Abstract
Springs are an important source of drinking water supply in mountainous karst areas of SW China. However, the quality of many spring waters has deteriorated greatly in recent years, which leads to a significant problem of drinking water scarcity. In this study, hydrochemistry and stable sulfur and oxygen isotopic compositions of SO42− (δ34S and δ18OSO4) of 38 representative samples of waters (incl. spring water, surface water, rainwater, and sewage) from the Hongjiadu Basin, Guizhou province, SW China, were investigated in order to identify the sources of contaminates in spring waters and trace the processes affecting the karst groundwater quality. Approximately 28% of the total investigated springs has been suffered from serious contamination and the concentrations of NO3−, SO42−, and total iron (TFe) in many spring waters have exceeded the standards for drinking water. The springs that have NO3− concentrations of > 30 mg/L are concentrated in residential and agricultural areas, suggesting that NO3− in spring water are mainly derived from chemical fertilizers, manure, and sewage. δ34S and δ18OSO4 data indicate that SO42− in spring water mainly originates from sulfide oxidation, acid rain, and sewage. Furthermore, the high δ34S and δ18OSO4 values of SO42− in some spring waters may be related to the occurrence of bacterial sulfate reduction. Some springs that are discharged from abandoned coal mines have SO42− concentrations of > 250 mg/L, demonstrating that mining activities have accelerated the deterioration of spring water quality. Also, springs with TFe concentrations of > 0.3 mg/L are discharged from coal-bearing strata, revealing that iron in spring waters is mainly derived from the oxidation of pyrite. Our results show that the karst spring waters are highly vulnerable to anthropogenic contaminations and human activities, such as agricultural fertilizing and sewage and waste disposal as well as mining activities, which exert a great impact on the quality of groundwater in karst areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Karst groundwater is a vital water resource and more than 10 million people in southwest China rely on it for drinking water supply (Ford and Williams 2007). However, karst groundwater is highly vulnerable to pollution caused by human activities and the restoration of contaminated groundwater is difficult. With the development of society and economy, the contamination of karst groundwater draws more and more attention (Yang et al. 2019; Wu et al. 2018; Jakóbczyk-Karpierz et al. 2016; Li et al. 2016; Vesper and White 2004). Groundwater is a “hidden resource” and the prevention and monitoring of groundwater pollution and restoration of water quality are more difficult than that of surface waters due to its inaccessibility (Marques et al. 2013). Springs are the primary way for the discharge of groundwater from karst aquifers and contaminants entering into the aquifers ultimately appear in spring water (Vesper and White 2004). Therefore, the monitoring of springs can provide more insights into the hydrogeological and hydrogeochemical processes that occur in underground environments (Pu et al. 2013).
The hydrochemical and isotopic compositions of spring water can provide important information about the factors that determine groundwater quality, such as lithology, water–rock interaction, and land use (Merchán et al. 2014; Marques et al. 2013; Vesper and White 2004). Stable sulfur and oxygen isotopes of sulfate (δ34S and δ18OSO4) have been widely used to identify sulfur sources and trace the sulfur cycling processes (Dugin et al. 2009; Otero et al. 2007), especially in small watersheds (Merchán et al. 2014; Otero et al. 2008), and to investigate the redox state of an aquifer (Li et al. 2013).
Springs are the primary source of drinking water supply for approximately 95% of the population in the Hongjiadu Basin. The basin was originally dominated by agricultural land but has undergone substantial changes in terms of land use in recent years due to the rapid development of coal mining and thermal power generation industries which have resulted in the serious deterioration of local groundwater quality (Ren et al. 2017). Moreover, the population within the basin has increased from 10,000 to 30,000 during the recent years, causing a sharp increase in the demand for drinking water. In view of the deteriorating water quality and increased demand for drinking water, it is crucial to investigate the current quality status of spring water and the relevant influencing factors within the basin. Therefore, a dual isotopes (δ34S and δ18OSO4) and hydrochemical analyses were used to investigate the effects of natural and anthropogenic factors on the quality of spring water in the basin. The specific objectives were to (1) identify the main sources of inorganic contaminants in spring water, (2) determine the effects of lithology and land use change on spring water quality, and (3) assess spring water quality and quantity with respect to the local demand for drinking water in the Hongjiadu Basin.
Study area
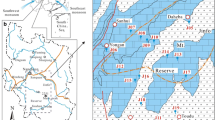
The Hongjiadu Basin is a small karst basin with a total area of 19.3 km2. It is located approximately 130 km northwest of Guiyang, the capital of Guizhou Province, SW China (Fig. 1a). The region is characterized by a subtropical monsoon climate with 80% of the total annual precipitation (~ 1400 mm) occurring during the rainy season (May–October) and an mean annual air temperature of 14.4 °C. The land is mainly used for forest, agriculture, and residential purposes. Small lakes are distributed in this region.
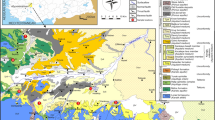
The Hongjiadu Basin is located in the upper reaches of the Wujiang River. The underlying bedrock is mainly composed of Permian to Triassic sedimentary carbonate rocks with coal-bearing strata intercalated into the Permian Longtan Formation. No gypsum-containing evaporites are found in the basin (Fig. 1b). The carbonate rocks are the major aquifers and more than 85% of groundwater is hosted in limestone aquifers. In general, groundwaters in the basin are discharged from unconfined aquifers. The atmospheric precipitation is the main recharge source of groundwater. Sulfur-rich coals are primarily distributed in groundwater recharge areas in the northeast and southeast parts of the basin (Fig. 1c). Because coal is the major energy resource, the region has been characterized by high rates of acid deposition for several years. The study area contains two groups of faults that preferentially trend NE–SW. These faults cut through the bedrocks and are the main channels for groundwater infiltration and transport, affecting the development of subsurface karst. The groundwater flows from the southwest to the northeast and finally discharges into the Wujiang River. The spring at sampling site 4 is the main outlet of groundwater and has the highest flow rate. However, the utilization of this spring is difficult as it directly discharges into the Wujiang River at a low elevation.
Sampling and analytical methods
A total of 38 water samples, including 29 springs (Fig. 2c), four surface water, and four sewage as well as one rainwater were collected from the Hongjiadu Basin in December 2016. According to the land use, the groundwater samples were classified into three major groups: samples from forest, agricultural, and residential areas (Table 1). Some springs are also affected by abandoned coal mines, such as springs 6-8.
In the field, water temperature, pH, electrical conductivity (EC), and dissolved oxygen (DO) were measured using a multi-parameter portable meter (WTW3430, Germany) with analytical precisions of 0.01 °C, 0.01, 0.1 μS/cm, and 0.01 mg/L, respectively. The HCO3− concentration was measured by a titration kit with a precision of 0.1 mmol/L. Water samples was filtrated on site using disposable 0.45 μm filters and placed in 50 mL high-density polyethylene bottles for the laboratory analyses of anions (filtered and unacidified), cations, and major/tracer metals (filtered and acidified to pH < 2 with HNO3). Samples for δ34S and δ18OSO4 analysis were collected in 10 L brown plastic bottles. The sulfate was extracted as BaSO4 by the addition of BaCl2 to the filtered water sample with the pH adjusted to < 2 (using HCl) to prevent the formation of BaCO3. The BaSO4 samples were freeze-dried in powder form and analyzed at the China University of Geosciences (Wuhan). Before sampling, all bottles were rinsed 3–4 times with filtered water. All water samples were stored at 4 °C in a dark environment before testing.
The concentrations of Ca2+, Mg2+, Na+, K+, and TFe were measured by an inductively coupled plasma atomic emission spectroscopy (ICP-AES, USA), and the concentrations of NO3−, SO42−, and Cl− were analyzed by an ion chromatography (Dionex ICS-1100, USA). The detection limits are 0.011, 0.013, 0.005, 0.02, 0.003, 0.05, 0.2, and 0.1 mg/L for Ca2+, Mg2+, Na+, K+, TFe, NO3−, SO42−, and Cl−, respectively. The samples for δ34S and δ18OSO4 analysis were measured using a combination of an elemental analyzer (Carlo Erba 1108, USA) and a stable isotope ratio mass spectrometer (MAT 253, USA), with analytical precisions of ± 0.2‰ and ± 0.5‰, respectively. All measurements were performed at the Karst Geological Resources and Environment Supervision and Monitoring Center of the Ministry of Land and Resources.
Results
Chemical and isotopic compositions
The data of chemical and isotopic compositions of water samples are listed in Table 1. The pH values of spring water samples range from 3.24 to 8.38 with a mean value of 7.28 which is lower than the mean values of surface water (8.31) and sewage (8.38) but higher than that of rainwater (5.48). The sample that was collected from spring 6 located in an abandoned coal mine has the lowest pH value of 3.24. The total dissolved solids (TDS = Na + K + Ca + Mg + Cl + SO4 + NO3 + HCO3) of both spring and surface water samples range between 47.9 and 2114 mg/L. The spring 6 sample also has the highest TDS, TFe, and SO42− concentrations but the lowest DO value. Samples of both springs 7 and 8 were also collected from areas of abandoned coal mines and have relatively low pH values and high TFe and SO42− concentrations. Except for samples of springs 6, 7, and 14 and rainwater, all samples have calculated charge balance errors (CBEs) within ± 5% (CBE = 100 × *(TZ+ – TZ−)/(TZ+ + TZ−), where TZ+ = Na+ + K+ + 2Ca2+ + 2Mg2+ and TZ− = Cl− + 2SO42− + NO3− + HCO3−). The calculated CBEs for samples of springs 6, 7, and 14 and rainwater are far exceeded the permissible range (± 5%), suggesting there are unanalyzed ions in spring waters (Edmond et al. 1995; Li et al. 2011).
The hydrochemical data of water samples from Hongjiadu Basin were plotted on a Piper diagram (Fig. 2). The dominant cations in the spring waters are Ca2+ and Mg2+ and the dominant anion is HCO3−. The studied spring waters are also rich in SO42−. These hydrochemical characteristics are similar to those from the Shuicheng Basin near the study area (Fig. 1b; Li et al. 2010). The hydrochemistry of spring water is mainly of Ca–Mg–HCO3 type (based on ions exceeding 20% of the total meq/L), followed by Ca–Mg–HCO3–SO4 type, and little of Ca–SO4 type. The molar ratios of [SO4]/[HCO3] for the spring water samples are 0.1–9.02. This is consistent with the values of karst groundwater from Guiyang city that has high concentrations of SO42− and complex sulfate sources (Lang et al. 2006).
The NO3− concentrations of spring waters range from 0.9 to 73.5 mg/L with a mean value of 23.8 mg/L. The mean value is higher than that of surface water (12.5 mg/L) and rainwater (1.68 mg/L) but lower than that of sewage samples (30.3 mg/L). In fact, springs 9, 16 from agricultural area and 27 from residential area have the highest NO3− concentrations (56.1–73.5 mg/L). The mean concentrations of Na+, Cl−, and K+ are in the following order: sewage > surface water > spring water > rainwater, successively. The TFe concentrations of springs 1, 6–8, and 14 samples (0.58–76.5 mg/L, mean 26.7 mg/L) have exceeded the limit (< 0.3 mg/L) of drinking water established by the Chinese government.
The δ34S values of sulfate in spring waters range from − 18.7 to + 0.3‰ (mean − 6.9‰) and the δ18OSO4 values from + 4.2 to + 10.3‰ (mean + 7.4‰). The δ34S and δ18OSO4 values of surface water samples range from − 8.1 to − 0.8‰ and + 7.7 to + 8.3‰, respectively. The δ34S and δ18OSO4 values are − 12.1‰ and + 6.5‰ respectively for rainwater and − 7.4‰ and + 7.6‰ respectively for sewage sample 35 and − 8.2‰ and + 8.3‰ respectively for sewage sample 36. The spring 7 sample collected from an abandoned coal mine has the highest δ34S value of + 0.3‰.
Effects of anthropogenic activities on spring water quality
The excessive application of chemical fertilizers is a major cause of non-point source pollution in China (Hou et al. 2017). The chemical fertilizers used in the Hongjiadu Basin mainly include formula fertilizer (Cl-containing fertilizer with a moderate Cl− concentration; N:P:K = 27:10:5), urea (total nitrogen ≥ 46.4%; Fig. 3a), and ammonium bicarbonate (total nitrogen ≥ 17.1%). If the fertilizer chemicals have not been totally taken up by crops, they will remain in the topsoil. In addition, manures including animal and human waste are applied to improve crop yields (Fig. 3b). The acids produced by the nitrification of chemical fertilizers and manure (NH4+ + 2O2 → NO3− + 2H2O + 2H+) can take part in the weathering of carbonate rocks, which increasing the NO3− concentration of groundwater (Singh et al. 2014; Barnes and Raymond 2009; Jiang et al. 2009).
In addition, our survey found that in several small factories and residential area, untreated wastewater (e.g., sewage sample 36 that have a NO3− concentration of up to 57.4 mg/L; Fig. 3c) are discharged directly into sinkholes through which the wastewater eventually enters into the groundwater. NO3− has a high solubility and mobility in the environment, resulting in the widespread NO3− contamination of spring water in agricultural and residential areas (Négrel and Pauwels 2004). Correspondingly, spring water samples obtained from agricultural and residential areas, such as those from springs 9, 16, and 27 have high NO3− concentrations (65.7, 73.5, and 66.1 mg/L respectively). According to the land use surrounding the springs, the mean NO3− concentrations of the 29 spring water samples vary in the order as follows: residential land (30.0 mg/L) > agricultural land (28.8 mg/L) > forestry land (9.43 mg/L), successively. Figure 4a shows that the NO3− concentration in the spring water samples is not related to lithology but rather to land use. All the spring water samples with NO3− concentrations of > 30 mg/L were obtained from agricultural and residential areas (Table 1 and Fig. 4a), suggesting an anthropogenic sources of NO3− in spring water. The relatively low concentration of NO3− in rainwater (Table 1) indicates that the rainwater is not one of main source of NO3− in spring water. Our data thus reveal that the NO3− of spring water mainly originates from chemical fertilizers, manure, and sewage.
Table 2 gives the Pearson correlation coefficients of K+, Na+, Cl−, SO42−, NO3−, and TFe concentrations of spring water samples. There are significant positive correlations between NO3− and Cl−, Cl− and Na+, and Na+ and K+, suggesting similar sources for these ions (Zhou et al. 2016; Jiang et al. 2009). In addition, the mean concentrations of K+, Na+, Cl−, and NO3− could be classified in three groups in terms of the land use surrounding all 29 springs: residential area (K+ = 4.81 mg/L; Na+ = 13.7 mg/L; Cl− = 16.0 mg/L) > agricultural area (K+ = 2.56 mg/L; Na+ = 5.03 mg/L; Cl− = 8.14 mg/L) > forestry land area (K+ = 1.93 mg/L; Na+ = 4.44 mg/L; Cl− = 2.72 mg/L). This further confirms that the K+, Na+, Cl−, and NO3− in spring waters mainly originate from anthropogenic inputs, namely, chemical fertilizers, manure and sewage.
There is also a highly positive correlation between TFe and SO42− concentrations (Table 2), which indicates the similarity in the sources of them. As the coal seams in the study area contain pyrite and other sulfur-bearing minerals, human activities, such as coal mining or water pumping, can alter the original redox conditions within the aquifer, leading to an increase of TFe and SO42− in groundwater (FeS2 + 7/2O2 + H2O → Fe2+ + 2SO42− + 2H+ and/or FeS2 + 14Fe3+ + 8H2O → 15Fe2+ + 2SO42− + 16H+; Liu et al. 2008). Springs with TFe concentrations exceeding the drinking water limit of 0.3 mg/L are located in area of coal-bearing strata (Fig. 1 and Table 1), which shows that the lithology is an important factor affecting spring water quality in the study area.
There is no clear relationships between SO42− and NO3−, SO42− and K+, SO42− and Na+, and SO42− and Cl− (Table 2). The samples with SO42− concentrations of > 100 mg/L are taken from springs in both areas of coal-bearing strata (e.g., springs 1 and 6; Fig. 4b) and residential areas (e.g., springs 24 and 25; Fig. 4b). The springs with SO42− concentrations exceeding the drinking water standard limit of 250 mg/L are only those that are discharged from abandoned coal mines (springs 6-8; Fig. 4b). This implies that human industrial activities have accelerated the deterioration of groundwater quality in the basin. This also reveals a more complex origin of SO42− in spring waters, as discussed in the following section.
Chemical weathering
The Ca2+, Mg2+, and HCO3− concentrations of groundwater in karst areas are mainly controlled by the weathering of carbonate rocks (Eqs. 1 and 2; Han and Liu 2004; Han et al. 2010). Waters affected by such carbonate rock weathering are enriched in 2 mol of HCO3− for each mol of (Ca2+ + Mg2+).
Under normal conditions, the equivalent concentration of (Ca2+ + Mg2+) in water is equal to that of HCO3−. In this study, we analyzed the correlation between the equivalent concentrations of (Ca2+ + Mg2+) and HCO3− of different water samples. As shown in Fig. 5a, all the equivalent concentrations of (Ca2+ + Mg2+) and HCO3− of spring water, surface water, and sewage deviate from the 1:1 line. This suggests that carbonic acid could not be the sole dissolution agent, i.e., other acids, possibly sulfuric and/or nitric acids, may have played a complementary role in the carbonate rock weathering. We also plotted (Ca2+ + Mg2+) versus (HCO3− + SO42− + NO3−) and found that most samples fall on the 1:1 line (Fig. 5b). This evidence shows that the sulfuric and nitric acids have played a relatively important role in carbonate weathering (Eq. 3).
Jiang (2012) reported that, together with carbonic acid, nitric acid from agricultural fertilizers, sewage, and soil and sulfuric acid from rainfall, fertilizers, sewage, and sulfide oxidation can participate in the carbonate weathering in the Nandong Underground River System which drains a lithological terrain similar to our study area. The weathering of carbonate rock by sulfuric and nitric acids leads to a noticeable increase of the NO3− and SO42− in the local water. Thus, elevated NO3− concentrations are observed in the spring water samples collected from the agricultural and residential areas. The SO42− concentrations of the samples collected from abandoned coal mines and residential areas are also elevated (Table 1). This suggests that human activities significantly impact spring water quality in the Hongjiadu Basin.
Discussion
Sources and influencing factors of SO4 2− in groundwater
The potential SO42− sources in groundwater mainly include (1) atmospheric precipitation, (2) sulfides, like the products of pyrite oxidation, (3) evaporites, like gypsum and anhydrite, (4) sulfate in soils, and (5) anthropogenic sources, such as sewage, chemical fertilizers, and manure. Under aerobic conditions, little sulfur isotopic fractionation occurs during the processes of mineral dissolution and/or precipitation, sulfide oxidation, soil adsorption, plant assimilation, and mineralization of organic sulfur. Hence, δ34S has been widely used as a tracer for identifying the sources of SO42− in groundwater (e.g., Li et al. 2013; Tuttle et al. 2009). Unlike δ34S, δ18O of SO42− is often affected by the oxygen exchange between SO42− and H2O and/or O2 during the process of sulfide oxidation, resulting in a change in the δ18OSO4 value (Kroopnick and Craig 1972). Therefore, the δ18OSO4 can be used to determine the oxidation or reduction state in karst aquifer environments (Samborska et al. 2013; Li et al. 2011). Under anaerobic, high-temperature conditions, bacterial sulfate reduction (BSR), and thermochemical sulfate reduction (TSR) result in a significantly increase of both δ34S and δ18OSO4 values and a decrease of SO42− in groundwaters (Bottrell et al. 2008; Strebel et al. 1990). Thus, the combined use of δ34S, δ18OSO4, and SO42− concentrations can not only enable the identification of SO42− sources but also facilitate the investigation of biogeochemical processes, such as BSR and TSR.
The δ34S and δ18OSO4 values of water samples as well as the potential SO42− end-members are plotted in Fig. 6. For spring waters, no correlation between δ34S and δ18OSO4 (R2 = 0.05; P > 0.05; n = 26) is found, indicating that the SO42− in spring waters originated from at least three different sources in the basin (Li et al. 2013; Samborska et al. 2013). As shown in Fig. 6, the δ34S and δ18OSO4 values of spring waters (− 18.7 to + 0.3‰ and + 4.2 to + 10.3‰, respectively) are out of the typical ranges of evaporite end-member (+ 10 to + 28‰ and + 14.5 to + 32.5‰, respectively; Krouse and Crinenko 1991). This suggests that evaporites are not the main source of SO42− in spring waters, which is consistent with the lithology within the basin (Fig. 1b). Previous studies show that sulfur contents in soils in the upper reaches of the Wujiang River are relatively low (e.g., Jiang et al. 2006; Han and Liu 2004). The Hongjiadu Basin is located in the upper reaches of Wujiang River, so the soil sulfur source can be precluded (Fig. 1a). Alternatively, atmospheric deposition, sulfide oxidation, and anthropogenic inputs are likely to be the potential sources of SO42− in spring waters.
Plots of δ34S vs. δ18OSO4 for water samples collected from the Hongjiadu Basin. Symbols are the same as those in Fig. 5
Atmospheric precipitation
Owing to the heavy use of sulfur-rich coal, the region of Guizhou Province has been greatly affected by acid rain that is characterized by low pH and high sulfate contents since the 1980s (Galloway et al. 1987). A large coal-fired power station that consumes approximately 2.54 MT coals per year is located in the basin (Fig. 7). The special topography of the basin prevents the timely dispersion of sulfur-containing gases and aerosols produced by coal combustion in this power plant. As a result, the deposition of combustion products (SO2 + 2OH → H2SO4 → SO42− + 2H+) with rainwater occurs in the basin. In the Hongjiadu Basin, the rainwater has a low pH of 5.48 and a higher SO42− concentration of 13.9 mg/L which is higher than that in the neighboring city of Guiyang (6.4 mg/L; n = 3; Liu et al. 2008) and the Nandong Underground River Basin (4.5 mg/L; n = 3; Jiang 2012). Figure 6 shows that the δ34S and δ18OSO4 values of most of spring water, surface water, and sewage samples fall within the typical ranges of rainwater (− 12 to + 9.4‰ and + 5 to + 17‰, respectively; Zhang et al. 2015; Xiao and Liu 2002). This further supports that acid rainwater is an important source of SO42− in spring water from the Hongjiadu Basin.
Because of the rapid conversion of rainwater and surface water into groundwater in karst areas, they should have similar chemical and isotopic characteristics (Liu et al. 2008). As mentioned above, springs in the Hongjiadu Basin are mainly recharged by atmospheric precipitation. However, great differences of both SO42− contents and δ34S values between the spring waters and rainwater are observed (Fig. 8), suggesting there are other sulfate sources and processes that control the chemical and isotopic properties of spring waters (LeDoux et al. 2016).
Plot of δ34S vs. 1/SO4 for spring water and rainwater samples collected from the Hongjiadu Basin. Symbols are the same as those in Fig. 5
Sulfide oxidation and bacterial sulfate reduction
Due to the special geological and hydrogeological features in karst areas, karst aquifers are vulnerable to contamination. Groundwaters in karst areas of southwest China are often reported to contain excess amounts of TFe and SO42−, which are mainly associated with the coal mining upstream (Liu et al. 2008).
The main sulfide mineral in coal-bearing strata from Guizhou Province is pyrite that has δ34S values of − 20.4 to − 2.51‰ (Ren et al. 2017; Liu et al. 2008). Although the typical range of δ18OSO4 values of sulfides reported in the literature is − 5 to +4‰ (Krouse and Mayer 2000), the varying proportions of O2 and H2O involved in sulfide oxidation often lead to a lower δ18OSO4 value during the rainy season but a higher value during the dry season (Li et al. 2011).
The springs 1, 14, and 18 discharged from coal-bearing strata (Fig. 1c) have δ34S values ranging between − 13.8 and − 4.1‰ which are within the range of pyrite in the region of Guizhou (− 20.4 to − 2.51‰). This suggests that the products of the oxidation of pyrite from the coal seams are probably the main source of SO42− in these three spring waters. In addition, the δ34S values of springs that are discharged from non-coal-bearing strata, for an example, springs 13 (− 10.9‰) and 20 (− 13.3‰), also fall within the range of pyrite from the Guizhou Province (− 20.4 to − 2.51‰). The coal seams in the study area are mainly distributed in the recharge areas of groundwater. This, together with the presence of numerous faults that provide channels for groundwater flowing through the basin (Fig. 1c), suggests that when groundwater discharged from non-coal-bearing strata are recharged by surface and groundwaters from coal-bearing strata, the sulfide in spring waters can have δ34S characteristics of the products of pyrite oxidation. Therefore, sulfide oxidation is another important processes affecting the SO42− in spring waters in downstream areas without the distribution of coal-bearing strata .
Samples 6–8 that collected from springs in abandoned coal mines have δ34S values of − 2.2‰, + 0.3‰, and − 12.3‰, respectively. Only the spring 8 has a δ34S value that is close to the mean value of groundwater from abandoned coal mines in Guizhou Province (− 13‰, n = 5; Jiang et al. 2006). Compared with − 13‰, the δ34S values for the springs 6 and 7 samples are much heavier. Six samples, including springs 1, 6–8, 14, and 18 that are discharged from coal-bearing strata have δ18OSO4 values of > + 4‰, suggesting that other processes may affect the isotopic ratios of SO42−, such as (1) the reduce of groundwater table in winter, resulting in greater participation of atmospheric O2 in the sulfide oxidation, (2) the occurrence of BSR and/or TSR, and (3) anthropogenic input of contaminants with higher δ18OSO4 values.
The participation of more O2 in the process of sulfide oxidation can result in an increase of δ18OSO4 values, but little change in δ34S (Zhang et al. 2015; Li et al. 2010). Tuttle et al. (2009) also reported that the lower groundwater level in winter can result in more O2 involving in sulfide oxidation, resulting in higher δ18OSO4 values in water samples collected from the Canadian River (+ 6.5 to + 17‰; mean + 10‰; n = 10). Such values are higher than those in this study (+ 4.2 to + 10.3‰; mean + 7.4‰; n = 26). The participation of O2 may partly explain the elevated values of δ18OSO4 but cannot fully explain the elevated values of δ34S, especially that of spring 6 and 7 samples. This suggests that there are other processes that determine the δ34S and δ18OSO4 values of spring water in the basin.
Previous studies have demonstrated that both BSR and TSR can increase the δ34S and δ18OSO4 values of residual sulfates (e.g., Watanabe et al. 2009; Tostevin et al. 2016). The temperature of all spring waters is 2.73–18.1 °C, and thus the effects of TSR should be insignificant (Tostevin et al. 2016; Worden and Smalley 1996). The occurrence of BSR in coal-bearing strata often results in high δ34S and δ18OSO4 values, and high TFe and low DO in groundwater (McMahon et al. 2010; Samborska et al. 2013; Puig et al. 2013). Compared with −13‰ and + 4‰, the spring 6 has higher δ34S and δ18OSO4 (− 2.2‰ and + 6.8‰, respectively). The TFe concentration of this spring water is as high as 76.7 mg/L, but the DO concentration as low as 0.23 mg/L. Also, the spring 7 has heavy δ34S and δ18OSO4 (+ 0.3‰ and + 6.9‰, respectively), and TFe concentration as high as 1.38 mg/L and the DO value as low as 0.36 mg/L. These hydrochemical and isotopic data support the occurrence of BSR in the waters of springs 6 and 7. In contrast, the higher concentration of SO42− in the springs 6 and 7 could be attributed to the rapid dissolution of secondary SO4-bearing minerals, such as jarosite (KFe3+(SO4)2(OH)6), coquimbite (Fe3+2(SO4)3⋅9H2O), pickeringite (MgAl2(SO4)4⋅22H2O), and roemerite (Fe2+Fe3+2(SO4)4⋅14H2O; Cravotta 1994; Sharma et al. 2013; Sun et al. 2013). Sharma et al. (2013) also reported that both the BSR and the dissolution of secondary SO4-bearing minerals occurred in an abandoned coal mine in Pennsylvania, USA. Hence, we suspect that the high δ34S, δ18OSO4 and SO42− contents of groundwater from the Hongjiadu Basin are associated with the occurrence of BSR and the dissolution of sulfate minerals.
Sewage, chemical fertilizers, and manure
The δ34S and δ18OSO4 values of the sewage samples from Hongjiadu Basin (mean − 7.8‰ and + 8.0‰, respectively) are similar to previously reported values of sewage in China (− 8 to − 4.3‰, and + 4.7 to + 7.5‰, respectively; Liu et al. 2008; Zhang et al. 2015). The SO42− concentrations in the sewage samples (e.g., sewage sample 36 that is directly discharged into the Hongjiadu karst aquifer; Fig. 3c) are as high as 220 mg/L, suggesting that sewage is another source of SO42− in spring water. The δ34S values (− 7.6 to − 4.5‰; Table 1) of most water samples of springs located in residential areas (e.g., springs 21, 24, 25, 27, and 29) are within the range of the published values of sewage (Fig. 6). This further confirms that the SO42− input of sewage into spring water in the study area is significant. The mean value of δ18OSO4 of sewage samples is + 8.0‰ (n = 2) which is heavier than that of spring waters (+ 7.4‰; n = 26). The mean values of δ18OSO4 of spring waters vary in the following order: residential area (+ 8.6‰) > agricultural area (+ 6.9‰) and forestry area (+ 6.9‰). This also shows that anthropogenic contaminants, likes sewage, result in an increase of δ18OSO4 values in spring waters from the Hongjiadu Basin. Studies of other regions, such as Manila, Philippines (Hosono et al. 2010), and Birmingham, UK (Bottrell et al. 2008), have also revealed that sewage input resulted in heavier δ18OSO4 values of groundwater.
Different raw materials and methods are used to produce chemical fertilizers in different countries, which results in significant differences in δ34S and δ18OSO4 values of chemical fertilizers that vary between − 6.5 and + 21.4‰ and + 7.7 and + 16.5‰, respectively (Vitòria et al. 2004). Previously published values of δ34S for chemical fertilizers in southwestern China are thus used as reference values in this study. Li et al. (2010) obtained δ34S values of + 10.9‰ and + 11.1‰ for two types of sulfur-containing chemical fertilizers used in the Shuicheng Basin which is near the Hongjiadu Basin (Fig. 1a). The δ34S values of all the samples from the Hongjiadu Basin, even those springs in agricultural areas (< −5‰) are much lower than these two reported values, which indicates that chemical fertilizers did not significantly contribute to the SO42− in the spring waters.
In contrast to chemical fertilizers, manure often has a relatively small difference in the δ34S values. The values reported in previous studies are − 0.9 to + 5.8‰ in the USA (Cravotta 1994), + 4‰ in the UK (Bartlett et al. 2010), and 0 to + 5‰ in Spain (Otero et al. 2007). Unfortunately, there are no reported δ34S and δ18OSO4 values of manure in the Honjiadu Basin and its neighboring areas. The δ34S and δ18OSO4 data of manure from other regions are thus used as references in this study, that is, − 0.9 to + 5.8‰ and − 3.8 to +6‰, respectively (Cravotta 1994; Bartlett et al. 2010; Otero et al. 2007). As shown in Fig. 6, none of the samples from the study area have δ34S and δ18OSO4 values within the range of reported values. Nevertheless, the input of manure cannot be totally excluded as a possible explanation for the increased δ34S values at individual sampling sites.
Assessment of spring water quality and quantity
Human activities have significantly impacted spring water quality in the Hongjiadu Basin. Thus, an assessment of spring water quality and quantity is important to determine whether they meet the local demand for drinking water. Table 3 presents the utilization status, discharge rates, indicators of water contamination and other information of the studied 29 springs. The main components that exceed the drinking water standards in spring water are TFe, SO42−, and NO3− and groundwaters from springs 1, 6–9, 14, 16, and 27 are not potable anymore (Table 3).
The daily water consumption per capita in Chinese villages is about 70 L. For a population of 28,500 in the Hongjiadu Basin, the daily water consumption is approximately 2 million liters. The daily discharge volume of potable spring water within the basin is approximately 4.2 million liters which is twice the required volume and should theoretically meet the local need for drinking water. However, as noted in “Study area”, the utilization of spring 4 which has the highest discharge rate in the basin is currently unfeasible. Excluding spring 4, the daily discharge volume of the basin springs is approximately 1.07 million liters which is just about half of the local need of drinking water.
A new industrial park is currently being planned in the study area, which would progressively increase the water demand and intensify human interference on the groundwater. In the absence of timely intervention by authorities in the effective management groundwater resource, the current shortage of drinking water in the Hongjiadu Basin is likely to be exacerbated.
Conclusions
The contaminants in spring waters from the Hongjiadu Basin are mainly NO3−, SO42−, and TFe, the concentrations of which have exceeded the standard limits for drinking water. The NO3−, Cl−, K+, and Na+ in spring waters mainly derived from chemical fertilizers, manure, and sewage. The results of δ34S and δ18OSO4 values show that the SO42− in spring waters mainly originates from acid rain, sulfide oxidation, and sewage. The springs suffered from NO3− contamination are located in agricultural and residential areas, revealing that the anthropogenic input significantly impacted the NO3− contamination of groundwater in the study area. The springs in residential areas and coal-bearing strata have high concentrations of SO42−, particularly those discharged from abandoned coal mines. This shows that the interference of human activities on natural processes have accelerated the deterioration of spring water quality. The BSR in several studied springs has increased the δ34S and δ18OSO4 values of the residual sulfates. Approximately 28% of the investigated 29 springs are unsuitable for drinking as some of the components in waters have exceeded the standard limits of drinking water. Except for the main spring that flows into the Wujiang River and is thus difficult to utilize, the daily total water discharge volume of springs in the Hongjiadu Basin is approximately 1.07 million liters, which can only satisfy half of the drinking water demand of the population in the basin. Considering that there is no abatement in agricultural activities in the study area and industrial activities are continually increasing, the shortage of drinking water in the Hongjiadu Basin is expected to be continued.
References
Barnes RT, Raymond PA (2009) The contribution of agricultural and urban activities to inorganic carbon fluxes within temperate watersheds. Chem Geol 266:318–327. https://doi.org/10.1016/j.chemgeo.2009.06.018
Bartlett R, Bottrell SH, Sinclair K, Thornton S, Fielding ID, Hatfield D (2010) Lithological controls on biological activity and groundwater chemistry in quaternary sediments. Hydrol Process 24:726–735. https://doi.org/10.1002/hyp.7514
Bottrell S, Tellam J, Bartlett R, Hughes A (2008) Isotopic composition of sulfate as a tracer of natural and anthropogenic influences on groundwater geochemistry in an urban sandstone aquifer, Birmingham, UK. Appl Geochem 23:2382–2394. https://doi.org/10.1016/j.apgeochem.2008.03.012
Cravotta CA (1994) Secondary iron-sulfate minerals as sources of sulfate and acidity: the geochemical volution of acidic ground water at a reclaimed surface coal mine in Pennsylvania. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation, American Chemical Society Symposium Series, vol 550, pp 345–364
Dugin K, Dongchan K, Bernhard M et al (2009) Identification of nitrate and sulfate sources in groundwater using dual stable isotope approaches for an agricultural area with different land use (Chuncheon, mid-eastern Korea). Agric Ecosyst Environ 132(3–4):223–231. https://doi.org/10.1016/j.agee.2009.04.004
Edmond JM, Palmer MR, Measures CI, Grant B, Stallard RF (1995) The fluvial geochemistry and denudation rate of the Guayana shield in Venezuela, Colombia, and Brazil. Geochim Cosmochim Acta 59(16):3301–3325. https://doi.org/10.1016/0016-7037(95)00128-M
Ford D, Williams P (2007) Karst hydrogeology and geomorphology. John Wiley & Sons, New York
Galloway JN, Zhao DW, Xiong JL et al (1987) Acid rain: China, United States, and a remote area. Science 236:1559–1562. https://doi.org/10.1126/science.236.4808.1559
Han G, Liu CQ (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem Geol 204:1–21. https://doi.org/10.1016/j.chemgeo.2003.09.009
Han G, Tang Y, Xu Z (2010) Fluvial geochemistry of rivers draining karst terrain in Southwest China. J Asian Earth Sci 38:65–75. https://doi.org/10.1016/j.jseaes.2009.12.016
Hosono T, Siringan F, Yamanaka T, Umezawa Y, Onodera SI, Nakano T, Taniguchi M (2010) Application of multi-isotope ratios to study the source and quality of urban groundwater in Metro Manila, Philippines. Appl Geochem 25:900–909. https://doi.org/10.1016/j.apgeochem.2010.03.009
Hou MY, Zhang L, Wang ZW et al (2017) Estimation of fertilizer usage from main crops in China. J Agric Resour Environ 34(4):360–367 (in Chinese). https://doi.org/10.13254/j.jare.2017.0061
Jakóbczyk-Karpierz S, Sitek S, Jakobsen R, Kowalczyk A (2016) Geochemical and isotopic study to determine sources and processes affecting nitrate and sulphate in groundwater influenced by intensive human activity - carbonate aquifer Gliwice (southern Poland). Appl Geochem 76:168–181. https://doi.org/10.1016/j.apgeochem.2016.12.005
Jiang Y (2012) Sources of sulfur in the Nandong underground river system, southwest China: a chemical and isotopic reconnaissance. Appl Geochem 27:1463–1470. https://doi.org/10.1016/j.apgeochem.2012.05.001
Jiang Y, Liu C, Tao F (2006) The role of sulfur cycling in carbonate weathering: isotope geochemistry of sulfur in the Wujiang River catchment, Southwest China. Chin J Geochem 25(1):278. https://doi.org/10.1007/BF02840283
Jiang Y, Wu Y, Groves C, Yuan D, Kambesis P (2009) Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J Contam Hydrol 109:49–61. https://doi.org/10.1016/j.jconhyd.2009.08.001
Kroopnick P, Craig H (1972) Atmospheric oxygen: isotopic composition and solubility fractionation. Science 175:54–55. https://doi.org/10.1126/science.175.4017.54
Krouse HR, Crinenko VA (eds) (1991) Stable isotopes: natural and anthropogenic sulphur in the environment. Wiley, New York
Krouse HR, Mayer B (2000) Sulphur and oxygen isotopes in sulphate. In: Cook PG, Herczeg AL (eds) Environmental tracers in subsurface hydrology. Kluwer Academic Press, Boston
Lang YC, Liu CQ, Zhao ZQ, Li SL, Han GL (2006) Geochemistry of surface and ground water in Guiyang, China: water/rock interaction and pollution in a karst hydrological system. Appl Geochem 21:887–903. https://doi.org/10.1016/j.apgeochem.2006.03.005
Ledoux STM, Szynkiewicz A, Faiia AM et al (2016) Chemical and isotope compositions of shallow groundwater in areas impacted by hydraulic fracturing and surface mining in the Central Appalachian Basin, Eastern United States. Appl Geochem 71:73–85. https://doi.org/10.1016/j.apgeochem.2016.05.007
Li XD, Liu CQ, Harue M, Li SL, Liu XL (2010) The use of environmental isotopic (C, Sr, S) and hydrochemical tracers to characterize anthropogenic effects on karst groundwater quality: a case study of the Shuicheng Basin, SW China. Appl Geochem 25:1924–1936. https://doi.org/10.1016/j.apgeochem.2010.10.008
Li XD, Liu CQ, Liu XL, Bao LR (2011) Identification of dissolved sulfate sources and the role of sulfuric acid in carbonate weathering using dual-isotopic data from the Jialing River, Southwest China. J. Asian Earth Sci 42:370–380. https://doi.org/10.1016/j.jseaes.2011.06.002
Li X, Gan Y, Zhou A, Liu Y, Wang D (2013) Hydrological controls on the sources of dissolved sulfate in the Heihe River, a large inland river in the arid northwestern China, inferred from S and O isotopes. Appl Geochem 35:99–109. https://doi.org/10.1016/j.apgeochem.2013.04.001
Li J, Qi Y, Zhong Y, Yang L, Xu Y, Lin P, Wang S, He J (2016) Karst aquifer characterization using storm event analysis for black dragon springshed, Beijing, China. Catena 145:30–38. https://doi.org/10.1016/j.catena.2016.05.019
Liu CQ, Lang YC, Satake H, Wu J, Li SL (2008) Identification of anthropogenic and natural inputs of sulfate and chloride into the karstic ground water of Guiyang, SW China: combined δ37Cl and δ34S approach. Environ Sci Technol 42:5421–5427. https://doi.org/10.1021/es800380w
Marques JM, Graça H, Eggenkamp HGM, Neves O, Carreira PM, Matias MJ, Mayer B, Nunes D, Trancoso VN (2013) Isotopic and hydrochemical data as indicators of recharge areas, flow paths and water–rock interaction in the Caldas da Rainha–Quinta das Janelas thermomineral carbonate rock aquifer (Central Portugal). J Hydrol 476(18):302–313. https://doi.org/10.1016/j.jhydrol.2012.10.047
McMahon PB, Carney CP, Poeter EP et al (2010) Use of geochemical, isotopic, and age tracer data to develop models of groundwater flow for the purpose of water management, northern High Plains aquifer, USA. Appl Geochem 25:910–922. https://doi.org/10.1016/j.apgeochem.2010.04.001
Merchán D, Otero N, Soler A, Causapé J (2014) Main sources and processes affecting dissolved sulphates and nitrates in a small irrigated basin (Lerma Basin, Zaragoza, Spain): isotopic characterization. Agric Ecosyst Environ 195:127–138. https://doi.org/10.1016/j.agee.2014.05.011
Négrel P, Pauwels H (2004) Interaction between different groundwaters in Brittany catchments (France): characterizing multiple sources through strontium- and sulphur isotope tracing. Water Air Soil Pollut 151:261–285. https://doi.org/10.1023/B:WATE.0000009912.04798.b7
Otero N, Canals À, Soler A (2007) Using dual-isotope data to trace the origin and processes of dissolved sulphate: a case study in Calders stream (Llobregat basin, Spain). Aquat Geochem 13:109–126. https://doi.org/10.1007/s10498-007-9010-3
Otero N, Sole A, Canals À (2008) Controls of δ34S and δ18O in dissolved sulphate: learning from a detailed survey in the Llobregat River (Spain). Appl Geochem 23:1166–1185. https://doi.org/10.1016/j.apgeochem.2007.11.009
Pu T, He Y, Zhang T, Wu J, Zhu G, Chang L (2013) Isotopic and geochemical evolution of ground and river waters in a karst dominated geological setting: a case study from Lijiang basin, South-Asia monsoon region. Appl Geochem 33:199–212. https://doi.org/10.1016/j.apgeochem.2013.02.013
Puig R, Folch A, Menció A, Soler A, Mas-Pla J (2013) Multi-isotopic study (15N, 34S, 18O, 13C) to identify processes affecting nitrate and sulfate in response to local and regional groundwater mixing in a large-scale flow system. Appl Geochem 32:129–141. https://doi.org/10.1016/j.apgeochem.2012.10.014
Ren K, Pan X, Zeng J, Jiao Y (2017) Distribution and source identification of dissolved sulfate by dual isotopes in waters of the Babu subterranean river basin, SW China. J Radioanal Nucl Chem 312:317–328. https://doi.org/10.1007/s10967-017-5217-y
Samborska K, Halas S, Bottrell SH (2013) Sources and impact of sulphate on groundwaters of Triassic carbonate aquifers, Upper Silesia, Poland. J Hydrol 486:136–150. https://doi.org/10.1016/j.jhydrol.2013.01.017
Sharma S, Sack A, Adams JP, Vesper DJ, Capo RC, Hartsock A, Edenborn HM (2013) Isotopic evidence of enhanced carbonate dissolution at a coal mine drainage site in Allegheny County, Pennsylvania, USA. Appl Geochem 29:32–42. https://doi.org/10.1016/j.apgeochem.2012.11.002
Singh KP, Gupta S, Mohan D (2014) Evaluating influences of seasonal variations and anthropogenic activities on alluvial groundwater hydrochemistry using ensemble learning approaches. J Hydrol 511:254–266. https://doi.org/10.1016/j.jhydrol.2014.01.004
Strebel O, Bottcher J, Fritz P (1990) Use of isotope fractionation of sulfate-sulfur and sulfate-oxygen to assess bacterial desulfurication in a sandy aquifer. J Hydrol 121:155–172. https://doi.org/10.1016/0022-1694(90)90230-U
Sun J, Tang C, Wu P, Strosnider WHJ, Han Z (2013) Hydrogeochemical characteristics of streams with and without acid mine drainage impacts: a paired catchment study in karst geology, SW China. J Hydrol 504:115–124. https://doi.org/10.1016/j.jhydrol.2013.09.029
Tostevin R, Craw D, Hale V et al (2016) Sources of environmental sulfur in the groundwater system, southern New Zealand. Appl Geochem 70:1–16. https://doi.org/10.1016/j.apgeochem.2016.05.005
Tuttle MLW, Breit GN, Cozzarelli IM (2009) Processes affecting δ34S and δ18O values of dissolved sulfate in alluvium along the Canadian River, central Oklahoma, USA. Chem Geol 265:455–467. https://doi.org/10.1016/j.chemgeo.2009.05.009
Vesper DJ, White WB (2004) Spring and conduit sediments as storage reservoirs for heavy metals in karst aquifers. Environ Geol 45(4):481–493. https://doi.org/10.1007/s00254-003-0899-6
Vitòria L, Otero N, Soler A et al (2004) Fertilizer characterization: isotopic data (N, S, O, C, and Sr). Environ Sci Technol 38(12):3254–3262. https://doi.org/10.1021/es0348187
Watanabe Y, Farquhar J, Ohmoto H (2009) Anomalous fractionations of sulfur isotopes during thermochemical sulfate reduction. Science 324:370–373. https://doi.org/10.1126/science.1169289
Worden RH, Smalley PC (1996) H2S-producing reactions in deep carbonate gas reservoirs: Khuff Formation, Abu Dhabi. Chem Geol 133:157–171. https://doi.org/10.1016/S0009-2541(96)00074-5
Wu Y, Luo ZH, Luo W, Ma T, Wang Y (2018) Multiple isotope geochemistry and hydrochemical monitoring of karst water in a rapidly urbanized region. J Contam Hydrol 218:44–58. https://doi.org/10.1016/j.jconhyd.2018.10.009
Xiao HY, Liu CQ (2002) Sources of nitrogen and sulfur in wet deposition at Guiyang, southwest China. Atmos Environ 36(33):5121–5130. https://doi.org/10.1016/S1352-2310(02)00649-0
Yang PH, Li Y, Groves C, Hong A (2019) Coupled hydrogeochemical evaluation of a vulnerable karst aquifer impacted by septic effluent in a protected natural area. Sci Total Environ 658:1475–1484. https://doi.org/10.1016/j.scitotenv.2018.12.172
Zhang D, Li XD, Zhao ZQ, Liu CQ (2015) Using dual isotopic data to track the sources and behaviors of dissolved sulfate in the western North China Plain. Appl Geochem 52:43–56. https://doi.org/10.1016/j.apgeochem.2014.11.011
Zhou J, Zhang Y, Zhou A, Liu C, Cai H, Liu Y (2016) Application of hydrochemistry and stable isotopes (δ34S, δ18O and δ37Cl) to trace natural and anthropogenic influences on the quality of groundwater in the piedmont region, Shijiazhuang, China. Appl Geochem 71:63–72. https://doi.org/10.1016/j.apgeochem.2016.05.018
Acknowledgments
We are grateful to anonymous reviewers and the editor for their constructive comments. We acknowledge Zhijun Wang and Amelia Huang for polishing the article.
Funding
This study is financially supported by the National Natural Science Foundation of China (grant no. 41702278) and the China Geological Survey Project (grant no. DD20160285).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Conducted hydrochemical and isotopic analysis of waters, Hongjiadu Basin, China
• Focused on chemical compositions, δ34S and δ18OSO4
• Bulk of spring water exceeds limits for NO3−, SO42−, and TFe in drinking water
• Method useful at revealing processes affecting spring water quality in karst basin
• Sewage, manure, chemical fertilizers, coal, acid rain, land use, etc. affected water quality
Rights and permissions
About this article
Cite this article
Ren, K., Pan, X., Zeng, J. et al. Contaminant sources and processes affecting spring water quality in a typical karst basin (Hongjiadu Basin, SW China): insights provided by hydrochemical and isotopic data. Environ Sci Pollut Res 26, 31354–31367 (2019). https://doi.org/10.1007/s11356-019-06272-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06272-x