Abstract
Fluoride (F) contamination study had been carried out to see its allocation in Kurmapalli watershed, Nalgonda district, Andhra Pradesh, India. The study area is located about 60 km SE of Hyderabad city. The groundwater is the main source of water for their living. The groundwater in villages and its surrounding are affected by fluoride contamination and consequently the majority of the people living in these villages has health hazards and is facing fluorosis. The purpose of this study is to identify the wells with high F, raise awareness in people, study the water chemistry, and also find out the source of F in groundwater. A total of 32 groundwater samples were collected from different wells in both shallow aquifers and deeper fractures zones during October 2004. The chemical analysis of groundwater has been done. Fluoride values vary from 0.7 to 19.0 mg/l. It is noted that the maximum value (19.0 mg/l) is one of the highest values found in groundwater in India and 78% of the total samples show F concentrations that exceeds the permissible limit value (1.5 mg/l). The highest value of F is found at Madanapur bore well which is located at central part of the watershed. The F value of this bore well was monitored from October 2004 to October 2006. During this period the F concentration varies from 17.8 to 21.0 mg/l with mean 19.3 mg/l. There is no correlation of F with chemical parameters except calcium. The Ca has shown inverse proportional with F. Water–rock interaction studies were also carried out to understand the behavior of F in groundwater at prominent F affected areas. Rock samples were collected and analyzed, and found their enrichment of F. The anthropogenic possibility of F is almost negligible. The rocks of this area are enriched in F from 460 to 1,706 mg/kg. It is indicated that the rock–water interaction is the main source of F in groundwater. The highest values of F are found in middle part of the region and are related to the occurrence of fluoride rich rocks and their chemical kinetic behavior with groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride helps for the human health as it helps in the normal mineralization of bones and formation of dental enamel (Cao et al. 2000). When the daily intake-dose of F is below 0.5 mg/l (deficiency) some health related problems could occur such as dental caries, lack of formation of dental enamel and deficiency of mineralization of bones, especially among the children. On the contrary, when F daily intake-dose exceeds 1.5 mg/l (poisoning effect), it can cause several health related problems, e.g., fluorosis, which equally affect both young and old (WHO 1984; Kharb and Susheela 1994). The incidence and severity of fluorosis is related to the F content in various components of environment, viz. air, soil, and water. Out of these, water (especially groundwater) is the major contributor to the problem (Agrawal et al. 1997). The problem of excess F concentration in groundwater resources has now become one of the most important toxicological and geo-environmental issues in India as well as several parts of the world (Nanyaro et al. 1984; Gaciri and Davies 1993; Gizaw 1996; Andezhath et al. 1999; Wang et al. 1999; Pillai and Stanley 2002; Madhnure et al. 2007). The high concentrations of F have been found in groundwater in China, Syria, Jordan, Ethiopia, Sudan, Tanzania, Kenya and Uganda (Smith et al. 1953; Ocherse 1953; Grech 1966; Latham and Grech 1967; Moller et al. 1970; Lester 1974; Olson 1979; Tekle-Haimanot et al. 1987; Fuhong and Shuqin 1988; Kahama 1997; Finkelman et al. 1999; Ando et al. 2001). The states of Gujarat, Rajasthan, Haryana, Punjab, Uttar Pradesh, West Bengal, Orissa, Madhya Pradesh, Andhra Pradesh, Tamilnadu and Karnataka in India are the most affected with this problem Ramesam and Rajagopalan (1985; Rao et al. 1993; Sisodia 1994; Misra 1997; Garg et al. 1998; Ahmed and Sreedevi 2002; Pillai and Stanley 2002; Jacks et al. 2005). A critical evolution of the occurrence of F in the natural waters of several states and Union territories of India indicates that the permissible limit value (1.5 mg/l) in the groundwater is exceeded as much as in 20 states (Chand Dinesh 2001). According to the ‘Survey of Status of Drinking Water Supply in Rural Habitation’ conducted by the Rajiv Gandhi National Drinking Water Mission (RGNDRM) in 1993, there are 9,741 villages in India having F content >1.5 mg/l in groundwater sources (Handa 1988; Susheela 1999). It is generally accepted that groundwaters are enriched in fluoride due to prolonged water–rock interactions (Nordstrom et al. 1989; Gizaw 1996; Frengstad et al. 2001; Carrillo-Rivera et al. 2002). The chemical composition of lithology, therefore, is regarded as an important factor determining the F concentration of groundwater. Studies have shown that F is generally enriched in groundwater of the bedrock aquifers of alkali granites and metamorphic rocks (Banks et al. 1998; Dowgiałło 2000; Botha and Van Rooy 2001; Shanker et al. 2003).
As we know that the fluoride incidence in groundwater is mainly a natural phenomenon, influenced basically by the local and regional geological setting and hydrogeological conditions (Handa 1975; Ramesam and Rajagopalan 1985; Tamata 1994; Srinivasa Rao 1997; Ahmed and Sreedevi 2002). The chief sources of F in groundwater are the fluoride-bearing minerals in the rocks and sediments. The important fluoride-bearing minerals are: fluorite (fluorspar), fluorapatite, cryolite, biotite, muscovite, lepidolote, tourmaline, hornblende series minerals, glaucophane-riebeckite, asbestos (chrysotile, actinolite, anthophyllite), sphene, apophyllite, zinnwaldite, etc. These minerals have sufficient amount of fluorine in their composition.
Since rocks are made up of minerals and the soil is derived from parent rocks, F is fairly abundant in rocks and soil. The F content varies in different geological formations i.e. in apatite rocks; amphiboles present in metamorphic rocks, in the groundwater present in the amphibole formations and weathered formations of pyroxene amphibolites and pegmatites (Sinha 1986; Srinivasa Rao 1997). The weathering and leaching processes, mainly by moving and percolating water, play an important role in the incidence of F in groundwater. The F concentration in groundwater depends upon the following factors like climate, relief, evaporation, precipitation, geology and geomorphology of the area (Liu and Wan Hua 1991). Usually fluorite is leached from rock by water under different pH conditions (Fuhong and Shuqin 1988). Thus pH plays an important role in dissociation and dissolution of F in groundwater (Saxena and Ahmed 2001, 2003). However, the present study area is fairly representative of the granitic aquifers that are affected by the F problems. The area has very high amount of F content in the groundwater and its values are <3.2 mg/l all over the area except the small patches where they are comparatively higher values. The purpose of this paper is to evaluate the quality of groundwater in Kurmapalli in particular intern of F. This study includes the source of F and also their chemical kinetic behaviour with groundwater.
Study area
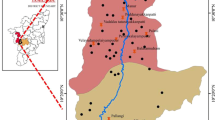
Kurmapalli watershed (study area) and the sampling points are shown in Fig. 1. It lies between longitude 78.68° and 78.84°E and latitude 16.83° and 16.98°N in Nalgonda District (Andhra Pradesh), India. This area covers about 100 km2, which is one of the most drought prone areas of Nalgonda district (Prasad et al. 2007). This basin is characterized by poor land soil scarce vegetation, erratic rainfall, and lack of soil moisture for most part of the year. Recurring drought coupled with increase in groundwater exploitation results in decline of groundwater levels (Rajani et al. 2006). The watershed forms part of semi-arid tract of Deccan plateau. Generally the area is being rained from July to December of the each year. During 2004, rainfall recorded at this watershed is only 400 mm. Out of 400 mm, only 276 mm is recorded during monsoon time i.e. from July to December 2004 and the rest is during summer months when the evaporation rate is quite high and is not useful for agricultural activity. The temperature continuously increases from the end of February to the hottest month (May) to between 35°C and over 46°C. In the coldest month (January), the values recorded ranged between 22 and 26°C. The major rock type in this area is gneissic complex. The slope of the area is 0–1% and having weathered pediplain with alkaline soil extending down the slope from the foot of mountain scarp. In this area 3rd order of stream passing through it and which appeared as good zone of groundwater, but unfortunately this area is highly contaminated with F presumably highest in granitic terrain in India. As a result of this, groundwater has turned into killing water for human race. Madanapur, one village of the study area, lies at 78.7368°E longitude and 16.9281°N latitude has been more affected with F which is considered to be one of the highest F concentrations in India.
Geological and hydrogeological setup
Granite and gneissic cover about 90% of the Kurmapalli watershed and followed by kankar and recent alluvium along the river course. In the northern part of the watershed has massive granite. The older gneisses of the area occupy the high hill ranges and also isolated hillocks, which are mostly of blocky nature and marked by scraps. Gneissic complex covers major part of the area forms the plains and occasionally forms low dome-shaped hillocks. Kankar is found in some patches of southeastern part of the area. Lineaments are found in all along the study area having NW–SE trend in general and at some places N–S trend has been seen. Drainage pattern is dendritic to sub-dendritic in nature. The dominant lineament directions control drainages at most of the places. The groundwater occurs in weathered portion of aquifer, joints and deep fractures. The groundwater is being exploited through dug wells tapping weathered zone and bore wells tapping from the fractured zones. The general trend of groundwater flow is towards the southeastern direction (Fig. 2). This flow pattern is disturbed at some places of the study area due to over exploitation and local geological settings. The thickness of the weathered zones varies from 5.6 to 20.0 m. The highly weathered part upto 20 m depths in central valley is the potential aquifer. The shallow aquifer gets direct recharge through rainfall and seepage from a number of irrigation tanks and fields. Most of the tanks are either got silted or could not store water due to breach of bunds. This has resulted into a low seepage rate as indirect recharge.
Materials and methods
About 32 water samples were collected during October 2004 from bore wells (Fig. 1), which are under use at 0.5 m below the water table, and were pumped more than 5 min. Methods of collection and analysis of water samples followed are essentially the same as given by Brown et al. (1983) and APHA (1985), which is shown in Table 1. These samples were collected in 1-l capacity polythene bottles. Prior to collection the bottles were thoroughly washed with diluted HNO3 acid, and then with distilled water in the laboratory before filling the bottle with sample. The bottle is rinsed to avoid any possible contamination in bottling and every other pre-cautionary measure has been taken. Fluoride was determined by ion selective electrode method using TISAB (Greenberg et al. 1998). The precise locations of the sampling points were determined in the field using GPS GARMIN-12, Global Positioning System (GPS). Some parameters like pH and electrical conductivity (EC) were measured during the sampling collection using portable kits. The chemical analyses of these groundwater samples were carried out at Geochemistry Laboratory, National Geophysical Research Institute (NGRI), Hyderabad using standard methods (Brown et al. 1983; APHA 1985). Five-rock samples were also collected from an exposure close to the highly affected fluoride well (Fig. 6). These rocks were crushed, powdered and messed. Sub-samples were used for chemical analysis at X-Ray Fluorescence Spectrometer (XRF) Laboratory, NGRI, Hyderabad using standard methods (Govil 1985). The analytical precision for the measurements of cations (Ca, Mg, Na, and K) and anions (HCO3, Cl, SO4, and F), indicated by the ionic balance error (IBE) was computed on the basis of ions expressed in me/l. The value of IBE was observed to be within a limit of ±5% (Mandel and Shiftan 1980; Domenico and Schwartz 1990).
Results and discussion
Chemistry of groundwater
The results of chemical analysis of groundwater are included in Table 2. The Table 3 gives the minimum, maximum, mean values of chemical constituents and standard deviation. The analytical results of the samples collected from the area indicate that the groundwater is generally alkaline in nature (pH 7.4–8.5). The EC varies from 490 to 2,000 μS/cm and total dissolved solids from 310 to 1,280 mg/l. Sodium is dominant ranging from 28 to 252 while Mg varies from 6 to 48 mg/l and Ca from 12 to 180 mg/l. Chloride concentration ranges from 34 to 385 mg/l. Bicarbonate is the next predominant anion, with a concentration varying between 128 and 456 mg/l.
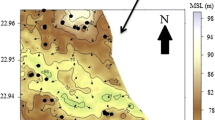
The F concentration varies from 0.7 to 19.0 mg/l and the highest concentrations are observed in the middle part of the watershed. The high fluoride groundwater in the area is concentrated mostly in and around Madanapur and at Polepalli (shown in Fig. 3). The F concentration exceeded the permissible limit (1.5 mg/l) in 78% of the total samples analyzed. The groundwater flows generally from NW to SE direction. The evaporation rate increases from recharge to discharge areas (Rajani et al. 2006). The dry climate with low rainfall favors salinization of groundwater along the flow direction. An attempt has been made to study the chemical character of the water samples of the area. Figure 4 shows the correlation of Ca and Mg with F content of groundwater and reflects the behavior of Ca and Mg with F in groundwater samples. The highest F (19.0 mg/l in October 2004) is observed in Madanapur bore well where Ca (19 mg/l) is comparatively low. The lowest Ca is more or less related with the calcium precipitation. Highest F likely to be form rock–water interaction where chemical kinetic is well suited and the rock has high value of F. In order to verify the correlation of F concentration with Na, Ca ions present in groundwater are established. These are clearly indicates the negative relation of F with Ca. However no other correlation existed can give any conclusion.
To establish the relationship of F with other water quality parameters, correlation analysis was performed. Acidity/alkalinity of the groundwater is a factor that controls the leaching of F from the fluoride bearing minerals. The correlation study made and the scatter plots brought out a positive correlation (r = 0.47) between the pH and F concentration, indicating that higher-alkalinity of the water promote the leaching of F and thus affect the concentration of F in groundwaters (Fig. 5a). There is a negative correlation with TDS in groundwater as shown in Fig. 5b. Groundwater with high F generally contains low levels of calcium. The calcium ion activity in the natural environment is controlled mainly by carbonate ion, which forms insoluble calcite. Thus the activities of calcium and F are negatively correlated with correlation coefficient (r = −0.47). Hence if rocks/soils and groundwater are low in calcium, the water can be enriched in F. The negative correlation between Ca and F is shown in Fig. 5c. The negative correlation coefficients was also observed between F and HCO3 (r = −0.39), and whereas no correlation observed between F and Na, which indicate the HCO3 and Na solubility may not favor the higher value of F in the study area (Fig. 5d, e).
Water–rock interaction studies
Geology of the study area is mainly granite and gneisses covered with black clayey soil. To study the influence of rock for F contamination, five-rock samples were collected from an exposure close to the elevated fluoride well (as shown in Fig. 6). Results of the chemical study are presented in Table 4. The chemical composition of rocks collected near to the F enriched area showed that the F concentrations ranged from 460 to 1,706 mg/kg. However the F value in sample no. R-2 is not traceable (Krishna et al. 2007). The variation of F in rock is well reflected in groundwater. It is indicated the possibility of F in groundwater is more where the rock is consistent with high in F. Hence a fairly good chemical kinetic condition in particular significant period in between mineral and groundwater exists in the study area. Deeper aquifer with good discharged found to be more in enriched in fluoride (as it is also observed in sample numbers 11, 7, 12 and 17 in Table 2). The dark mineral fraction of gneisses (separated in a high intensity magnetic field) contains high concentrations of F. This in combination with the relative stability of fluorapatite during the weathering process indicates that the main source of F− in groundwater is from granite and gneisses rocks. Fluorine is lost during weathering at approximately the same rate as other elements from the mineral phase (Handa 1975; Nordstrom et al. 1989; Ahmed and Sreedevi 2002; Saxena and Ahmed 2003).
The seasonal depths to water level and F values were collected from the elevated F affected area (around Madanapur village). It is presented in Table 5. This table indicates the depth to water level varies from 5.26 to 16.21 m below groundwater level (bgl) corresponding to F values vary from 2.4 to 21.0 mg/l in June 2005 in and around Madanapur village. The trends of these parameters are also shown in Fig. 6. This indicates, F is approximately proportional to the water column of aquifer. It means that when the depth to water level below ground level is decreasing then F value is increasing trend in the study area.
Conclusions
-
Fluoride (21.0 mg/l) is found in groundwater at Madanapur village, Kurmapalli watershed, Nalgonda district, Andhra Pradesh, which is one of the highest F in groundwater of granite terrain in India.
-
Concentration of F in rock samples varies from 460 to 1,706 mg/kg. This indicates that rock is enriched in F.
-
The lack of Ca in groundwater may decrease the possibility of creating precipitation of CaF2; fluoride is inversely proportional to calcium; and also the formation of NaF, which has a high solubility, could enhance the high concentration of fluoride. This indicates that source of fluoride in groundwater is due to rock-water interaction.
-
Middle part of the study area is more affected by F contamination in groundwater as well as deeper aquifers are enriched in F.
References
Agrawal V, Vaish AK, Vaish P (1997) Groundwater quality: focus on fluoride and fluorosis in Rajasthan. Curr Sci 73(9):743–746
Ahmed S, Sreedevi PD (2002) Cyclic variation of fluoride contents in a granite aquifer in semi-arid region. ICWRMA, Kuwait
Andezhath SK, Susheela AK, Ghosh G (1999) Fluorosis management in India: the impact due to networking between health and rural drinking water supply agencies. IAHS-AISH Publ 260:159–165
Ando M, Tadano M, Yamamoto S, Tamura K, Asanuma S, Watanabe T (2001) Health effects of fluoride pollution caused by coal burning. Sci Total Environ 271:107–116
APHA (1985) Standard methods for the examination of water and wastewater, 16th edn. Am pub Health Asso, Washington, p 100
Banks D, Frengstad B, Midtgard AK, Krog JR, Strand T (1998) The chemistry of Norwegian groundwaters: the distribution of radon, major and minor elements in 1,604 crystalline bedrock groundwaters. Sci Total Environ 222:71–91
Botha FS, Van Rooy JL (2001) Affordable water resource development in the Northern Province, South Africa. J African Earth Sci 33:687–692
Brown E, Skougstad MW, Fishmen MJ (1983) Method for collection and analyzing of water samples for dissolved mineral and gases. US Govt. Printing Office, Washington, p 75
Cao J, Zhao Y, Lin JW, Xirao RD, Danzeng SB (2000) Environmental fluoride in Tibet. Environ Res 83:333–337
Carrillo-Rivera JJ, Cardona A, Edmunds WM (2002) Use of abstraction regime and knowledge of hydrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosı basin. Mexico J Hydrogeol 261:24–47
Chand Dinesh (2001) International Workshop on fluoride in drinking water: strategies. Management and mitigation, Bhopal, pp 13–27
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York
Dowgiałło J (2000) Thermal water prospecting results at Jelenia Go′ra-Cieplice (Sudetes, Poland) versus geothermometric forecasts. Environ Geol 39:433–436
Finkelman RB, Belkin HE, Zheng B (1999) Health impacts of domestic coal use in China. Proc Natl Acad Sci USA 96:3427–3431
Frengstad B, Banks D, Siewers U (2001) The chemistry of Norwegian groundwaters: the dependence of element concentrations in crystalline bedrock groundwaters. Sci Total Environ 277:101–117
Fuhong R, Shuqin J (1988) Distribution and formation of high-fluorine groundwater in China. Environ Geol Water Science 12(1):3–10
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412
Garg VK, Dahiya S, Chaudhary A, Shikha D (1998) Fluoride distribution in groundwaters of Jind district, Haryana, India. Ecol Evn Cons 41(1–2):19–23
Gizaw B (1996) The origin of high bicarbonate and fluoride concentrations in waters of the main Ethiopian Rift Valley. J Afr Earth Sci 22:391–402
Govil PK (1985) X-ray fluorescence analysis of major, minor and selected trace elements in new IWG reference rock samples. J Geol Soc India 26:38–42
Grech P (1966) Fluorosis in young persons: further survey in northern Tanganyika, Tanzania. Brit J Radiol 39:760–764
Greenberg E, Connors Joseph J, Jenkins David (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Handa BK (1975) Geochemistry and genesis of fluoride containing groundwater in India. Groundwater 13(3):275–281
Handa BK (1988) Fluoride occurrence in natural waters in India and its significance. Bhu-Jal News 3(2):31–37
Jacks G, Bhattacharya P, Chaudhary V, Singh KP (2005) Controls on the genesis of some high-fluoride groundwaters in India. Applied Geochemistry 20:221–228
Kahama RW (1997) Fluorosis in children and sources of fluoride around Lake Elementaita region of Kenya. Fluoride 30:19–25
Kharb P, Susheela AK (1994) Fluoride ingestion in excess and its effect on organic and certain inorganic constituents of soft tissues. Med Sci Res 22:43–44
Krishna AK, Murthy NN, Govil PK (2007) Multielement analysis of soils by wavelength-dispersive X-ray fluorescence spectrometry. Atomic Spectroscopy 28(6):202–214
Latham MC, Grech P (1967) The effect of excessive fluoride intake. Am J Public Health 4:651–660
Lester F (1974) Fluoride myelopathy, with a review of literature. Ethiop Med J 12:39–49
Liu Y, Wan Hua Z (1991) Environmental characteristics of regional groundwater in relation to fluoride poisoning in north China. Environ Geol Water Sci 18(1):3–10
Madhnure P, Sirsikar DY, Tiwari TN, Ranjan B, Male DB (2007) Occurrence of fluoride in the groundwaters of Pandharkawada area, Yavatmal district, Maharashtra, India. Curr Sci 92(5):675–679
Mandel S, Shiftan ZL (1980) Groundwater resources investigation and development. Academic Press, New York
Misra SK (1997) Water quality monitoring and surveillance in rural areas of Rajasthan. Public Health Engineering Department, Govternment of Rajasthan, India
Moller IJ, Pindborg JJ, Gedalia I, Roed-Peterson B (1970) The prevalence of dental fluorosis in the people of Uganda. Arch Oral Biol 15:213–255
Nanyaro JT, Aswathanarayana U, Mungere JS, Lahermo P (1984) A geochemical model for the abnormal fluoride concentrations in waters in parts of northern Tanzania. J Afr Earth Sci 2:129–140
Nordstrom DK, Ball JW, Donahoe RJ, Whittemore D (1989) Groundwater chemistry and water–rock interaction at Stripa. Geochim Cosmochim Acta 53:1727–1740
Ocherse T (1953) Chronic endemic fluorosis in Kenya, East Africa. Br Dent J 95:57–61
Olson B (1979) Dental findings in high-fluoride areas in Ethiopia. Community Dent Oral Epidemiol 7:51–56
Pillai KS, Stanley VA (2002) Implications of fluoride—an endless uncertainty. J Environ Biol 23:81–87
Prasad RK, Mondal NC, Banerjee P, Nandakumar MV, Singh VS (2007) Deciphering potential groundwater zone in hard rock through the application of GIS. Environ Geol 55:467–475
Rajani KVN, Swamy MV, Rao VK (2006) Estimation of groundwater resources—a case study of Kurmapalli watershed in Nalgonda and Rangareddy districts of Andhra Pradesh. In: Rao BV, Das GJ, Sarala S, Girdhar MVSS (eds) Proceeding 2nd international conference on hydrology and watershed management (5–8th December 2006), vol I. BS Publications, Hyderabad, pp 118–126
Ramesam V, Rajagopalan K (1985) Fluoride ingestion into the natural waters of hard-rock areas, Peninsular India. J Geol Soc India 26:125–132
Rao NVR, Rao N, Rao KSP, Schuiling RD (1993) Fluorine distribution in waters of Nalgonda District, Andhra Pradesh, India. Environ Geol 21:89–95
Saxena VK, Ahmed S (2001) Dissolution of fluoride in groundwater: a water-rock interaction study. Environ Geol 40:1084–1087
Saxena VK, Ahmed S (2003) Inferring the chemical parameters for the dissolution of Fluoride in groundwater. Environ Geol 43(6):731–736
Shanker R, Thussu JL, Prasad JM (2003) Geothermal studies at Tattapani hot spring area, Sarguja district, central India. Geothermics 16:61–76
Sinha RK (1986) Industrial Minerals, 2nd edn. Oxford and IBH, New Delhi, p 379
Sisodia P (1994) Health hazards due to non-optimal fluoride content in groundwaters in Rajasthan. Rajasthan Voluntary Health Association, Jaipur
Smith DA, Harris HA, Kirk R (1953) Fluorosis in the Butan, Sudan. J Trop Med Hyg 8:57–58
Srinivasa Rao N (1997) The occurrence and behavior of fluoride in the groundwater of the lower Vamsadhara River basin, India. Hydrological Sci J 42(6):877–892
Susheela AK (1999) Fluorosis management programme in India. Curr Sci 77(10):1250–1256
Tamata SR (1994) Possible mechanism for concentration of fluoride in groundwater. Bhu-jal News pp 5–11
Tekle-Haimanot R, Fekadu A, Bushra B (1987) Endemic fluorosis in the Ethiopian Rift Valley. Trop Geogr Med 39:209–217
Wang XC, Kawahara K, Guo XJ (1999) Fluoride contamination of groundwater and its impact on human health in Inner Mongolia area. Aqua 48:146–153
HO W (1984) Guideline of drinking quality. World Health Org, Washington, pp 333–335
Acknowledgments
Authors wish to thank Dr. V. P. Dimri, Director, NGRI for his permission to publish this paper. Thanks to Ministry of Water Resources, New Delhi (Ref.No. MOWR-21/80/2004-R&D/928-941, Date 25th March 2004) for their finical supports and also to the anonymous reviewers who have provided the valuable suggestions to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mondal, N.C., Prasad, R.K., Saxena, V.K. et al. Appraisal of highly fluoride zones in groundwater of Kurmapalli watershed, Nalgonda district, Andhra Pradesh (India). Environ Earth Sci 59, 63–73 (2009). https://doi.org/10.1007/s12665-009-0004-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0004-x