Abstract
The leaves and petioles of Paulownia elongata x fortunei are residual fractions from the tree plantations commercially destined to the production of wood and their valorization could contribute to the rational utilization of this resource. The saccharidic fraction is the most abundant in both parts of the plant and the sugar profile is very similar, but the ethanol extractives are more abundant in leaves. Non isothermal processing was selected since it provided better results than isothermal extraction with shorter times. For this reason, optimization of autohydrolysis under non isothermal operation (140–240 °C) was performed for both materials: leaves and petioles. The final autohydrolysis temperature highly influenced the saccharidic, proteic, phenolic and volatile composition of the extracts. Operating under selected conditions leaves provided extracts with more antioxidant compounds than petioles. The proposed technology provides a variety of commercially valuable components, which could contribute to the integral use of this energetic crop following a biorefinery approach.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The valorization of underused parts of the Paulownia tree (leaves, petioles) is interesting in order to provide a rational utilization of the raw material. Leaves and petioles are known for their biological properties. The extraction of phenolic bioactives with antioxidant properties is proposed using a green aqueous extraction based on the operation at high pressure. This process has not been reported previously for these materials and allows the solubilization of both phenolic and hemicellulosic fractions. The optimal extraction conditions (maximal temperature during heating) leading maximal extraction yields, and phenolic content and antiradical activity of the extracts were selected. The autohydrolysis treatment could serve as a first stage in a biorefinery process for the fractionated utilization of these materials.
Introduction
Paulownia is a genus of broadleaved trees, family Paulowniaceae (previously in the family Scrophulariaceae) comprising nine species and a few natural hybrids [1] that are native to China and Southeast Asia [2]. The economic potential is based on the value of its wood, its high biomass production [3] as well as the new uses and related products being developed [4]. Due to their rapid growth, short rotation and relative resistance to climatic conditions, interest in this genus has grown considerably, and intensive plantations of several species and hybrids within Paulownia can be found worldwide [5]. Paulownia elongata x fortunei is a sterile Cotevisa 2® interspecific paulownia hybrid clone, non-invasive or harmful to native flora (referred to herein as Paulownia COT-2). Paulownia COT-2 shows improved growth rate compared to each of the parental varieties, reaching heights above 18 m in 3 years.
Paulownia sp. could be an interesting source of secondary metabolites, among other compounds, flavonoids, lignans, phenolic acids and terpenoids [6, 7], have been isolated from several parts of the plant (leaves, flowers, fruits, wood, bark, roots and seeds) and have been traditionally used in Chinese herbal medicine to treat a variety of ailments and diseases because of their numerous biological activities: antioxidant, anti-inflammatory, antimicrobial, anticancer [1, 8].
The biorefinery utilization of Paulownia wood has been proposed [4, 9]. However, the total valorization of the underutilized fractions, such as leaves and petioles has not been reported. The leaves are large, especially those of the first year of growth, more than 60 cm wide, which represents a great ecological resource in the fight against air pollution. Their high nitrogen content makes them useful as fodder or fertilizer.
The development of greener efficient processes is increasingly demanded and the use of water as solvent is particularly promising [10]. Water extraction processes under pressurized conditions (below the supercritical conditions, normally 10 to 100 bar), and high temperatures (in the range of 100 to 374 °C) is called subcritical water or pressurized hot water [11]. At these conditions water presents unique properties, especially the high ionic product and the low dielectric constant which makes subcritical water more similar to less-polar organic solvents and allowing a faster extraction and a better extraction yield [12]. The change in properties of water under subcritical conditions favours the extraction of apolar compounds, and the partial breakage of the polysaccharidic structures. Autohydrolysis leads to a solid phase primarily composed of cellulose and lignin and a liquid phase rich in hemicellulose-derived compounds. This liquid phase can be further subjected to acid hydrolysis to convert the solubilized oligomeric hemicelluloses into monosaccharides and/or furans or levulinic acid [13]. Also the phenolic fraction resulting from depolymerization of acid soluble lignin fractions are solubilized and should be removed for saccharide purification. They can be valorised as antioxidants, being as potent and less susceptible to volatilisation than synthetic antioxidants [14]. The remaining solid contains cellulose, with higher accessibility for enzyme hydrolysis [4]. Autohydrolysis has been proposed for lignocellulosics such as wood [15, 16], agroindustrial wastes [17,18,19], seaweeds [20] and mushrooms [21, 22] and has recently been proposed as a pretreatment before bioethanol production from Paulownia wood [4].
Autohydrolysis or extraction with pressurized hot water under subcritical conditions is a useful technique proposed for the valorization of leaves, to obtain antioxidant extracts rich on bioactive compounds [23,24,25], and provided higher extraction yields and also higher phenolic content in the extracts than other assisted techniques [26].
The aim of this work is to optimize an autohydrolysis treatment of Paulownia elongata x fortunei (COT-2) leaves and petioles, usually discarded in plantations, to extract valuable components and explore the fractionation of the material for a possible biorefinery based process.
Materials and Methods
Raw Material
Paulownia elongata x fortunei (COT-2) leaves and petioles, kindly provided by Maderas Álvarez Oroza (Nois, Lugo, Spain), were collected in June 2017 and dried at room temperature for 15 days. The leaves were collected together with the petiole (part that connects the leaf to the trunk) in one of the Maderas Álvarez Oroza’s plantations. The petioles have a woody aspect, different from that of the leaves and for this reason, both fractions were analyzed and processed independently. Before storage, samples were crushed and stored in a cool and dry place.
Raw Material Characterization
Moisture and ash contents were gravimetrically determined by ISO 638 and ISO 776 methods, respectively.
Mineral (Ca, Mg, Fe, Cd, Cu, Fe, Zn, and Pb) content was determined by atomic absorption (SpectrAA-220 Fast Sequencial, Varian) and Na and K were determined by atomic emission. Hg was analysed by cold vapor atomic absorption.
Total nitrogen was measured by elemental analysis (FlashEA 1112 Elemental analyzer, Thermo, USA) using 130 mL/min of He as carrier gas and 100 mL/min as reference gas. The oxygen flow was 250 mL/min, and the temperatures of the oxidation and reduction ovens were 900 °C and 680 °C, respectively. Total nitrogen result was converted to protein using the factor 6.25.
The ethanol extractives content was gravimetrically determined after Soxhlet extraction.
For the quantification of carbohydrates, a preeliminary acid hydrolysis was performed. This consists of two stages, a first in which the sample was brought into contact with 72% sulfuric acid for 60 min in a water bath at 30 °C in order to break the polysaccharides. The second stage was carried out to obtain monosaccharides and was carried out with 4% sulfuric acid for 60 min in an autoclave. The liquor obtained was filtered through 0.45 µm cellulose acetate membranes and analyzed by HPLC using a refractive index detector (Model 1200, Agilent Technologies, USA) for the determination of carbohydrates. Aminex HPX-87H column from Biorad with 0.003 M H2SO4 at 0.6 mL/min as mobile phase operating at 50 °C was used to determine glucose, trehalose, arabinose and acid groups (acetic, formic, galacturonic acid). CarboSep CHO 682 column from Transgenomics with ultra-pure water at 0.4 mL/min as mobile phase at 80 °C was used to determine xylose, galactose, mannose and rhamnose, after neutralization with BaCO3. External standards for all the compounds were used.
Hydrothermal aqueous extraction under non isothermal conditions
Crushed leaves or petioles were mixed with distilled water at a liquid to solid mass ratio of 15:1 (w:w). Accordingly, 15 g of leaves and 20 g of petioles were contacted with 225 g and 300 g of distilled water, respectively. The raw material and solvent were stirred at 150 rpm in a 600 mL pressurized reactor (Parr Instrument Company, Illinois, USA) at final heating temperatures in the range of 140–240 °C. When the maximum treatment temperature was reached, the system was cooled with water through a stainless steel coil located inside the vessel. Finally, the separation of solid and liquid phases was carried out by filtration.
Aqueous Extracts Characterization
Total Phenolic Content (TPC) was determined by the Folin-Ciocalteau method, described by Singleton and Rossi [27]. Gallic acid was used as standard and analyses were performed by triplicate. In this method 0.5 mL of liquor was mixed with 3.75 mL of distilled water, 0.25 mL of Folin-Ciocalteu’s reagent diluted 1:1 with distilled water and 0.5 mL of sodium carbonate (10%, w/v) were added. Samples were incubated at room temperature in the darkness for 1 h and the absorbance was measured at 765 nm in a Thermo Scientific Evolution 201 spectrophotometer.
Trolox Equivalent Antioxidant Capacity (TEAC) was determined according to Re et al. [28]. The ABTS radical (2,2’-azinobis-(3-ethyl-benzothiazoline-6-sulfonate)) was produced by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulfate. The ABTS radical solution was diluted with PBS until an absorbance of 0.7 to 734 nm was reached against a phosphate buffered saline (PBS) solution. Then, 1 mL of the diluted solution was added to 10 μL of sample. The mixture was incubated for 6 min at 30 °C. At the end of this time, the decrease in absorbance at 734 nm was measured in a Thermo Scientific Evolution 201 spectrophotometer.
Soluble protein content was determined according to Bradford [29]. One mL of reagent (PanReac) was added to a 100 μL of sample and after 5 min the absorbance was measured at 595 nm using bovine serum albumin (Sigma) as standard.
Saccharidic fraction were determined by HPLC using the method described in the previous section (2.1.1) for carbohydrates determination. Aliquots of the liquors obtained at each temperature of treatment were subjected to posthydrolysis with 4% sulphuric acid in autoclave (Mod 4001756, Selecta, Spain) at 121 °C for 20 min and cooled by a water bath, to hydrolyze the oligomers. The content of oligomers in liquors is determined by difference between the content of monomers in the autohydrolysis liquor and in the posthydrolysis liquor.
GC-MS
GC-MS was performed with a HP 6890 Series II gas chromatograph coupled to a HP 5973 mass selective detector and equipped with a capillary ZB-5 column (30 m × 0.25 mm inner diameter × 0.25 μm film thickness). The samples were first filtered (0.45 μg Millipore filter) and then were injected into the GC-MS (1 μL of sample), applying 1:7 split mode with the injection port at 300 °C. Helium was used as carrier gas with a flow of 0.9 mL/min. The oven temperature was as follows: 45 °C for 1 min, 8 °C/ min to 200 °C, 15 °C/min to 310 °C and 310 °C for 10 min. The total run time was 37.71 min. Compounds were identified from the chromatograms by computer matching of mass spectra of the peaks with the NIST 17 mass spectral library.
Statistical Analysis
Experimental results were expressed as the mean ± standard deviation (SD) of three experiments (n = 2).
Results and Discussion
Characterization of Paulownia Leaves and Petioles
According to data in Fig. 1, petioles have higher carbohydrate content (55 %) than leaves (31%). However, the leaves contain more ethanol extractives, ash and protein. Attending to carbohydrate fraction, glucose is the major compound in both materials, and depending on the raw material, followed by xylose, galactose, arabinose, rhamnose and galacturonic acid. In a smaller proportion, mannose, acetic and formic acid were identified. This is interesting because polyphenols in plants can be found in conjugated form with sugar residues bound to the hydroxyl groups, being glucose, galactose, arabinose, rhamnose, xylose and glucuronic and galactouronic acids, some of the compounds to which they are associated more frequently. In both leaves and petioles K was the most abundant element, reaching 23.7 and 61.7 mg/kg, respectively, followed by Ca and Mg. Among microelements Zn and Fe were the most important (Table 1).
Hydrothermal Processing
Non Isothermal Conditions
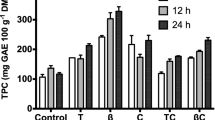
Non isothermal operation was performed for leaves and petioles. Total time extraction of different temperatures studied is shown in Table 2. Higher extraction yields were attained with higher temperatures, requiring lower exposure time. The maximum extraction yields were attained at 200 °C for leaves and at 220 °C for petioles (Fig. 2a). Protein content in leaves is 1.54 mg/100 g extract at 140 °C and increases progressively until 200 °C reaching its maximum of 2.23 mg/100 g extract, for petioles the maximum was reached at 180 °C with 1.74 mg/100 g extract (Fig. 2b). The antioxidant capacity and total phenol content of the leaves is higher than those of petioles (Fig. 2c and d). In petioles, the results improved with increasing temperature, reaching 14.4 g Trolox/100 g of extract and 4.8 g GAE/100 g extract at 240 °C. Likewise, in leaves operating at a maximum of 240 °C led to a total phenol content of 19.54 g GAE/100 g extract. However, the antioxidant capacity was maximun at 140 °C, about 81.3 g Trolox/100 g extract. At this temperature total phenol content was 18.05 g GAE/100 g extract. The phenolic content is in the range of that extracted from S.ebulus leaves during 30 min at 140 °C [24].
Tables 3, 4 show the composition of monosaccharides (a) and oligosaccharides (b) in the aqueous extracts obtained after autohydrolysis at different temperatures in leaves and petioles, respectively. Monosaccharides were determined directly from liquor and the oligomeric content was determined by difference with monomeric content. The content in monosaccharides is higher in the autohydrolysis extracts from petioles, whereas the opposite trend was observed in case of oligosaccharides, that were higher in leaves.
For leaves, the monosaccharide content (Table 3) showed an important decrease at temperatures higher than 180 °C. Glucose and trehalose showed a maximum at 140 °C with 3.58 and 3.80 g/100 g extract, whereas rhamnose and mannose showed a maximum at 180°C with 2.19 and 1.73 g/100 g extract. Acid groups increase their concentrations with temperature as expected, reaching 7.05 g formic acid/100 g extract and 3.11 g acetic acid/100 g extract at 240 °C. The major component in oligomeric fractions (Table 3) was glucose (GlOS) (16.05 g/100 g extract at 140 °C) that showed a steady decrease in the studied range, followed by rhamnose (11.40 g/100 g extract at 140 °C) with a marked decrease at temperatures higher than 220 °C. The oligomeric forms in sugars decreased with temperature, except galacto-oligomers (GOS), which showed a maximum at 200 °C (4.69 g/100 g extract). The concentration of others oligosaccharides identified were below 3 g/100 g extract.
For petioles, the monosaccharide content (Table 4) presented a similar behaviour to that of leaves, obtaining slightly higher values, in case of glucose the maximum was 5.32 g/100 g extract at 140 °C and in rhamnose was 2.35 g/100 g extract at 200 °C. Formic acid and acetic acid accounted for more than 10 g/100 extract at 240 °C.
The oligosaccharide content (Table 4) showed differences compared to those determined in leaves. In case of glucose in oligosaccharides, the maximum content reached (4.92 g/100 g extract at 240 °C) corresponded to less than half determined in leaves. The same decline showed the oligosaccharides of rhamnose reaching their maximum at 180 °C (3.17 g/100 g extract). Conversely, the oligosaccharides of xylose (XOS) and arabinose (AOS) were significantly higher than those of leaves. It should be noted that xylose oligosaccharides increased strongly with temperature up to 220 °C, where it reached its maximum 10.12 g/100 g extract and then fell down sharply. Arabinose-containing oligosaccharides were maximum at 180 °C (6.25 g/100 g extract). Galactose-containing oligosaccharides presented similar values, above 3 g/100 g extract, at the range of temperatures 180–220 °C. At low temperatures, oligosaccharides of galacturonic acid were present at 7 g/100 g extract, then decreased sharply. Other oligomers identified present contents below to 2 g/100 g extract.
Temperature is the key parameter in pressurized hot water extraction, since both solubility of non polar compounds and kinetics are enhanced but the thermolabile compounds could be degraded [25, 30]. At high temperatures some properties of the water could be altered such as the polarity and dielectric constant, which promotes the solubility of less-polar compounds due to the breakage of the hydrogen bonds of the water molecules [31, 32]. Also, as the water temperature rises, the viscosity and the surface tension of the water decrease and diffusivity of water increases, resulting in increased mass transfer rate of the solute from the sample matrix into the solvent [33]. Consequently, the cell walls become more permeable, favoring the solubility of bioactive compounds including substances insoluble in water previously.
As expected, operational conditions reported for wood processing are more severe. Operation at 160°C for 2.5 h provided maximum xylo-oligomers concentration from Paulownia elongata [9] and non isothermal heating up to 210 °C (40 min) during autohydrolysis of wood could be suitable for obtaining oligomeric sugar solutions and cellulose to be further hydrolyzed and converted into ethanol from Paulownia tomentosa [4]. The sugar solubilization depends on the material, but also on the component [34].
The selected temperatures were in the range of the optimal for other lignocellulosic residues processed by autohydrolysis at temperatures 200–240 °C providing solubilization around 30% [19]. Aqueous pressurized liquid extraction of olive leaves extracts was performed at 200 °C to maximize the extraction of antioxidants, since the amount of phenolic compounds increased with the extraction temperature [23]. For the extraction of fractions from boldo leaves, high pressure water extraction during 3 h at 125 °C was selected, since boldine yields decreased at 175 °C [30]. During pressurized microwave-assisted hydrothermal extraction of bioactive components of Lawsonia inermis leaves, extraction at 120 °C for 30 min provided higher yields, particularly of lawsone (2-hydroxy-1,4-naphthoquinone) [35]. In these latter cases extraction at higher temperatures and longer treatment time caused degradation of the target compounds.
Composition of the Liquid Phase
Data from GC–MS analysis of the compounds identified in the liquid phase from autohydrolysis of leaves and petioles are shown in Fig. 3a and b, respectively. The compounds identified include carboxylic acids, alcohols, aldehydes, ketones, phenolic and nitrogen containing compounds, confirming that the autohydrolysis process induced changes in the chemical structure. Particularly as an increase in the carbohydrate thermal derived compounds, and in the lignin-derived fraction. A larger number of compounds have been found in the hydrolysates produced from both materials at 240 °C, but the maximal contents were mostly observed in those produced at 220 °C. The presence of acetic acid increasing with temperature was found in hydrolysates from both materials, generated as a result of hydrolysis of the hemicellulosic fraction. In liquors from petioles, also formic acid was an important constituent, with a maximum at 200 °C. Furfural and 1-hydroxy-2-propanone are among the major thermal decomposition products of xylan, with maximum values at the higher temperatures. Hydroxymethylfurfural was only found in samples produced at the highest temperature tested. Furaneol, a product of Maillard reaction, was also detected at the highest temperature in petioles and increased with temperature in leaves. Phenolic derivatives (2-methylphenol, catechol), furan compounds (furfural, 5-methyl-2-furancarboxaldehyde, 1H-pyrrole-2-carboxaldehyde...), were the major volatile constituents found in the hydrolysates. Furfural was more abundant in samples from leaves and increased with autohydrolysis temperature.
a Most abundant compounds (a) (area > 1.500.000) identified in the liquid phase from autohydrolysis of leaves from Paulownia COT-2 and (b) most abundant compounds (area > 800.000) identified in the liquid phase from autohydrolysis of petioles from Paulownia COT-2, determined by GC–MS analysis regarding retention time
Other compounds identified have been reported in other thermally processed materials, such as 5-methyl-2-furancarboxaldehyde in aqueous leaves extracts [36] and in smoke from sucrose [37], (3-methyl-oxiran-2-yl)-methanol, as an intermediate in phenol oxidation [38], and 3-methyl-1, 2-cyclopentanedione from thermal treatment of lignocellulosic biomass.
Added valued compounds such as catechol and its derivatives were also identified after thermochemical processing at 220 °C and 240 °C in both leaves and petioles, and could be used as commodity organic chemicals. Vanillin, an added value compound widely used in pharmaceutical and food industry, is present in petioles samples at 220 °C. Also 3-pyridinol was detected in leaves and petiole samples at temperatures from 180 °C onwards, a pyridine widely identified in thermal degradation processes.
All these compounds that can be extracted from the paulownia petioles and leaves represent an opportunity to take advantage of the resources and by-products obtained from this energy crop, within the framework of biorefineries and the circular economy.
Conclusions
Based on the results from non isothermal aqueous treatment of paulownia leaves on the extraction of the phenolic fraction and the antiradical properties of the extracts, maximum yields were attained at 200 and 220 °C for leaves and petioles, respectively. Hardly no influence of temperature was noticed on the phenolic content in extracts from leaves, but the maximum for petioles occurred at the highest tested temperature. Oligosaccharides form glucose and rhamnose predominated in leaves extracts at the lower temperatures tested, whereas oligomers from arabinose and xylose were the most abundant in extracts from petioles at 180 and 220 °C, respectively.
Thus, the proposed non isothermal operation provides a variety of commercially valuable components which could contribute to the integral use of this energetic crop following a biorefinery approach.
References
Yadav, N.K., Vaidya, B.N., Henderson, K., Lee, J., Stewart, W.M., Dhekney, S.A., Joshee, N.: A review of Paulownia biotechnology: a short rotation, fast growing multipurpose bioenergy tree. Am. J. Plant Sci. 4, 2070–2082 (2013). https://doi.org/10.4236/ajps.2013.411259
García-Morote, F.A., López-Serrano, F.R., Martínez-García, E., Andrés-Abellán, M., Dadi, T., Candel, D., Rubio, E., Lucas-Borja, M.E.: Stem biomass production of Paulownia elongata × P. fortunei under low irrigation in a semi-arid environment. Forests 5, 2505–2520 (2014). https://doi.org/10.3390/f5102505
San José, M.C., Cernadas, M.J., Corredoira, E.: Histology of the regeneration of Paulownia tomentosa (Paulowniaceae) by organogénesis Histología de la regeneración por organogénesis en Paulownia tomentosa (Paulowniaceae). Rev. Biol. Trop. 62, 809–818 (2014). https://doi.org/https://doi.org/10.15517/rbt.v62i2.10845
Domínguez, E., Romaní, A., Domingues, L., Garrote, G.: Evaluation of strategies for second generation bioethanol production from fast growing biomass Paulownia within a biorefinery scheme. Appl. Energy. 187, 777–789 (2017). https://doi.org/10.1016/j.apenergy.2016.11.114
Milenković, I., Tomšovský, M., Karadžić, D., Veselinović, M.: Decline of Paulownia tomentosa caused by Trametes hirsuta in Serbia. For. Pathol. 48, (2018). https://doi.org/https://doi.org/10.1111/efp.12438
Schneiderová, K., Šmejkal, K.: Phytochemical profile of Paulownia tomentosa (Thunb). Steud. Phytochem. Rev. 14, 799–833 (2015). https://doi.org/10.1007/s11101-014-9376-y
Ryu, H.W., Park, Y.J., Lee, S.U., Lee, S., Yuk, H.J., Seo, K.-H., Kim, Y.-U., Hwang, B.Y., Oh, S.-R.: Potential anti-inflammatory effects of the fruits of Paulownia tomentosa. J. Nat. Prod. 80, 2659–2665 (2017). https://doi.org/10.1021/acs.jnatprod.7b00325
He, T., Vaidya, B., Perry, Z., Parajuli, P., Joshee, N.: Paulownia as a medicinal tree: traditional uses and current advances. Eur J. Med. Plants. 14, 1–15 (2016). https://doi.org/10.9734/ejmp/2016/25170
Yan, J., Joshee, N., Liu, S.: Utilization of hardwood in biorefinery: a kinetic interpretation of pilot-scale hot-water pretreatment of Paulownia elongata woodchips. J. Biobased Mater. Bioenergy. 10, 339–348 (2016). https://doi.org/10.1166/jbmb.2016.1609
Díaz Reinoso, B., González Muñoz, M.J., Domínguez González, H.: Introduction. In: Domínguez, H. and González-Muñoz, M. (eds.) Water Extraction of Bioactive Compounds: From Plants to Drug Development. pp. 1–50. Elsevier (2017)
Pinto, D., Vieira, E.F., Peixoto, A.F., Freire, C., Freitas, V., Costa, P., Delerue-Matos, C., Rodrigues, F.: Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. (2021). https://doi.org/https://doi.org/10.1016/j.foodchem.2020.127521
Wiboonsirikul, J., Adachi, S.: Extraction of functional substances from agricultural products or by-products by subcritical water treatment. Food Sci. Technol. Res. 14, 319–328 (2008). https://doi.org/10.3136/fstr.14.319
Santos, T.M., Alonso, M.V., Oliet, M., Domínguez, J.C., Rigual, V., Rodriguez, F.: Effect of autohydrolysis on Pinus radiata wood for hemicellulose extraction. Carbohydr. Polym. 194, 285–293 (2018). https://doi.org/10.1016/j.carbpol.2018.04.010
Cruz, J.M., Conde, E., Domínguez, H., Parajó, J.C.: Thermal stability of antioxidants obtained from wood and industrial wastes. Food Chem. 100, 1059–1064 (2007). https://doi.org/10.1016/j.foodchem.2005.11.012
González, J., Cruz, J.M., Domínguez, H., Parajó, J.C.: Production of antioxidants from Eucalyptus globulus wood by solvent extraction of hemicellulose hydrolysates. Food Chem. 84, 243–251 (2004). https://doi.org/10.1016/S0308-8146(03)00208-5
Cruz, J.M., Domínguez, H., Parajó, J.C.: Anti-oxidant activity of isolates from acid hydrolysates of Eucalyptus globulus wood. Food Chem. 90, 503–511 (2005). https://doi.org/10.1016/j.foodchem.2004.05.018
Cruz, J.M., Domínguez, H., Parajó, J.C.: Assessment of the production of antioxidants from winemaking waste solids. J. Agric. Food Chem. 52, 5612–5620 (2004)
González-Muñoz, M.J., Conde, E., Domínguez, H., Torres, M.D.: Recovery of phytochemical compounds from natural and blanched green broccoli using non-isothermal autohydrolysis. Int. J. Food Sci. Technol. 54, 1276–1282 (2019). https://doi.org/10.1111/ijfs.14066
Conde, E., Moure, A., Domínguez, H., Parajó, J.C.: Production of antioxidants by non-isothermal autohydrolysis of lignocellulosic wastes. LWT - Food Sci. Technol. 44, 436–442 (2011). https://doi.org/10.1016/j.lwt.2010.08.006
González-López, N., Moure, A., Domínguez, H.: Hydrothermal fractionation of Sargassum muticum biomass. J. Appl. Phycol. 24, 1569–1578 (2012). https://doi.org/10.1007/s10811-012-9817-1
Parada, M., Rodríguez-Blanco, A., de Ana, F., Magán, F., Domínguez, H.: Sequential extraction of Hericium erinaceus using green solvents. LWT Food Sci. Technol. 64, 397–404 (2015). https://doi.org/10.1016/j.lwt.2015.06.008
Rodríguez-Seoane, P., González-Muñoz, M.J., Falqué, E., Domínguez, H.: Pressurized hot water extraction of β-glucans from Cantharellus tubaeformis. Electrophoresis. 39, 1892–1898 (2018). https://doi.org/10.1002/elps.201700399
Herrero, M., Temirzoda, T.N., Segura-Carretero, A., Quirantes, R., Plaza, M., Ibañez, E.: New possibilities for the valorization of olive oil by-products. J. Chromatogr. A. 1218, 7511–7520 (2011). https://doi.org/10.1016/j.chroma.2011.04.053
Cvetanović, A., Zeković, Z., Švarc-Gajić, J., Razić, S., Damjanović, A., Zengin, G., Delerue-Matos, C., Moreira, M.: A new source for developing multi-functional products: biological and chemical perspectives on subcritical water extracts of Sambucus ebulus L. J. Chem. Technol. Biotechnol. 93, 1097–1104 (2018). https://doi.org/10.1002/jctb.5468
Pagano, I., Piccinelli, A.L., Celano, R., Campone, L., Gazzerro, P., Russo, M., Rastrelli, L.: Pressurized hot water extraction of bioactive compounds from artichoke by-products. Electrophoresis. 39, 1899–1907 (2018). https://doi.org/10.1002/elps.201800063
Zeković, Z., Cvetanović, A., Švarc-Gajić, J., Gorjanović, S., Sužnjević, D., Mašković, P., Savić, S., Radojković, M., Đurović, S.: Chemical and biological screening of stinging nettle leaves extracts obtained by modern extraction techniques. Ind. Crops Prod. 108, 423–430 (2017). https://doi.org/10.1016/j.indcrop.2017.06.055
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965)
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C.: Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237 (1999). https://doi.org/10.1016/S0891-5849(98)00315-3
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). https://doi.org/10.1016/0003-2697(76)90527-3
Del Valle, J.M., Rogalinski, T., Zetzl, C., Brunner, G.: Extraction of boldo (Peumus boldus M.) leaves with supercritical CO2 and hot pressurized water. Food Res. Int. 38, 203–213 (2005). https://doi.org/10.1016/j.foodres.2004.09.010
Giombelli, C., Iwassa, I.J., da Silva, C., Bolanho Barros, B.C.: Valorization of peach palm by-product through subcritical water extraction of soluble sugars and phenolic compounds. J. Supercrit. Fluids. 165, 104985 (2020). https://doi.org/10.1016/j.supflu.2020.104985
Ko, M.J., Nam, H.H., Chung, M.S.: Subcritical water extraction of bioactive compounds from Orostachys japonicus A Berger (Crassulaceae). Sci Rep 10, 10890 (2020). https://doi.org/10.1038/s41598-020-67508-2
Kim, D.S., Lim, S.B.: Kinetic study of subcritical water extraction of flavonoids from Citrus unshiu peel. Sep Purif Technol (2020). https://doi.org/10.1016/j.seppur.2020.117259
Tsubaki, S., Iida, H., Sakamoto, M., Azuma, J.-I.: Microwave heating of tea residue yields polysaccharides, polyphenols, and plant biopolyester. J. Agric. Food Chem. 56, 11293–11299 (2008). https://doi.org/10.1021/jf802253s
Zohourian, T.H., Quitain, A.T., Sasaki, M., Goto, M.: Extraction of bioactive compounds from leaves of Lawsonia inermis by green pressurized fluids. Sep. Sci. Technol. 47, 1006–1013 (2012). https://doi.org/10.1080/01496395.2011.641056
Luo, F., Yang, D., Chen, Z., Megharaj, M., Naidu, R.: Characterization of bimetallic Fe/Pd nanoparticles by grape leaf aqueous extract and identification of active biomolecules involved in the synthesis. Sci. Total Environ. 562, 526–532 (2016). https://doi.org/10.1016/j.scitotenv.2016.04.060
Sung, W.-C.: Volatile constituents detected in smoke condensates from the combination of the smoking ingredients sucrose, black tea leaves, and bread flour. J. Food Drug Anal. 21, 292–300 (2013). https://doi.org/10.1016/j.jfda.2013.07.005
Zhong, J., Wang, J., Gong, M., Lin, T., Liu, Z., Zhang, X., Chen, Y.: Kinetic and degradation mechanism study on Sr2CeO4-promoted photo-oxidation of gaseous benzene. Sep. Purif. Technol. 57, 57–62 (2007). https://doi.org/10.1016/j.seppur.2007.01.021
Acknowledgements
The authors thank the financing granted by the Ministry of Economy, Industry and Competitiveness of Spain through the project CTM2015-68503-R. PRS thanks to the Ministry of Economy, Industry and Competitiveness of Spain, the PIF grant BES-2016-076840, Sheila Gómez for her technical assistance and Ángel Álvarez for his help in collecting samples. C. del Pozo expresses her gratitude to the Universitat Autònoma de Barcelona for funding her PhD contract through PIF Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodríguez-Seoane, P., del Pozo, C., Puy, N. et al. Hydrothermal Extraction of Valuable Components from Leaves and Petioles from Paulownia elongata x fortunei. Waste Biomass Valor 12, 4525–4535 (2021). https://doi.org/10.1007/s12649-020-01298-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01298-6




 ) leaves and (
) leaves and ( ) petioles against ABTS
) petioles against ABTS