Earth Sci 66:2075–2084
Charlet L, Polya DA (2006) Arsenic hazard in shallow reducing groundwaters in southern Asia. Elements 2:91–96
Chen CJ, Chen CW, Wu MM, Kuo TL (1992) Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer 66:888–892
Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY (1996) Dose response relationship between ischemic heart disease mortality and longterm arsenic exposure. Arterioscler Thromb Vasc Biol 16:504–510
Chiou HY, Hsueh YM, Liaw KF et al (1995) Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up-study in Taiwan. Cancer Res 55:1296–1300
Choong TSY, Chuah TG, Robiah Y, Gregory Koay FL, Azni I (2007) Arsenic toxicity, health hazards and removal techniques from water: an overview. Desalination 217:139–166
Chudaev O, Chudaeva V, Sugimori K, Kuno A, Matsuo M (2006) Geochemistry of recent hydrothermal systems of Mendeleev Volcano, Kuril Islands, Russia. J Geochem Explor 88:95–100
Duker AA, Carranza EJM, Hale M (2005) Arsenic geochemistry and health. Environ Int 31:631–641
Eleni I, Aletrari M, Eftychia C (2006) Risk assessment of the dietary intake of lead, cadmium, mercury and nitrates in cyprus and the relevant uncertainty. In: proceedings of the AOAC Europe Section, International Workshop, November 6-7, Limassol
Erfurt-Cooper P, Cooper M (2009) Health and wellness tourism: spas and hot springs. Aspects of tourism, vol 40. Channel View Publications, Bristol
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, Englewood Cliffs
Ghafouri MR (2003) Mineral water and mineral springs of Iran. University of Tehran, Tehran
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na–K–Ca–Mg geoindicators. Geochim Cosmochim Acta 52:2749–2765
Guha Mazumder DN, Haque R, Ghosh N et al (2000) Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India. Int J Epidemiol 29:1047–1052
Hindmarsh JT, McCurdy RF (1986) Clinical and environmental aspects of arsenic toxicity. CRC Crit Rev Clin Lab Sci 23:315–347
Karimi H, Moore F (2008) The source and heating mechanism for the Ahram, Mirahmad and Garu thermal springs, Zagros mountains, Iran. Geothermics 37:84–100
Krauskopf KB, logue K (2002) Environmental geochemistry, environmental science, encyclopedia of physical science and technology, 3rd edn. Academy Press, London, pp 519–545
Leybourne MI, Goodfellow WD, Boyle DR (1998) Hydro geochemical, isotopic and rare earth element evidence for contrasting water–rock interactions at two undisturbed Zn–Pb massive sulphide deposits, Bathurst Mining Camp, NB, Canada. J Geochem Exp 64:237–261
Marini L (2000) Geochemical techniques for the exploration and exploitation of geothermal energy, Università degli Studi di Genova. Dipartimento per lo Studio del Territorio e delle sue Risorse, Genova, Italia
McArthur JM, Banerjee DM, Hudson-Edwards KA, Mishra R, Purohit R, Ravenscroft P, Cronin A, Howarth RJ, Chatterjee A, Talukder T, Lowry D, Houghton S, Chadha DK (2004) Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: the example of West Bengaland its worldwide implications. Appl Geochem 19:1255–1293
Milton AH, Hasan Z, Rahman A (2001) Chronic arsenic poisoning and respiratory effects in Bangladesh. J Occup Health 43:136–140
Minissale A (1991) Thermal springs in Italy: their relation to recent tectonics. Appl Geochem 6:201–212
Modabberi S, Jahromi Yekta SS (2014) Environmental geochemistry and sources of potentially toxic elements in thermal springs in the Sabalan volcanic field, NW Iran. Environ Earth Sci 71:2821–2835
Mroczek EK (2005) Contributions of arsenic and chloride from the Kawerau geothermal field to the Tarawera River, New Zealand. Geothermics 34:218–233
Mukherjee SC, Rahman MM, Chowdhury UK et al (2003) Neuropathy in arsenic toxicity from groundwater arsenic contamination in West Bengal-India. J Environ Sci Health Part A Environ Sci Eng 38:165–183
Mukherjee A, Bhattacharya P, Savage K, Foster A, Bundschuh J (2008) Distribution of geogenic arsenic in hydrologic systems: controls and challenges. J Contam Hydrol 99:1–7
Mukherjee A, Fryar AE, Scanlon BR, Bhattacharya P, Bhattacharya A (2011) Elevated arsenic in deeper groundwater of western Bengal basin, India: extents and controls from regional to local-scale. Appl Geochem 26:600–613
Mutlu H (1998) Chemical geothermometry and fluid–mineral equilibria for the Ömer-Gecek thermal waters, Afyon area, Turkey. J Volcanol Geotherm Res 80:303–321
Navi P, Taheri M, Yazdi M (2012) Introduction of Kharaqan Hot springs for Health Tourism. In: Proceedings of 1st Symposium on Irans Geoheritage, January 23, The Geological Survey and Mineral Exploration of Iran, 13 p
Nicolli HB, Suriano JM, Gomez Peral MA, Ferpozzi LH, Baleani OA (1989) Groundwater contamination with arsenic and other trace elements in an area of the Pampa, Province of Cordoba, Argentina. Environ Geol Water Sci 14:3–16
Nicolli HB, Bundschuh J, Blanco MC, Tujchneider OC, Panarello HO, Dapeña C, Rusansky JE (2012) Arsenic and associated trace-elements in groundwater from the Chaco-Pampean plain, Argentina: results from 100 years of research. Sci Total Environ 429:36–56
Pearcy CA, Chevis DA, Haug TJ, Jeffries HA, Yang N, Tang J, Grimm DA, Johannesson KH (2011) Evidence of microbially mediated arsenic mobilization from sediments of the Aquia aquifer, Maryland, USA. Appl Geochem 26:575–586
Pehlivan R (2002) The effects on human health and hydro geochemical characteristics of the Kirkgeçit and Ozancik hot springs, Çanakkale, Turkey. Environ Geochem Health 25:205–217
Pentecost A, Jones B, Renaut RW (2003) What is a hot spring? Can J Earth Sci 40:1443–1446
Piper AM (1944) A graphical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Pitkanen P, Kaija J, Blomqvist R, Smellie JAT, Frape SK, Laaksoharju M, Negral PH, Casanova J, Karhu J (2002) Hydro geochemical interpretation of groundwater at Palmottu. Paper EUR 19118 EN, European Commission, Brussels, pp 155–167
Polya DA, Gault AG, Diebe N, Feldman P, Rosenboom JW, Gilligan E, Fredericks D, Milton AH, Sampson M, Rowland HAL, Lythgoe PR, Jones JC, Middleton C, Cooke DA (2005) Arsenic in shallow Cambodian groundwaters. Miner Mag 69:807–823
Rahman MM, Tondel M, Ahmad SA, Axelson O (1998) Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol 148:198–203
Rahman MM, Chowdhury UK, Mukherjee SC et al (2001) Chronic arsenic toxicity in Bangladesh and West Bengal, India—a review and commentary. Clin Toxicol 39:683–700
Rahman M, Naidu R, Bhattacharya P (2009) Arsenic contamination in groundwater in the Southeast Asia region. Environ Geochem Health 31:9–21
Ravenscroft P, Brammer H, Richards K (2009) Arsenic pollution: a global synthesis. Wiley, UK
Raychowdhury N, Mukherjee A, Bhattacharya P, Johannesson K, Bundschuh J, Bejarano Sifuentes G, Nordberg E, Martin RA, Rosario Storniolo AD (2013) Provenance and fate of arsenic and other solutes in the Chaco-Pampean Plain of the Andean foreland, Argentina: from perspectives of hydrogeochemical modeling and regional tectonic setting. J Hydrol. doi:10.1016/j.jhydrol.2013.07.003
Robinson B, Duwig C, Bolan N, Kannathasan M, Saravanan A (2003) Uptake of arsenic by New Zealand watercress (Lepidium sativum). Sci Total Environ 301:67–73
Scanlon BR, Nicot JP, Reedy RC, Kurtzman D, Mukherjee A, Nordstrom DK (2009) Elevated naturally occurring arsenic in a semiarid oxidizing system, Southern High Plains aquifer, Texas, USA. Appl Geochem 24:2061–2071
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith AH, Goycolea M, Haque R et al (1998) Marked increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water. Am J Epidemiol 147:660–669
Taheri M, Yazdi M, Navi P (2012a) Health hazards and arsenic pollutants in Kharaqan Hot springs, Qazvin. In: proceedings of 4th Symposium of Iranian Society of Economic Geology, August 30–31, Birjand university
Taheri M, Yazdi M, Navi P, Sadati N (2012b) Application of remote sensing for alteration mapping in Avaj area. In: proceedings of 16th Symposium of geological society of Iran, September 4–6, Shiraz University, p 8
Taylor SR (1964) Abundance of elements in the continental crust. Geochim Cosmochim Acta 28:1273–1286
Thakur JK, Thakur RK, Ramanathan A, Kumar M, Singh SK (2011) Arsenic contamination of groundwater in Nepal—an overview. Water 3:1–20
Tondel M, Rahman M, Magnuson A, Chowdhury IA, Faruquee MH, Ahmad SA (1999) The relationship of arsenic levels in drinking water and the prevalence rate of skin lesions in Bangladesh. Environ Health Perspect 107:727–729
USNRC (1999) Arsenic in drinking water. DC, United States National Research Council, National Academy Press, Washington
Vaughan DJ (2006) Arsenic. Elem (Int Mag Miner Geochem Petrol) 2(2):71–75
Wang CH, Hsiao CK, Chen CL et al (2007) A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol 222:315–326
Webster JG (1999) The source of arsenic (and other elements) in the Marbel-Matingao river catchment, Mindanao, Philippines. Geothermics 28:95–111
Wedepohl KH (eds) (1969–1974) Handbook of Geochemistry. Springer, Berlin
Welch AH, Westjohn DB, Helsel DR, Wanty RB (2000) Arsenic in ground water of the United States: occurrence and geochemistry. Ground Water 38(4):589–604
Woolson EA (1983) Emissions, cycling and effects of arsenic in soil ecosystems. In: Fowler BA (eds) Biological and environmental effects of arsenic. Elsevier, New York, pp 51–139
World Health Organization (2003) Total dissolved solids in drinking water. Background document for preparation of WHO guidelines for drinking-water quality. World Health Organization, Geneva
World Health Organization (2006) Guidelines for safe recreational water environments. Swimming pools and similar environments. World Health Organization, Geneva
World Health Organization (2011) Arsenic in drinking-water. World Health Organization, Geneva
Yazdi M, Taheri M, Navi P, Sadati N (2013) Landsat ETM+ imaging for mineral potential mapping: application to Avaj area, Qazvin, Iran. Int J Remote Sens 34(16):5778–5795
Yoshizuka K, Nishihama S, Sato H (2010) Analytical survey of arsenic in geothermal waters from sites in Kyushu, Japan, and a method for removing arsenic using magnetite. Environ Geochem Health 32:297–302
Yousefi H, Noorollahi Y, Ehara S, Itoi R, Yousefi A, Fujimitsu Y, Nishijima J, Sasaki K (2010) Developing the geothermal resources map of Iran. Geothermics 39:140–151
Zhang G, Liu CQ, Liu H, Jin Z, Han G, Li L (2008) Geochemistry of the Rehai and Ruidian geothermal waters, Yunnan Province, China. Geothermics 37:73–83
Acknowledgments
The authors would like to express their thanks to the Geological Survey of Iran and Shahid Beheshti University for providing the funds for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yazdi, M., Taheri, M. & Navi, P. Environmental geochemistry and sources of natural arsenic in the Kharaqan hot springs, Qazvin, Iran. Environ Earth Sci 73, 5395–5404 (2015). https://doi.org/10.1007/s12665-014-3794-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3794-4