Abstract
The search for cleaner production technologies and sustainable development tools has prompted increasing interest in the use of new, renewable raw materials for biorefineries and energy production. Thus, biodiesel production from the pulp oil (fruit oil) of Acrocomia totai Mart., a species of palm tree, is currently being investigated. However, the bioenergy potential of biomass from different parts of this species is still unknown, and may not be limited to oil from the pulp. Therefore, the aim of this work was to evaluate the leaves of A. totai as a secondary source of lipids and energy. Deciduous and fresh leaves were submitted to the Soxhlet and Bligh & Dyer extractions, and the presence of 1.0 to 5.8% of unsaturated fatty acids was observed by GC-FID analyses. The crude extract (CE) of the leaves was obtained by exhaustive extraction with methanol and then partitioned with different solvents. CE and its fractions were characterized by 1H nuclear magnetic resonance (NMR) spectroscopy, and the thermal properties were determined. The average specific heat of the grease fractions was 2.0 J g−1 K−1 and the calorific value of the polar and non-polar fractions varied from 15.7 to 34.1 MJ kg−1. For the whole leaves, the calorific value of the deciduous leaves was higher than that of the fresh leaves due to their lower water content, as determined by thermogravimetric-differential thermal analyses (TG-DTA) and infrared (IR) spectroscopy. The residual pies from the Soxhlet extractions of the deciduous and fresh leaves were characterized by TGA-DTA and scanning electron microscopy (SEM), revealing a lignocellulosic fibrous material with calorific values of 14.2 MJ kg−1 and 9.3 MJ kg−1, respectively, and an average specific heat of 1.6 J g−1 K−1. These thermal properties are comparable to those of sugarcane bagasse, a widely used type of biomass for industrial large-scale bioenergy production in Brazil. Therefore, these results indicated that it may be possible to harvest the deciduous leaves, a residue from the “macaúba” cultivation, for energy cogeneration in biodiesel refineries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass is a renewable raw material, of biological origin or from non-fossil materials that can be used as an energy source [1, 2]. Nowadays, the global energy system depends heavily on fossil fuels, and in 2016, petrol derivatives contributed to 81% of the total primary energy supply of the world. Even though the supply of renewables has been increasing over time, only a 1% increase has been observed since 2000. Among renewable energy sources, biomass is the majority, with a 13% share in the global energy mix, followed by hydropower at 3%, and all other renewable sources (solar, wind, geothermal, tidal, and others) contribute to only 2%. Africa is the continent with the highest rate of use of energy from renewable sources in its global energy matrix, with 48% of the total. It is followed by America (12.7 %), then Asia (11.8 %), Oceania (11.5 %), and Europe (10.5 %) [3].
Several agricultural crops and woods from reforestation are important sources of biomass for energy purposes. In terms of crop production in 2016, sugarcane, corn, wheat, rice, and soybean accounted for the most significative ones, with a world average production of 1891, 1060, 749, 741, and 335 million tons, respectively [3]. For fuel production purposes, sugars and oils from sugarcane, corn, and oleaginous crops can be industrialized to produce liquid bioethanol and biodiesel. Biofuels can also be produced using by-products or residues like plant straws, husks, stalk, and bagasses, using chemical and biotechnological processes based mainly on cellulose degradation and the production of fermentable sugars [4,5,6].
In Brazil, hydropower accounts for most of the electricity produced in the country, while biomass power plants, which are mainly fueled by sugarcane bagasse, accounted for a sizeable 9.2% of overall capacity, even though different sources of biomasses are available in large amounts throughout the country. On the other hand, ethanol is a remarkable example of success in the sector of biofuels for use in internal combustion engines (mainly automobiles). It is mainly produced using the process of fermentation in sugars from the sugarcane juice bagasse at competitive prices [4]. In the USA, ethanol is mainly produced by fermentation processes in sugars obtained from corn. In both countries, the production of biodiesel using soybean oil has also grown, especially during periods of high international oil prices [5].
One of the major drawbacks of the use of crops for energy production is the competition with the production of food for humans and livestock. Recently, the use of corn and soybeans in the production of biofuels has boosted the prices of these commodities, causing inflation in the markets of human food products, animal feed, beef, pork, and poultry meat [7]. Therefore, it is of the utmost importance that we make an effort to find new sources of biomass for energy production that will not interfere with the food market, avoiding the risk of leading many regions to dangerous food security levels, especially in developing countries [8]. To address this need, the use of agricultural non-food raw materials has gained special attention. Sugarcane bagasse is one of the most successful cases of materials for cogeneration of energy. While the juice of the plant is used for sugar and ethanol production, the residual bagasse can be used in thermoelectric power generation [4]. This approach has been adopted by several Brazilian mills at a large scale, with interesting economic and environmental gains [4, 9, 10]. Moreover, the lignocellulosic material can be used in the production of more sugars and ethanol, using enzymatic or chemical degradation of cellulose [7]. Based on this success, other lignocellulosic biomasses are gaining attention as possible sources of energy that do not pose a threat or competition to the traditional food chain raw materials [7, 8].
“Macaúba” (macaw palm) is the popular name of the palm trees belonging to the genus Acrocomia, native to South and Central America. It has been widely researched in recent years as a source of vegetable oil, which is extracted from its pulp and almonds [11, 12]. The oil can be used in biodiesel production or in various cosmetic applications. Much of the macaúba palm farming is carried out in small farms or on native trees [13, 14]. Commercial large-scale cultivation is a fast-approaching reality, given the large number of studies aimed at adapting and domesticating the species for this purpose [15, 16]. However, the possible use of other non-fruiting parts of this palm, such as the leaves, for energy purposes has not been reported in the literature.
With the growing need to diversify the energy matrix and create new business and household income possibilities, macaúba leaves show the right properties for biomass production, because they grow in large quantities and fall from the trunk of the tree, forming massive structures of biomass, known as “deciduous”. Thus, in addition to harvesting fruits for large-scale oil production, falling leaves could be harvested to provide a cheap raw material for energy cogeneration [12, 14].
Due to the need for maintaining the sustainability of natural resources for the supply of raw materials, food production, livestock feed, biofuel synthesis, and textiles, new sources of renewable products should be inserted in the global market taking into account environmental awareness. In this sense, the present work aimed to study the potential of Acrocomia totai Mart. leaves, both fresh and deciduous, as a source of oleaginous material and biomass for energy production. It is noteworthy to note that no report on the energetic potential of the A. totai leaves was found in the literature. The oleaginous material was extracted with different techniques, characterized, and the resulting cake had its thermal and combustion properties determined.
Material and Methods
Leaves Harvesting and Pre-treatment
The macaúba palm tree (Acrocomia totai Mart.) originated in the municipality of Moreira Sales, Paraná, Brazil (longitude S 24° 3' 23, 64"; latitude W 53° 3' 26, 32"). An exsicata was authenticated by Dr. N. T. V. Junqueira and deposited in the herbarium of the State University of Maringá (No. 29683). Deciduous leaves (DL) were collected after the natural fall from the palm trunk (forage), and fresh leaves (FL) were collected directly from the plant at a relative humidity of 68%, with clear and fully sunny skies in April (Autumn) at 23 °C. After harvesting, the FL were dried at room temperature (25 °C) until constant weight, and the DL water content was determined according to the standard gravimetric method [17] on a dry basis (d.b.) at 105 °C for 24 h.
After dried, the leaves were ground in a knife mill (CienLab), and samples with mesh size 28 (0.59 mm) were obtained and submitted to the Bligh & Dyer (BD) extraction, Soxhlet extraction (SE), and methanol exhaustive extraction (ME).
Soxhlet Extraction
The oil extractions from deciduous leaves were performed in replicates (n = 3) employing the Soxhlet technique [18]. A total of 10 g of DL was placed in a cellulose cartridge for extraction with n-hexane PA (300 mL) with its boiling point 68 °C as the extraction temperature. The extraction operating times were 4, 8, 16, and 24 h, and the sample was exhausted within 48 h of extraction. After the extraction, the solvent was recovered from the oil with a Fisatom 802 rotary evaporator. Quantification of the oil was performed by gas chromatography (GC) as described below.
Bligh & Dyer Extraction
BD extraction from DL and FL was performed in replicates (n = 3) with an adapted Bligh & Dyer [19] methodology. In an Erlenmeyer flask, 15 g of sample were weighed along with 30 mL of chloroform and 60 mL of methanol. The flask was capped and shaken for 5 min. Then, 30 mL of chloroform was added, followed by a further 2 min of intense stirring. In sequence, 30 mL of distilled water was added, and the resulting mixture was stirred again for 5 min. Finally, the sample was filtered on filter paper, washed with 50 mL of chloroform. The liquid was then transferred to a separatory funnel and allowed to settle for 24 h. Then, the chloroform fraction was separated from the aqueous phase and allowed to evaporate at room temperature.
Exhaustive Extraction with Methanol
To obtain the crude extract (CE), 1.22 kg of ground A. totai leaves were subjected to exhaustive extraction at room temperature (25 °C) using distilled methanol, according to a modified protocol [17]. Then, the methanolic mixture was submitted to evaporation under reduced pressure at 600 mmHg and 45 °C, obtaining 167.4 g of CE. This procedure was repeated 6 times, using 4 L of methanol in each extraction.
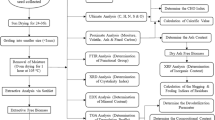
First, the CE (167.4 g) was washed with hexane, obtaining the hexanic fraction (MH, 26.0 g). In sequence, the remaining fraction was washed with chloroform and ethyl acetate, obtaining the respective chloroform (MC, 4.7 g) and ethyl acetate fractions (MA, 5.3 g). Finally, the crude extract was washed with methanol, obtaining the methanolic fraction (MM, 81.7 g). The remaining solid (RF, 19.6 g) was called the remaining fraction, as shown in Fig. 1.
Chromatographic Analysis
The fatty acid (FA) composition analysis was performed in a Varian CP-3800 gas chromatograph (GC) coupled with a flame ionization detector (FID) and a specific capillary column for separating esters (BP-X70-SGE; 30 m × 0.25 mm). Helium was used as the carrier gas on a 1:10 split ratio. The analysis was performed with a temperature-programmed column: the initial temperature was 110 °C and the temperature was subsequently ramped up to 160 °C at 8 °C min−1 and then to 230 °C at 3.5 °C min−1. The temperatures of the detector and injector were kept constant at 220 and 260 °C, respectively. The components of the analyzed sample were identified by comparing the retention times in the capillary column with the retention times of a standard sample (fatty acid methyl ester mix). The chosen internal standard (IS) was 23:0 methyl tricosanoate (Sigma–Aldrich) [12].
Nuclear Magnetic Resonance
1H-NMR spectra were obtained on a Bruker AVANCE III HD model equipment, operating at 500.13 MHz, a field of 11 Tesla with a 5-mm probe and direct field gradient detection. For the analysis, 20 mg of samples were dissolved in 600 μL of deuterated solvent (CDCl3, CD3OD, D2O) (Cambridge Isotope Laboratories, Inc) with tetramethylsilane (TMS) as the internal reference [20].
Infrared Spectrum Analysis
The spectra were obtained by the technique in the medium infrared range between 450 and 4000 cm−1 with a resolution of 1 cm−1 and 64 accumulations per sample. For the analysis, tablets of the samples and KBr were prepared by mixing 200 mg of KBr with 2 mg of sample (CE), previously dried in an oven at 105 °C. The sample and KBr were placed in a tablet maker and submitted to a 5-ton pressure, gradually increasing each 30 s until maximum pressure (5-ton), remaining for an additional 30 s. Then, the tablets were submitted to a Fourier transform infrared spectroscopy (FTIR), performed on a Perkin Elmer Frontier model equipment [21].
Scanning Electron Microscopy
Scanning electron microscope (SEM) images were obtained in a scanning electron microscope (FEI-Quanta 250) located in the Research Support Center Complex (COMCAP/UEM) at the State University of Maringá (UEM, Brazil). A priori analysis, the samples were dried at 100 °C for 24 h. The samples were placed on a stub with double-sided carbon tape on the surface and coated with a thin layer of gold before the analysis. The SEM images were obtained applying an acceleration voltage of 15 kV and a current intensity of 30 μA. [22].
Determination of Specific Heat Capacity (cp)
Specific heat capacity was determined for the samples of the deciduous leaves, fresh leaves, 4 h extraction pie, 48 h extraction pie, hexanic fraction, chloroform fraction, ethyl acetate fraction, methanolic fraction, and crude extract. Measurements were performed on a calorimeter based on the thermal relaxation method. This consists of analyzing the temperature variation in the sample as a function of time after the application of a heat pulse, where the sample is fixed (substrate). In this assembly, the sample was fixed to the thermal reservoir by copper wires, and a diode laser (Coherent, model 3-1050, 635 nm, power up to 10 mW) was used to generate a temperature difference between the system (substrate + sample) and the thermal reservoir. This temperature difference was measured using a differential thermocouple connected to a Keithley model 2182 nanovoltmeter. The temperature controller (Lakeshore, model 340) was responsible for reading the thermal reservoir and controlling its temperature. The experiment was monitored by a microcomputer through a GPIB (General-Purpose Interface Bus) interface. By adjusting the thermal relaxation curves, the specific heat capacity value of the sample was obtained [23].
Calorific Value
The calorific value for the samples of the deciduous leaves, fresh leaves, 4 h extraction pie, 48 h extraction pie, hexanic fraction, chloroform fraction, ethyl acetate fraction, methanolic fraction, and crude extract were analyzed according to the methodology of NBR 11956 [24]. A digital calorimetric pump was used (Parr 6200) located in COMCAP/UEM. For this analysis, ground samples and sieves were packed in steel capsules (approximately 5.00 × 10−4 kg) and were placed inside the calorimetric pump for combustion.
Thermogravimetric Analysis
The crude extract and fractions from exhaustive extraction, a residual cake from the shortest hexane extraction (4 h) and exhaustion (48 h), DL, and FL were characterized in thermogravimetric (TG) and thermogravimetric differential (DTG) terms (COMCAP/UEM). The experiments were carried out in a simultaneous Netzsch TGA-DSC equipment (STA 409 PG TA model), over a temperature range of 25 to 900 °C in air atmosphere. For each analysis, approximately, 15 mg of sample was deposited in alumina crucibles. The crucible sterilization was performed in an air atmosphere at 1500 °C in a muffle furnace [9].
Statistical Analysis
Results were expressed by means of values ± standard deviation of the determinations. A comparison of means was performed by the Tukey test analysis of variance (ANOVA) with 5% confidence level (p < 0.05).
Results and Discussion
Oleaginous Content Extraction
Table 1 shows the oil content extracted with the Soxhlet and the BD techniques. The oleaginous content was determined in the Soxhlet extraction of DL using n-hexane for 48 h, resulting in the value of 5.79 ± 1.29%. For the extraction in the time of 4 h, 1.04 ± 0.53% of oleaginous content was observed. To date, no content of grease material in the leaves of A. totai has been reported in the literature, but studies have shown that oil extracted from macaúba (Acrocomia aculeata) fruits with different solvents have high oil contents that varied between 23.4 and 30.0% [14, 25].
Extraction by the Bligh & Dyer technique is faster than the Soxhlet technique, and it resulted in similar extraction values for the grease material content, which are 5.47% and 1.07% for the DL and FL, respectively. The lowest yield by the Bligh & Dyer method with the macaúba FL can be explained by the presence of moisture, which impaired the extraction phenomenon [26]. In addition, a moisture content of 64.30% was observed for the FL and 11.39% for the DL, meaning that weighing an equal amount of DL and FL will increase the oil yield in the DL, as noted by the presented results.
Fatty Acid Composition
The fatty acid composition was obtained by gas chromatography (GC-FID). A predominance of polyunsaturated fatty acids was observed in the grease material obtained from the DL (92.62 ± 13.25 mgFatty Acid gExtract−1) and FL (73.38 ± 8.85 mgFatty Acid gExtract−1), extracted with the Bligh & Dyer technique as show in Table 2. In turn, the grease material extracted from DL in the Soxhlet technique showed a majority of saturated fatty acids (137.65 ± 4.99 mgFatty Acid gExtract−1) in its composition. This difference was attributed to the temperature and the extraction time, both higher in the Soxhlet technique relative to the BD technique. This impaired the stability of the pi bonds that are characteristic of the polyunsaturated compounds. The composition, mostly polyunsaturated fatty acids in the DL and FL, was similar to the oils extracted from the pulp and almonds of the macaúba fruits, in which Souza et al. [12] found high concentrations of unsaturated (63.5%) and polyunsaturated (14.8%) fatty acids, corresponding to 78.3% of the total unsaturated fatty acids in unripe almonds. For macaúba acid (24.9 mg KOH g−1) and neutral pulp oil (2.7 mg KOH g−1), Silva et al. [14] observed the presence of 55.8–68.4% for unsaturated acids and 18.8–10.7% for polyunsaturated acids, respectively, including oleic acid, linoleic acid, and linolenic acid [11, 13].
For the extraction performed with the FL using the BD technique, a predominance of linoleic acid (43.8 ± 1.93 mgFatty Acid gExtract−1) was observed, followed by palmitic acid (26.71 ± 1.09 mgFatty Acid gExtract−1), oleic acid (15.33 ± 1.2 mgFatty Acid gExtract−1), stearic acid (13.24 ± 0.64 mgFatty Acid gExtract−1), γ-linolenic acid (11.81 ± 0.95 mgFatty Acid gExtract−1), and linolenic acid (10.7 ± 5.34 mgFatty Acid gExtract−1), as show in Table 2. The grease from the DL obtained via Bligh & Dyer extraction presented the following quantities of fatty acids in descending order: palmitoleic acid (42.41 ± 1.8 mgFatty Acid gExtract−1), 11,14-eicosadienoic acid (29.7 ± 4.01 mgFatty Acid gExtract−1), oleic acid (29.27 ± 1.08 mgFatty Acid gExtract−1), gondoic acid (24.21 ± 2.44 gondoic acid), gamma-linolenic acid (23.53 ± 3.88 mgFatty Acid gExtract−1), and stearic acid (21.07 ± 0.88 mgFatty Acid gExtract−1). For the analysis of the grease material extracted in a Soxhlet, the majority composition (> 10 mg g−1) in decreasing order was stearic acid (76.44 ± 2.16 mgFatty Acid gExtract−1), oleic acid (67.63 ± 1.97 mgFatty Acid gExtract−1), palmitic acid (43.6 ± 1.99 mgFatty Acid gExtract−1), palmitoleic acid (22.88 ± 0.53 mgFatty Acid gExtract−1), gondoic acid (14.59 ± 0.22 mgFatty Acid gExtract−1), and gamma-linolenic acid (10.61 ± 0.9 mgFatty Acid gExtract−1), as show in Table 2. It is worth mentioning that 11,14-eicosadienoic acid was not detected in the macaúba FL extract. It is important to point out that the acid composition may vary when different solvent or extraction methods are employed [25]. Thus, Trentini et al. [25] found similar yields when using n-hexane and dichloromethane as solvents in the Soxhlet extraction from macaúba pulp oil, but extraction with compressed propane provided achieving ~ 86% of the yield obtained via the Soxhlet. However, a difference was observed in the fatty acid composition, i.e., 63.1 g in 100 g−1 of oil for hexane solvent, 56.5 g in 100 g−1 oil for dichloromethane, and an average for compressed propane ~ 60.0 g in 100 g−1 of oil in oleic acid content, when compared to the three extractions, respectively.
Scanning Electron Microscopy of the A. totai Leaves
The crushed leaves of A. totai were analyzed by SEM and the images are presented in Fig. 2. The surfaces of the fresh leaves’ (Fig. 2a) fibers are covered in impurities specific to the fibers, such as waxes and other types of fatty acids [27, 28], with significant presence of leaf parenchyma cells [29]. It can also be observed that the deciduous leaves (Fig. 2b) showed defined fibers and a compact structure in contrast to the FL, indicating a shrinkage of the DL which is a common phenomenon in fibrous plants [27, 30]. This shrinkage is related to the loss of unbound water molecules between the material’s fibers and is important for food analysis and nutritional values such as vitamins and minerals [30]. The SEM images of the residual DL extraction pies extracted for 4 h (Fig. 2c) and 48 h (Fig. 2d) revealed that the n-hexane extraction removed the exterior wax layer and degraded the parenchyma cells, thus exposing the compact fibrous structure [29].
As shown in Fig. 2, the SEM analysis showed that the solvent promoted the opening of the cells of the leaf parenchyma, facilitating the removal of the grease matter and corroborating the differences between the fatty materials obtained by the Soxhlet and Bligh & Dyer extractions.
Hydrogen NMR Analysis of CE and Fractions
Exhaustive extraction is a soft technique that aims to obtain high levels of grease extraction with significant released amounts of extractable material, and is considered a comprehensive method for screening the major secondary metabolites from the biomass without introducing significant chemical changes [31]. 1H analyses were performed on the crude extract and on the fractions obtained from FL, because it is a non-destructive technique that is fast, selective, and capable of simultaneously detecting a large number of constituents in the complex mixtures [32]. Table 3 presents the results of the NMR analysis for the crude extract and the fractions from the FL of A. totai. Several diagnostic signals in the mixtures from the FL of A. totai were observed in the 1H NMR spectrum. Among them, signs of terminal methyl group protons (–(CH2)n–CH3) were observed in the region between δH 0.77 and 0.99 [12]. Also, there were characteristics of pentacyclic triterpenes and steroids with unsaturation, which can be confirmed by the signals of double linkage (–CH=CH–) observed in the region between δH 5.12 and 5.37 [33]. Evidence of long chains (–(CH2)n–) was observed in the region between δH 1.61 and 1.68, as a characteristic of mixtures of fatty compounds, such as triacylglycerols found in vegetable oils [33]. Signs of heteroatoms were also observed in the region between δH 3.25 and 3.89, assigned to OCH2 and OCH ester groups, and CHOH and CH2OH groups from the glycerol part of glyceroglycolipids and phospholipids [20], and were also observed in the region between δH 1.99 and 2.05, which is a characteristic of allylic hydrogens (–CH2–CH=CH–)[34]. This result was coherent with the previous study with A. totai leaves, in which the phytochemical composition of the crude extract was described, leading to the identification of triterpenes, phytosteroids, and fatty acids [33].
At high field, 1H NMR analyses of the methanol fraction and crude extracts showed signs of terminal methyl groups (–(CH2)n–CH3) in the region between δH 1.05 and 1.30. In the middle field, signs of anomeric hydrogens of sugar units were observed in the region between δH 3.12 and 4.48 [32]. In the low field region from δH 6.51 to 7.50, there were mainly signs of aromatic and olefinic hydrogens of flavonoids. Signs of stilbenoids can be seen in the region between 5.12 and 5.41 [32, 35].
Fourier Transform Infrared Analysis of the Residual Soxhlet Extraction Pies
The infrared spectra obtained through the analysis of the FTIR from the residual Soxhlet extraction pies of A. totai DL showed the presence of compounds with common functional groups, as shown in Fig. 3. A broadband was observed in the region 3700-3400 cm−1, which suggests an O–H stretch that is a characteristic of natural fibers [36]. For the macaúba fibers evaluated in this study, the band was observed at 3451 cm−1, and this band was attributed to the substituent groups of phenols, alcohols, and carboxylic acids. The spectra also indicated the presence of bands in the region between 2918 and 2848 cm−1 that were related to the stretches of C–H carbon sp3 bonds [37]. In the region of 1737 cm−1, a carbonyl (C=O)-free band was observed, and this band’s intensity can be attributed to the intermolecular interactions between the carboxylic groups of hemicellulose and the lignin structure [38]. In the region of 1716 cm−1, there is an ester carbonyl stretch (C=O) [36, 38]. A C–H absorption band was also observed at 1377 cm−1 (–C–CH3). The presence of a band at 1635 cm−1 indicated the presence of β-glycosidic bonds between the sugar units at 1160 cm−1. Furthermore, the infrared spectra showed results similar to those previously observed for macaúba palm cake (A. aculeata) [39].
Some signals may be linked to hemicellulose, cellulose, and lignin, like the presence of the band at 1416 cm−1, which is a characteristic of symmetrical deformation in methylene groups (CH2) and angular deformation in –(CH2)– at 720 cm−1 [40]. The broadband at 1033 cm–1 was associated with the stretch of the C–O bond that is also a characteristic of cellulose [36]. The signal in the region of 1480 cm−1 signaled the axial deformation of C–H and the bands between 1040 and 1160 cm−1 C–O are indicative of alcohols and phenols [38, 39].
Specific Heat Capacity (cp)
The results obtained for the specific heat analyses are presented in Table 4. It is possible to note that the specific heat obtained for the DL and FL was equal to 1.6 J g-1 K−1 for both samples, but a slightly lower value was observed for the 4-h extraction pie (1.3 J g−1 K−1), and a higher value was observed for the pie of 48 h (1.8 J g−1 K−1).
The specific heat of the hexane, chloroform, and ethyl acetate fractions of the extracts of the leaves of A. totai showed slightly different averages due to the difference in polarity of the lipids extracted in each phase, as observed in the NMR analysis of 1H and GC-FID, and the mean value observed in each fraction was 2.2 J g−1 K−1. Thus, the values obtained ranged from 2.2 J g−1 K−1 in the non-polar fraction (hexane) to 1.9 J g−1 K−1 in the polar fraction (ethyl acetate). This demonstrates a decrease in heat capacity due to the removal of extracted substances with the solvent employed. Compared with commercial oils, the specific heat of chestnut oil, canola, peanut, and sunflower are 2.35, 2.20, 2.04, and 2.07 J g−1 K−1, respectively [41].
The specific heat observed in the crude extract and the methanol fraction was 2.1 ± 0.1 and 2.3 ± 0.1 J g−1 K−1, respectively. The observed value for the polar fraction (methanol fraction) was higher than those of the non-polar fractions studied (hexane, chloroform, and ethyl acetate). Since methanol has the highest dielectric constant among the evaluated solvents, its ability to reduce the power of the electric field on the solute (EB) provides better solvation, thus promoting more effective extraction of metabolites, especially carbohydrates, from the vegetable’s tissues [42].
Calorific Values
Table 4 shows the calorific values in kilojoule/kilogram for the analyzed samples. The values we found showed a lower calorific value for FL (9349.80 kJ kg−1) when compared to DL (14,215.34 kJ kg−1). The presence of 64.30% moisture in the FL justifies the lower calorific value observed when compared to the DL, which presented 11.39% moisture. Results reported in the literature on the analysis of the calorific values of dry sugarcane leaves by Kumar et al. [43] demonstrate a value of 18,080 kJ kg−1, while a value of 14,215.34 kJ kg−1 was observed in the DL. When compared to other biomasses that are consolidated in the market, such as rice (15,320 kJ kg−1), wheat straw (19,120 kJ kg−1) [44], and sugarcane bagasse (16,100 kJ kg−1) [34], the calorific value of the macaúba leaves is similar to the amount of energy released in the burning of these raw materials. Thus, the calorific value indicates the potential of the biomass for bioenergy production as cogeneration and is similar to the values reported for the endocarp and kernel cake residues from the macaúba fruit [10].
The calorific value of the DL was also determined after performing the extractions with n-hexane. The calorific values of the residual pies from 4 h and 48 h extraction were observed to be 17,213.61 kJ kg−1 and 17,092.62 kJ kg−1, respectively. This difference was attributed to the difference in the amount of grease material extracted, which was 3.62 ± 0.53% for 4 h extraction in the Soxhlet, and 19.98 ± 0.47% after exhaustion at 48 h. Therefore, there was greater removal of fatty material in exhaustion and the calorific value of DL after extraction was reduced when compared to the residual pie at 4 h. Evartisto et al. [45] produced charcoal from the carbonization of the A. aculeata fruit and reported 31,250.27 kJ kg−1 for the epicarp and 32,904.06 kJ kg−1 for the endocarp. Evaristo et al. [10] studied the potential of macaúba palm residues such as husk, pulp cake, endocarp, and kernel cake obtained after the macaúba fruit processing, and reported that it has great potential as feedstock and also in solid biofuel production. Thus, it seems that several parts of the macaúba tree can be employed in processes of energy cogeneration.
The calorific values were determined as shown in Table 4. The n-hexane fraction, chloroform fraction, and ethyl acetate fraction were extracted from the crude extract in a polarity gradient solvent, since different solvents were able to differentiate extract composition (as previously discussed) with distinguished calorific values [33]. The results showed that the n-hexane (34,059.56 kJ kg−1), chloroform (32,609.20 kJ kg−1), and ethyl acetate (29,585.10 kJ kg−1) fractions presented the highest-to-lowest calorific values. As observed by 1H NMR analyses, these fractions are rich in low-polarity compounds, mainly fatty acids, that contribute to a higher calorific value. Considering that the polar fraction (methanol) may contain water and other high-polarity compounds, a lower calorific value was expected, as Yan et al. [46] reported, and the result of 15,733.68 kJ kg−1 for the calorific value of the methanolic fraction agrees with the literature. The crude extract is composed of a mixture of compounds with high molecular mass, which reduced the value of the calorific power (5316.39 kJ kg−1) when compared with the fractions extracted with solvents [1, 47].
Thermal Analysis: Thermogravimetry/Derived Thermogravimetry (TG/DTG), Differential Thermal Analysis (DTA)
Figure 4 presents the TG and differential thermal analysis (DTA) thermograms for the leaves of A. totai, fractions, residual pies, and crude extract. It was observed in the thermograms of DL, FL, and pies from the 4-h and 48-h Soxhlet extractions (Fig. 4a) that there was a similar behavior during thermal decomposition, that is, the loss of mass in three stages. In the range of approximately 25 to 150 °C, there was a 10% reduction in the mass attributed to water loss [48], concordant with the DTA curve (Fig. 4b), which presents an endothermic peak at a maximum temperature of 150.5 °C. In the second event, the reduction of mass in the temperature range between 150 and 530 °C was related to the decomposition of the organic matter in the biomass that is present in all of the analyzed samples. In this period, the loss of mass was approximately 60%, corresponding to the thermal degradation of hemicellulose, cellulose, and lignin, that is, the compounds that were observed in the infrared analysis. The thermal degradation of hemicellulose and cellulose into volatile compounds is related to a peak at 300 °C, and lignin decomposes at a temperature of 450 °C [34]. For the thermographic curves of the macaúba fruit residues, Evaristo et al. [10] observed that the residues with less lignin concentrations, such as pulp cake (29.2%) and husk residues (27.5%), presented steeper weight losses than the endocarp (31.6%) and the kernel cake (37.2%), between 260 and 300 °C. The authors also observed that, above 310 °C, all residues show the greatest reduction in mass.
In the third event, in the temperature range between 530 and 900 °C, a residual mass loss of 5.2 to 12.1% was observed, in which, the complete degradation of lignin is thought to occur at temperatures up to 900 °C [49]. The DTA curve (Fig. 4b) revealed endothermal and exothermic peaks corresponding to the decomposition recorded in the TG curve, which was also evidenced by the high specific heat, as shown previously in Table 5.
For the samples obtained from the exhaustive and fractioned extraction from the crude extract (hexane fraction, chloroform fraction, ethyl acetate fraction, and methanol fraction), a similar behavior was observed in the thermograms (Fig. 4c) of the MH, MC, and MA fractions. During thermal decomposition, three events were present in the decomposition process. The first mass reduction event, in the temperature range between 25 and 150 °C, was attributed to the loss of water by desorption [48, 49], and a decrease of approximately 2% in mass for the MH and MC samples and 5% for the MA sample was observed. In the second event, in the temperature range between 150 and 470 °C, the mass loss was approximately 85%. This was found to be the degradation of the organic matter present in the biomass, such as hydrocarbons, esters, aromatics, ketones, carboxylic acids, aldehydes, and alcohols [49]. A third event in the temperature range between 470 and 900 °C was observed. This corresponded to a mass loss of approximately 4%, as shown in Table 5.
The crude extract (Fig. 4c) presented a small mass reduction of 38%, and in the first event, in the temperature range between 25 and 120 °C, there was a higher decrease of 18.3%, which suggests the presence of water and volatile compounds [49]. In the second event, in the temperature range between 120 and 470 °C, one can observe only an 11.4% loss of mass. This decomposition can be associated with a wide variety of secondary metabolites, with low molecular mass [37]. In addition, it can be related to the same compounds that were lost in the second event of the hexane, chloroform, and acetate fractions, because all of the compounds present in these fractions were originally in the crude extract. The analysis of Fig. 4 combined with Table 5 allowed us to infer that the use of solvents n-hexane, ethyl acetate, and chloroform promoted the extraction of low-polarity compounds, mainly fatty acids, as inferred by the NMR and infrared analysis. Compounds such as low molecular mass greases have high-energy potential and combustibility. This explains the high rate of mass loss with heating when compared to the crude extract or methanol fractions, which have a greater amount of cellulosic and lignin material [10, 37, 49].
Conclusion
Renewable sources of energy are essential in the coming years due to their sustainability. Therefore, this work shows that the A. totai leaves can be an interesting source of lipids and energy, as determined by their compositional and thermodynamic properties. It was observed that the macaúba leaves have a calorific potential similar to that of the sugarcane bagasse and other biomasses, which may be explained by the large presence of fatty acids, lipid material, and low water content. Thus, the cultivation of the macaúba trees to harvest their fruits for biodiesel production, which is already a common practice in many biofuel companies, may be intercropped with the collection of deciduous leaves for use in processes of energy cogeneration, with decreasing consumption of traditional fuels, less CO2 emissions, and cost savings without any big modifications in the refinery layout or agricultural practices in farms.
References
Hameed S, Sharma A, Pareek V, Wu H, Yu Y (2019) A review on biomass pyrolysis models: Kinetic, network and mechanistic models. Biomass Bioenergy 123:104–122. https://doi.org/10.1016/j.biombioe.2019.02.008
Nunes LJR, Causer TP, Ciolkosz D (2020) Biomass for energy: a review on supply chain management models. Renew Sust Energ Rev 120:109658. https://doi.org/10.1016/j.rser.2019.109658
World Bioenergy Association - The global voice of bioenergy. Global Bioenergy Statistics 2018. Avaliable in <https://bit.ly/2yyykNF>. Access: 18 April 2020
Griebenow C, Ohara A (2019) Agora Energiewende & Instituto E+ Diálogos Energéticos: Report on the Brazilian Power System. 155/01-CP-2019/EM. Avaliable in: <https://www.agora-energiewende.de/fileadmin2/Projekte/2019/Brazil_Country_Profile/155_CountryProf_Brazil_EN_WEB.pdf> Access: 09 Oct 2020
Kumar D, Singh V (2019) Bioethanol production from corn, corn (Third Edition) Chapter 22, Chem Technol 615-631 https://doi.org/10.1016/B978-0-12-811971-6.00022-X
Muscat A, Olde EM, Boer IJM, Ripoll-Bosch R (2020) The battle for biomass: a systematic review of food-feed-fuel competition. Glob Food Sec 25:100330. https://doi.org/10.1016/j.gfs.2019.100330
Koradiya M, Duggirala S, Tipre D, Dave S (2016) Pretreatment optimization of Sorghum pioneer biomass for bioethanol production and its scale-up. Bioresour Technol 199:142–147. https://doi.org/10.1016/j.biortech.2015.08.156
Zhang Z, O’Hara IM, Mundree S, Gao B, Ball AS, Zhu N, Bai Z, Jin B (2016) Biofuels from food processing wastes. Curr Opin Biotechnol 38:97–105. https://doi.org/10.1016/j.copbio.2016.01.010
Alonso DM, Hakim SH, Zhou S, Won W, Hosseinaei O, Tao J, Garcia-Negron V, Motagamwala AH, Mellmer MA, Huang K, Houtman CJ, Labbé N, Harper DP, Maravelias CT, Runge T, Dumesic JA (2017) Increasing the revenue from lignocellulosic biomass: maximizing feedstock utilization. Sci Adv 3:e1603301. https://doi.org/10.1126/sciadv.1603301
Evaristo AB, Grossi JAS, Carneiro ACO, Pimentel LD, Motoike SY, Kuki KN (2016) Actual and putative potentials of macauba palm as feedstock for solid biofuel production from residues. Biomass Bioenergy 85:18e24–18e24. https://doi.org/10.1016/j.biombioe.2015.11.024
Iha OK, Alves FCSC, Suarez PAZ, Oliveira MBF, Menegheti SMP, Santos BPT, Soletti JI (2014) Physicochemical properties of Syagrus coronate and Acrocomia aculeate oils for biofuel production. Ind Crop Prod 62:318–322. https://doi.org/10.1016/j.indcrop.2014.09.003
Souza GK, Diório A, Johann G, Gomes MCS, Pomini AM, Arroyo PA, Pereira NC (2019) Assessment of the physicochemical properties and oxidative stability of kernel fruit oil from the Acrocomia totai palm tree. J Am Oil Chem Soc 96:51–61. https://doi.org/10.1002/aocs.12175
Poetsch J, Lewandowski DHI, Oberländer D, Hilger T (2012) Acrocomia aculeate - a sustainable oil crop. Rural 21:41–44. Available in: https://energypedia.info/images/6/6e/EN_Acrocomia_aculeata_%E2%80%93_a_sustainable_oil_crop_RURAL21_2012-3.pdf. Access: 9 Jun, 2020
Silva LN, Cardoso CC, Pasa VMS (2016) Production of cold-flow quality biodiesel from high-acidity on-edible oils - esterification and transesterification of macauba (Acrocomia aculeata) oil using various alcohols. Bioenergy Res 9:864–873. https://doi.org/10.1007/s12155-016-9740-4
Granja MMC, Motoike SY, Andrade APS, Correa TT, Picoli EAT, Kuki KN (2018) Explant origin and culture media factors drive the somatic embryogenesis response in Acrocomia aculeata (Jacq.) Lodd. ex Mart., an emerging oil crop in the tropics. Ind Crop Prod 117:1–12. https://doi.org/10.1016/j.indcrop.2018.02.074
Fernández-Coppel IA, Evaristo AB, Guimarães AC, Martín-Gild J, Navas-Gracia LM, Martín-Ramos P (2018) Life cycle analysis of macauba palm cultivation: a promising crop for biofuel production. Ind Crop Prod 125:556–566. https://doi.org/10.1016/j.indcrop.2018.09.036
AOAC, Official methods of analysis of the Association of Official Analytical Chemists International. 18th ed., Gaithersburg, 2005.
Soxhlet F (1879) Weight analysis of milk fat. Dinglers Polytechnisches J 232:461–465
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Sarpal AS, Teixeira CMLL, Silva PRM, Lima GM, Silva SR, Monteiro TV, Cunha VS, Daroda RJ (2015) Determination of lipid content of oleaginous microalgal biomass by NMR spectroscopic and GC–MS techniques. Anal Bioanal Chem 407:3799–3816. https://doi.org/10.1007/s00216-015-8613-6
del Río JC, Evaristo AB, Marques G, Martín-Ramos P, Martín-Gil J, Gutiérrez A (2016) Chemical composition and thermal behavior of the pulp and kernel oils from macauba palm (Acrocomia aculeata) fruit. Ind Crop Prod 84:294–304. https://doi.org/10.1016/j.indcrop.2016.02.018
Lima HHC, Kupfer VL, Moises MP, Guilherme MR, Rinaldi JC, Felisbino SL, Rubira AF, Rinaldi AW (2018) Bionanocomposites based on mesoporous silica and alginate for enhanced drug delivery. Carbohydr Polym 196:126–134. https://doi.org/10.1016/j.carbpol.2018.04.107
Medina AN, Caldeira AMF, Bento AC, Baesso ML, Sampaio JA, Catunda T, Gandra FG (2002) Thermal relaxation method to determine the specific heat of optical glasses. J Non-Cryst Solids 304:299–305. https://doi.org/10.1016/S0022-3093(02)01038-4
ABNT, Brazilian Association of Technical Standards (Associação Brasileira de Normas Técnicas), Standard test methods for gross calorific value of coal and coke - method of test. ABNT, NBR 11956:1990. https://www.astm.org/Standards/D5865.htm
Trentini CP, Santos KA, Silva EA, Garcia VAS, Cardozo-Filho L, Silva C (2016) Oil extraction from macauba pulp using compressed propane. J Supercrit Fluids 126:72–78. https://doi.org/10.1016/j.supflu.2017.02.018
Law SQK, Peter BC, Gregory JS, Martin JO (2017) Centrifugal recovery of solvent after biphasic wet extraction of lipids from a concentrated slurry of Nannochloropsis sp. biomass. Algal Res 24:299–308. https://doi.org/10.1016/j.algal.2017.04.016
Corrêa AC, Carmona VB, Simão JA, Galvani F, Marconcini JM, Mattoso LHC (2019) Cellulose nanocrystals from fibers of macauba (Acrocomia aculeata) and gravata (Bromelia balansae) from Brazilian pantanal. Polymers 11:1785. https://doi.org/10.3390/polym11111785
Vianna SA (2017) A new species of Acrocomia (Arecaceae) from Central Brazil. Phytotaxa 314:045–054. https://doi.org/10.11646/phytotaxa.314.1.2
Vianna SA, Carmelo-Guerreiro SM, Noblick LR, Colombo CA (2017) Leaf anatomy of the Acrocomia (Arecaceae): an additional contribution to the taxonomic resolution of a genus with great economic potential. Plant Syst Evol 303:233–248. https://doi.org/10.1007/s00606-016-1369-4
Mayor L, Sereno M (2004) Modelling shrinkage during convective drying of food materials: a review. J Food Eng 61:373–386. https://doi.org/10.1016/S0260-8774(03)00144-4
Bart HJ, Pilz S (2011) Industrial scale natural products extraction, 1st ed. Wiley-VCH Verlag GmbH & Co, Germany
Bilia AR (2014) Nuclear magnetic resonance as analytical tool for crude plant extracts plant analysis: chemical and biological. EAC: Applications, Theory and Instrumentation. https://doi.org/10.1002/9780470027318.a9913
Souza GK, Kischkel B, Freitas CF, Negri M, Back D, Johann G, Hioka N, Schuquel ITA, Santin SMO, Pomini AM (2019) Antiproliferative activity and energy calculations of a new triterpene isolated from the palm tree Acrocomia totai. Nat Prod Res. https://doi.org/10.1080/14786419.2019.1696331
Zanatta ER, Reinehr TO, Awadallak JA, Kleinübing SJ, Santos JBO, Bariccatti RA, Arroyo PA, Silva EA (2016) Kinetic studies of thermal decomposition of sugarcane bagasse and cassava bagasse. J Therm Anal Calorim 125:437–445. https://doi.org/10.1007/s10973-016-5378-x
Rencoret J, Kim H, Evaristo AB, Gutierrez A, Ralph J, del Río JCC (2018) Variability in lignin composition and structure in cell walls of different parts of macaúba (Acrocomia aculeata) palm fruit. J Agric Food Chem 66:138–153. https://doi.org/10.1021/acs.jafc.7b04638
Mothé CG, Miranda IC (2009) Characterization of sugarcane and coconut fibers by thermal analysis and FTIR. J Therm Anal Calorim 97:661–665. https://doi.org/10.1007/s10973-009-0346-3
Fernandes FHA, Santana CP, Santos RL, Correia LP, Conceição MM, Macêdo RO, Medeiros ACD (2013) Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J Therm Anal Calorim 113:443–447. https://doi.org/10.1007/s10973-012-2807-3
Scheufele FB, Módenes AP, Borba CE, Ribeiro C, Espinoza-Quiñones FR, Bergamasco R, Pereira NC (2016) Monolayer–multilayer adsorption phenomenological model: Kinetics, equilibrium and thermodynamics. Chem Eng J 284:1328–1341. https://doi.org/10.1016/j.cej.2015.09.085
Vieira SS, Magriotis ZM, Santos NAV, Cardoso MG, Saczk AA (2012) Macauba palm (Acrocomia aculeata) cake from biodiesel processing: an efficient and low cost substrate for the adsorption of dyes. Chem Eng J 183:152–161. https://doi.org/10.1016/j.cej.2011.12.047
Nandanwar RA, Chaudhari AR, Ekhe JD (2016) Nitrobenzene oxidation for isolation of value added products from industrial waste lignin. J Chem Biol Phys Sci 6:501–513 https://www.jcbsc.org/admin/get_fileenv.php?id=292
Fasina OO, Colley Z (2008) Viscosity and specific heat of vegetable oils as a function of temperature: 35 °C to 180 °C. Int J Food Prop 11:738–746. https://doi.org/10.1080/1094291070158627356
Arantes LJ, Silva MB, Silva EM (2003) Avaliation of surfaces machined by different dieletric fluids in the electroerosion machining. Rev Esc Minas 56:91–96. https://doi.org/10.1590/S0370-44672003000200005
Kumar M, Sabbarwal S, Mishra PK, Upadhyay SN (2019) Thermal degradation kinetics of sugarcane leaves (Saccharum officinarum L) using thermo-gravimetric and differential scanning calorimetric studies. Bioresour Technol 279:262–270. https://doi.org/10.1016/j.biortech.2019.01.137
Iftikhara M, Asghara A, Ramzana N, Sajjadib B, Chenb W (2019) Biomass densification: effect of cow dung on the physicochemical properties of wheat straw and rice husk based biomass pellets. Biomass Bioenergy 122:1–6. https://doi.org/10.1016/j.biombioe.2019.01.005
Evaristo AB, Martino DC, Ferrarez AH, Donato DB, Carneiro ACO, Grossi JAS (2016) Energy potential of the macaw palm fruit residues and their use in charcoal production. Ciênc Florest SM 26:571–577. https://doi.org/10.5902/1980509822757
Yan WH, Duan PG, Wang F, Xu YP (2016) Composition of the bio-oil from the hydrothermal liquefaction of duckweed and the influence of the extraction solvents. Fuel 185:229–235. https://doi.org/10.1016/j.fuel.2016.07.117
Mushrif SH, Vasudevan V, Krishnamurthy CB, Venkatesh B (2015) Multiscale molecular modeling can be an effective tool to aid the development of biomass conversion technology: a perspective. Chem Eng Sci 121:217–235. https://doi.org/10.1016/j.ces.2014.08.019
Garcia DP, Caraschi JC, Ventorim G (2016) Thermal decomposition of wood pellets by TGA. Holos 32:327–339. https://doi.org/10.15628/holos.2016.3886
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Acknowledgments
The authors are thankful to CAPES (Coordination for the Improvement of Higher Education Personnel), CNPq (National Counsel of Technological and Scientific Development, (Process no. 150602/2019-7)), and the Araucaria Foundation for the financial support of the research and to the Complex of Research Support Centers (COMCAP/UEM) for the support in the analysis.
Author information
Authors and Affiliations
Contributions
The statement to specify the contribution of each co-author is as follows:
Conceived and designed the experiments: Gredson Keiff Souza, Armando Mateus Pomini;
Performed the experiments: Gredson Keiff Souza, Alexandre Diório Hugo Henrique Carline de Lima, Rogerio Santos Maniezzo;
Analyzed the data: Gredson Keiff Souza, Alexandre Diório, Hugo Henrique Carline de Lima;
Contributed reagents/materials: Andrelson Wellington Rinaldi, Nehemias Curvelo Pereira, Armando Mateus Pomini;
Drafted or revised the manuscript: Gredson Keiff Souza, Alexandre Diório, Andrelson Wellington Rinaldi, Nehemias Curvelo Pereira, Armando Mateus Pomini.
All authors approved the manuscript, this submission.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
This manuscript describes an original work, has not been published before, and is not under consideration by any other journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souza, G.K., Diório, A., de Lima, H.H.C. et al. Assessment of Natural and Post-Extraction Biomass from Acrocomia totai leaves: a Renewable Source of Energy. Bioenerg. Res. 14, 1025–1037 (2021). https://doi.org/10.1007/s12155-020-10208-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10208-6