Abstract
Paulownia tomentosa, a member of the plant family Paulowniaceae and a rich source of biologically active secondary metabolites, is traditionally used in Chinese herbal medicine. Flavonoids, lignans, phenolic glycosides, quinones, terpenoids, glycerides, phenolic acids, and miscellaneous other compounds have been isolated from different parts of P. tomentosa plant. Recent interest in this species has focused on isolating and identifying of prenylated flavonoids, that exhibit potent antioxidant, antibacterial, and antiphlogistic activities and inhibit severe acute respiratory syndrome coronavirus papain-like protease. They show cytotoxic activity against various human cancer cell lines and inhibit the effects of human cholinesterase, butyrylcholinesterase, and bacterial neuraminidases. Most of the compounds considered here have never been isolated from any other species of plant. This review summarizes the information about the isolated compounds that are active, their bioactivities, and the structure–activity relationships that have been worked out for them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant Paulownia tomentosa (Thunb.) Siebold & Zucc. ex Steud. is a very adaptable and extremely fast-growing timber tree native to central and western China traditionally used in Chinese Medicine. This deciduous tree is now grown in many areas worldwide, mostly as a decorative ornamental tree (Erbar and Gűlden 2011; Zhu et al. 1986).
Two related varieties of P. tomentosa have been described—var. tsinlingensis has a round to shallowly cordate leaf base and a glabrous or sparsely hairy lower leaf surface, whereas var. tomentosa is characterized by a cordate leaf blade base and an abaxial surface that is densely hairy when mature (Hong et al. 1998).
Paulownia was named paulownia in honour of Anna Paulowna (1795–1865), queen consort of The Netherlands and a daughter of Tsar Paul I of Russia. Nowadays, it is commonly known under its synonym Bignonia tomentosa (Zhu et al. 1986) or as the princess-tree, empress-tree, foxglove tree, royal paulownia, kiri, or mao pao tong (yuan bian zhong) (Erbar and Gűlden 2011; Hong et al. 1998).
The genus Paulownia was first assigned to family Bignoniaceae by Swiss botanist Thunberg (1781). It was then transferred to the Scrophulariaceae family by the Dutch scholars Zuccarini and Siebold (1835) (Zhu et al. 1986). At last, Paulownia has been categorized as a family of its own, Paulowniaceae, based on data from the latest molecular phylogenetic studies. This family includes only one genus and between six and ten species. It was originally introduced in 1949 by the research of Nakai (Erbar and Gűlden 2011).
Biological activity and traditional uses of P. tomentosa extracts
According to both legends and records, people were already using Paulownia for various purposes about 2,600 years ago. Chinese herbal medicine has used P. tomentosa traditionally to relieve bronchitis, especially by reducing coughing, asthma, and phlegm (Zhu et al. 1986). It has also been used to treat conjunctivitis, dysentery, enteritis, erysipelas, gonorrhea, hemorrhoids, parotitis, traumatic bleeding, and tonsillitis (Jiang et al. 2004; Si et al. 2009). Solutions prepared from the leaves and fruit extracted in water have been used in daily applications to promote the healthy growth of hair and turn grey hair dark. An extract prepared from the wood and leaves may relieve swollen feet. Pharmacological experiments have shown that extracts from the fruit can reduce blood pressure. Nowadays, injections and tablets prepared from Paulownia flowers and fruit are used for the herbal treatment of chronic bronchitis and other kinds of inflammation (Zhu et al. 1986).
More than 130 physiologically active constituents have been isolated from different parts of the Paulownia plant. Their biological activity has been tested using both the isolated compounds and different types of extracts. For example, n-butanol, EtOAc, and MeOH extracts obtained from the fruit have displayed antiradical activity in anti-DPPH and peroxynitrite assays, due to mainly the presence of flavonoids and phenolic glycosides, but not of all compounds present in these extracts have been identified) (Šmejkal et al. 2007b). An MeOH extract obtained from the fruit inhibited hAChE (IC50 = 1.44 mg/ml) and BChE (IC50 = 0.97 mg/ml) significantly more strongly than CHCl3 and water extracts (possibly due to the content of phenolic glycosides and C-prenylated dihydroflavonols and flavanones) (Cho et al. 2012). Significant concentration-dependent anti-inflammatory properties of EtOH extracts of the bark of the tree have also been observed recently using a lipopolysaccharide (LPS)-induced nitric oxide production inhibition model in the murine macrophages cell line RAW264.7 (Si et al. 2011a). Kim et al. (2010a, b) showed potential of aqueous extract of P. tomentosa to suppress glutamate induced toxicity in primary cultured rat cortical cells (with possible sesquiterpene lactone as active substance). The bio-activities of individual compounds and, where possible, the structure–activity relationships are in Tables 1, 2, 3 and 4 and discussed in separate chapters.
P. tomentosa flavonoids and their biological activity
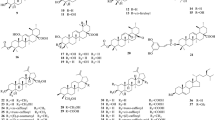
Flavonoids represent the most numerous group of secondary metabolites isolated from P. tomentosa. They can be divided into simple non-prenylated flavonoids 1–18 (Table 1), C-prenylated and C-geranylated flavonoids 19–59 (Tables 2, 3, respectively), and flavonoid glycosides 60–65. Compounds 16, 17, 19, 20, 25–31, 33–36, 38–59 have never been isolated from any other species. Most of the isolated flavanones are characterized by a 2S configuration in contrast to the dihydroflavonols, for which 2R, 3R isomer is often observed.
Some of the flavonoid compounds found in P. tomentosa have been categorized as dietary flavonoids. Consuming these compounds is believed to deliver health benefits. The activities of these flavonoids are frequently been reviewed, for example in the papers of Romano et al. (2013), Marzocchella et al. (2011), Ross and Kasum (2002), and Havsteen (2002). Some simple flavonoid substances isolated from fruit of P. tomentosa are commonly observed as the lipophilic components of different plant exudates. Flavonoids 1–18 show broad-spectrum pharmacological activities (Table 1). Many papers report their potential role as anticancer compounds for use against human cervical and breast (Bulzomi et al. 2010, 2012), hepatoma, leukemic (Zhao et al. 2012), osteosarcoma (Chen et al. 2013), gastric carcinoma (Lu et al. 2013), colon adenocarcinoma (Sánchez-Tena et al. 2013), or prostatic (Zhang and Coultas 2013) cancer cell lines. Their antioxidant properties could explain their promising cardioprotective (Paneerselvam et al. 2010; Psotová et al. 2004) and neuroprotective effects (Losi et al. 2004). Antimicrobial (Betts et al. 2013; Jiang et al. 2004; Mankovskaia et al. 2013) and antiviral (Khachatoorian et al. 2012) bio-activities against different pathogens have also been discovered. In some cases, no specific biological activity has been observed (Kim et al. 2010a, 2010b; Tang et al. 1994).
Paulownia tomentosa is also a rich source of prenylated and geranylated flavonoids (19–59), whose occurrence is limited to relatively a few plant families (Yazaki et al. 2009). There are sometimes misunderstandings in the naming of these compounds. A compound containing both a phenolic skeleton and a terpenoid side chain is often designated as a “prenylated” phenolic substance, even though it contains not a prenyl, but rather a geranyl or other type of terpenoid side-chain. Prenylated flavonoids are biosynthesized by a combination of several pathways: the acetate, shikimate, and mevalonate (Fig. 1). It is now known that the prenyl and geranyl moieties are biosynthesized via mevalonate or deoxyxylulose pathway. The connection of terpenoid and flavonoid biosynthetic pathways is provided by prenyltransferases (Šmejkal 2014; Andersen and Markham 2006; Kuzuyama et al. 2005). The side chain can later be converted into different moieties by several reactions. It is unclear, whether the described modifications of the prenyl side-chain are natural—sunlight, the presence of oxygen and an elevated temperature can all affect the terpenoid metabolism—or are artefacts of isolation. Some of the changes in the prenyl moiety have been observed after treatment of an extract, or during the separation of a mixture in the acidic environment of silica gel (Navrátilová et al. 2013; Šmejkal 2014) (Fig. 2).
Most of the prenylated flavonoids isolated from P. tomentosa belong to C-geranylated group of flavonoids (21–52). Some of them have their side-chain further modified by hydroxylation (35–44, 50, 51, 53–59), methoxylation (45–47, 49, 50), oxidation (47, 48, 52), cyclization (51, 53–59), or reduction (19, 20, 47–52). Interestingly, these compounds have been isolated from the leaves, flowers and fruit, but they are most commonly isolated from the roots, root bark, or bark of different plants (Botta et al. 2005). Compounds 23, 24, and 28 have been found in the yellow dendritic trichomes on the adaxial side of the P. tomentosa leaves. On the other hand, no significant detected concentration of secondary metabolites has been detected in the white dendritic trichomes on the adaxial side of the leaves or the brown dendritic trichomes on the flower buds (Kobayashi et al. 2008). Glandular hairs found on the young reproductive organs of P. tomentosa are rich in flavonoids, with concentrations over 1,000 times greater than those on the surfaces of the young leaves (Kobayashi et al. 2008). Some seasonal variations in the appropriate time for harvesting the fruit have been discovered. Autumn is the best time to obtain high concentrations of flavonoids whereas early summer is better for phenylpropanoid glycosides (Holubová and Šmejkal 2011).
The antioxidant (Asai et al. 2008; Šmejkal et al. 2007a, b; Zima et al. 2010), antibacterial (Navrátilová et al. 2013; Šmejkal et al. 2008b), antiphlogistic (Hošek et al. 2010), cytotoxic (Kollár et al. 2011; Šmejkal et al. 2007a, 2008a, 2010), and severe acute respiratory syndrome coronavirus papain-like protease (SARS-CoV PLpro) activities (Cho et al. 2013) of isolated geranylated flavonoids have been described recently, together with their inhibitory effect on both human acetylcholinesterase and butyrylcholinesterase (Cho et al. 2012). The great ability of several geranylated flavanones to interact with bacterial sialidase isolated from Clostridum perfringens (Cp-NanI), a bacterium causing various gastrointestinal diseases, has recently been reported (Lee et al. 2014).
Possible relationships between structure and activity have been proposed for each of these biological activities. Nevertheless, it is difficult to evaluate real structure–activity relationships only by comparing the published studies, because the study conditions and the assays employed are often different. Generally, the addition of an isoprenoid chain renders the derivate molecule more pharmacologically effective than the parent compound, probably because the prenyl group increases the lipophilicity and confers a strong affinity for biological membranes (Botta et al. 2005; Epifano et al. 2007; Chen et al. 2014).
Interestingly, neither the geranyl side-chain nor its substitution affects the antioxidant activity of flavonoids. The spatial arrangement of the substitution of the flavanone skeleton is more important. For example, the antiradical activity is increased by 2′-hydroxyl substitution of the ring B, whereas 4′-methoxyl substitution diminishes it. The general rules postulated for the antioxidant activity of flavonoids in vitro are applicable (Havsteen 2002; Chen et al. 2002), there are many review publications that touch on this topic, and it is not aim of this paper to delve deeply into this (Plaza et al. 2014). The type of antioxidant assays used for the experiment could also be a factor that significantly affects this activity (Zima et al. 2010).
Numerous reports about structure related antimicrobial activity have been published, but comparison shows the results to be widely conflicting (Cushnie and Lamb 2005). Based on several studies, it has been postulated that hydroxylation at position C-5 or C-7 of ring A and positions C-2′ or C-4′ of ring B increases the antibacterial activity (Šmejkal et al. 2008b; Navrátilová et al. 2013). However, contrary to this assumption, 45 and 50 had no significant antibacterial activity (Navrátilová et al. 2013). Interestingly, some C-geranylated flavonoids do not able inhibit the growth of Gram-negative bacteria (21, 22, 25–27, 35, 38, 45, 47–48, 50, 53, and 54). Resistance to these compounds is probably due to the more complex structure and hydrophilic nature of G-cell walls (Navrátilová et al. 2013; Šmejkal et al. 2008b). Furthermore, substitution of the geranyl side-chain with carbonyl, hydroxyl or methoxyl groups diminishes the antibacterial activity in a manner similar to what is seen when the geranyl substituent at C-6 forms a ring by reacting with the hydroxyl group at C-7. Compounds 45, 47, 48, 50, 53, and 54 exhibit some degree of activity in the range of the concentrations tested on the Gram-positive bacteria Staphyloccocus aureus and various methicillin resistant strains of S. aureus. The level of activity varied in depending on both the structure and the bacterial strain used in the assay. Flavonoid structures like 24 are more effective in protecting plants from water loss because of their reduced polarity (Kobayashi et al. 2008; Navrátilová et al. 2013). Compounds 45, 47, 48, 50, and 53 were also tested for their ability to affect the initiation of the eukaryotic translation via dual-luciferase reported assay (firefly and renilla), but only 45 showed a modest activity in comparison with anisomycin (Navrátilová et al. 2013).
The cytotoxicity of the prenylated flavonoids obtained from P. tomentosa has been tested in more than 20 different cell lines. The type of prenylation strongly affects the cytotoxicity of a flavonoid in the traditional P-388 murine leukemia model. The unmodified 3-prenyl group and the presence of corresponding ortho-dihydroxy or trihydroxy substitution of the flavonoid ring B are crucial to its activity against P-388 as compared with other prenyl substituents (Hakim et al. 1999, 2002, 2006). Similar findings have been observed for modified C-6 geranyl groups and the substitution of the ring B for other cell lines tested (Šmejkal et al. 2010). However, replacing the para-hydroxy group of the ring B of a C-8 prenylated flavanone with several different acyl substituents resulted in greater cytotoxicity (Aniol et al. 2012; Šmejkal 2014). It has also been found that cytotoxic activity is diminished by the presence of a C-3 hydroxyl substituent on the ring C, 4′-methoxy substitution of the ring B or a para-hydroxy substituted ring B (Šmejkal et al. 2008a). For this reason, it is important to emphasize that the relative importance of the substitution of ring B can differ according to the cell line used. Modification of a prenyl or geranyl side-chain can not only change the direct cytotoxicity, but it may also affect the proliferation cells or trigger apoptosis (Kollár et al. 2011; Šmejkal et al. 2007a, 2008a, 2010; Šmejkal 2014).
Prenylated flavonoids 55–59, modified with an unusual 3,4-dihydro-2H-pyran moiety, have been found to inhibit the severe acute respiratory syndrome corona virus PLpro enzyme more effectively than their parent compounds, the precursors from which were they derived (Cho et al. 2013).
The presence of a geranyl group at the C-6 position (21, 22, 24–26, 30, 31, 33, and 34) seems to be crucial for the hAChE and BChE inhibitory effects. The most effective inhibitor was 22. All of the geranylated flavonoids, apart from 26, inhibited hAChE dose-dependently. It appears that greater inhibition is observed when ring B of the flavanone bears free hydroxyl groups (Cho et al. 2012).
The crystal structure of the bacterial sialidase Cp-NanI catalytic domain in a complex with the geranylated flavonoid diplacone (22) provides structural insights into the binding mode of natural flavonoid-based inhibitors at atomic resolution. It shows how the geranyl and C-3′ hydroxyl groups of 22 contribute to the stability of the enzyme-inhibitor complex. Time-dependent competitive inhibition patterns have been observed. Structural comparison of the human sialidases Neu1–Neu4 with the Cp-NanI–diplacone (22) complex suggests that the interaction between human sialidases and 22 is likely to be unfavourable because of polar or ionic repulsion (Lee et al. 2014).
Six flavonoid glycosides have been extracted from the stem bark 60–62 (Si et al. 2009) and flowers 63–66 (Scogin 1980) of P. tomentosa. Apigenin-7-O-β-d-glucopyranoside (synonym: cosmosiin) (Kurkina et al. 2011) (60) shows anti-HIV activity in vitro on H9 cells (Tang et al. 1994) and enhances the secretion of adiponectin, the phosphorylation of the tyrosine residue of insulin receptor-β, and the translocation of GLUT5 (Rao et al. 2011). Compound 60 shows no significant hepatic Glc-6-phosphatase inhibitory activity in vitro (Kumar et al. 2010). Apigenin-7-O-β-d-glucuronopyranoside (61) was more potent than apigenin (2) and omeprazole in an assay analysing the inhibition of reflux esophagitis and gastritis promoted surgically and by application of indomethacine in rats (Min et al. 2005). No information about the biological activity of apigenin-7-O-[β-d-glucuronopyranosyl (1 → 2)-O-β-d-glucuronopyranoside] (62) has been found in the literature. Delphinidin-3-O-glucoside (synonym: myrtillin) (63) protects microglia from inflammation-induced stress signaling (Carey et al. 2013), is cytotoxic against the MCF-7 (breast) cancer cell line (Vareed et al. 2006), and has an the affinity for the estrogenic ERβ receptor (Hidalgo et al. 2012). Delphinidin-3,5-di-O-glucoside (64) and cyanidin-3,5-di-O-glucoside (65) are potent antioxidants (Lee et al. 2011; Tanaka et al. 2013). Compounds 63 and 65 are potent ACE inhibitors in vitro (Hidalgo et al. 2012; Persson et al. 2009).
P. tomentosa lignans and their biological activity
Only three lignans: (+)-paulownin (66), (+)-sesamin (67), (+)-piperitol (68) have been isolated from the wood of P. tomentosa (Ina et al. 1987; Takahashi and Nakagawa 1966; Zhu et al. 1986) (Fig. 3). Several reports have described their pharmacological effects (Zhang et al. 2014; Pan et al. 2009). Compound 66 is a promising antifungal agent that acts against the basidiomycetes Fomitopsis palustris and Trametes versicolor (Kawamura et al. 2004). Sesamin (67) has been previously isolated from the wood of P. tomentosa, and P. kawakamii and the hybrid of P. elongata × P. fortunei (Takahashi and Nakagawa 1966; Zhu et al. 1986). Compound 67 is the major lignan in sesame, and has various biological activities, such as antioxidant, angiogenic (Chung et al. 2010), antiphlogistic (Chatrattanakunchai et al. 2000), antihypertensive (Nakano et al. 2006), insecticidal (Nascimento et al. 2004), neuroprotective (Khan et al. 2010), and anticarcinogenic (Yasuda and Sakaki 2012). However, this compound showed no significant ability to reduce the formation of atherosclerotic lesions in the ApoE (−/−) gene-knockout mouse, whereas specific dietary polyphenols, especially quercetin and theaflavin were more active (Loke et al. 2010). It has been suggested that some of the biological effects attributed to sezamine (67) may, in fact, be caused by its metabolites and that 67 may be acting as a proactive substance in the body (Yasuda and Sakaki 2012). The metabolism of sesamim (67) by cytochrome P450 and UDP-glucuronosyltransferase has been found remarkably different in humans as compared to other animals. Further investigation into the safety of taking sesamin (67) with therapeutic drugs that are metabolised by CYP2C9 is also needed (Yasuda and Sakaki 2012).
P. tomentosa phenolic glycosides and their biological activity
A total of thirteen phenylpropanoid glycosides (69–81) with multifarious bioactivities, natural compounds derived biosynthetically from the amino acid phenylalanine, have been isolated from different parts of the P. tomentosa plant (Table 4) (Damtoft and Jensen 1993; Kang et al. 1994; Ota et al. 1993; Schilling et al. 1982; Si et al. 2008b, d, Si et al. 2011b; Sticher and Lahloub 1982; Šmejkal et al. 2007b; Zhu et al. 1986). Phenylpropanoids are of great interest for fabricating effective tonic, anticancer, hepatoprotective, immunostimulating, antimicrobial, and anti-inflammatory phytopreparations (Kurkin 2003; Galvez et al. 2006; Panossian and Wagner 2005; Fu et al. 2008; Pan et al. 2003).
P. tomentosa quinones and their biological activity
A new furanoquinone, methyl-5-hydroxy-dinaphthol[1,2–2′,3′]furan-7,12-dione-6-carboxylate (MHDDC) (82) (Fig. 4) identified in P. tomentosa stem bark (MeOH extract) has been shown to possess antiviral activity against poliovirus types 1 and 3, using HeLa cells in vitro (Kang et al. 1999). Its cathepsin K and L inhibitory activities in vitro have recently been discovered. The 5-OH functional group may have a favourable effect on this reduction potential which prevents the degradation of bone the matrix carried out by osteoclasts (Park et al. 2009).
The naphtoquinone plumbagin (83) has been detected in the leaves and fruit of P. tomentosa (Babula et al. 2006). It has been used in traditional systems of medicine since ancient times (Pile et al. 2013). It has exhibited promising antimalarial activity in vitro with IC50 of 580 (270–640) nM and 370 (270–490) nM, respectively, against 3D7 chloroquine-sensitive P. falciparum and K1 chloroquine-resistant P. falciparum clones, using an assay based on SYBR Green I. Toxicity testing indicated relatively low toxicity at dose levels up to 100 mg/kg body weight (for a single oral dose) and 25 mg/kg (for daily dose given for consecutive 14 days) for acute and subacute toxicity, respectively. Based on the results of in vivo antimalarial testing, plumbagin administered at a the dose of 25 mg/kg of body weight for 4 consecutive days exhibited moderate to weak antimalarial activity with regard to its ability to reduce parasitaemia and prolong survival time (Sumsakul et al. 2014). Other published studies of plumbagin (83) described effects such as antifungal, antibacterial, cytotoxic (Krishnaswamy and Purushothaman 1980), anticoagulant (Santhakumari et al. 1978), anthelmintic (antischistosomal) (Zhang and Coultas 2013) and an anti-inflammatory effect on the amelioration of experimental ulcerative colitis in mice at a dose of 8–10 mg/kg (Pile et al. 2013).
P. tomentosa terpenoids and their biological activity
Six iridoids: 7-β-hydroxyharpagide (84), paulownioside (85), catalpol (86), aucubin (87), tomentoside (88) and 7-hydroxytomentoside (89) have been isolated from the leaves of P. tomentosa (84–87) (Adriani et al. 1981; Franzyk et al. 1999), the bark of the trunk and roots (86) (Plouvier 1971) and parts of the young plant (85, 86, 88, and 89) (Damtoft and Jensen 1993) (Fig. 5). Compound 86 shows neuroprotective activity (Li et al. 2004), a cardioprotective effect against myocardial infarction—specifically reperfusion damage (Huang et al. 2013a, b), and radioprotective effects (Chen et al. 2013). It also increases glucose utilization by increasing the secretion of β-endorphin from the adrenal gland (Shieh et al. 2011). Compounds 86 and 87 possess antispasmodic activity (Deurbina et al. 1994). Aucubin (87) had antioxidant and pancreas-protective effects on streptozotocin-induced diabetes in rats (Jin et al. 2008) and it may improve obesity-induced atherosclerosis by attenuating the TNF-α-induced inflammatory response (Park 2013). A weak antibacterial effect (against Streptococcus pneumoniae and MG-hemolytic streptococcus, MIC 28.946 mg/ml) (Zheng et al. 2012) and neuroprotective effects of 87 on diabetes and diabetic encephalopathy (Xue et al. 2012) have also been observed.
The sesquiterpenic lacton isoatriplicolide tiglate (90) has been isolated from P. tomentosa flowers. It has neuroprotective effects against glutamate-induced neurotoxicity (Kim et al. 2010a, 2010b) and cytotoxic activity against several cancer cell lines: A549 lung carcinoma; SK-OV-3 adenocarcinoma; SK-Mel-2 malignant melanoma; XF498 central nervous system tumors; HCT15 colon adenocarcinoma, against which its effect is comparable to that of reference substance adriamycin (Moon and Zee 2001); human breast cancers MDA-MB-231, MCF7, HS578T, and T47D; and the HeLa, SiHa and C33A cervical cancer cell lines (Jung et al. 2012).
Seven phytosterols have been isolated from P. tomentosa leaves: ursolic acid (91) (Zhu et al. 1986; Zhang and Li 2011), 3-epiursolic acid (92), pomolic acid (93), corosolic acid (94), maslinic acid (95), β-sitosterol (96), and daucosterol (97) (Zhang and Li 2011). Most of these show various biological activities, e.g., 91 is a potentially useful for treating Alzheimer’s disease (because of its ability to block the interactions of the amyloid β-CD36) (Wilkinson et al. 2011). It can prevent the recruitment of the monocytes that accelerate atherosclerosis, a major complication of diabetes, in mice (Ullevig et al. 2011), and it also possesses antibacterial (Wong et al. 2012), anti-trypanosomal, and anti-leishmanial properties (Bero et al. 2011). Compound 91 has also shown cytotoxic effects against K562 and K562/ADR human chronic myelogenous leukaemia, HL60 and HL60/ADR human acute myelocytic leukemia cancer cells, and the human colon cancer cell lines SW480 and SW620 (Shan et al. 2011). Compounds 93–96 also show promising cytotoxic potential, e.g., 93 is effective against the human chronic myelogenous leukaemia cell line 562 and also against cells derived from chronic myeloid leukaemia patients (Vasconcelos et al. 2007). Compound 94 shows cytotoxic effects against osteosarcoma MG-63 cells (Cai et al. 2011) and immunosuppressive activity on myeloid-derived suppressor cells in the murine sarcoma model (Horland et al. 2013). Compound 95 is effective against HT29 human colon cancer cells (Reyes-Zurita et al. 2011) and 96 promotes apoptosis in various cancer cells (Hac-Wydro 2013). Anti-inflammatory activities have been revealed for compounds 91, 92, and 96 (Deepak and Handa 2000), and 93 (Schinella et al. 2008). An immunomodulatory effect of 97 in protecting mice against candidiasis disseminated by the CD4+ Th1 immune response has been seen (Lee et al. 2007). Other recently discovered pharmacological effects include antimalarial (95) (Moneriz et al. 2011), hypotensive and endothelium-dependent vasorelaxant effects (93) (Estrada et al. 2011). Compound 95 is potentially useful for treating cerebral ischemic injuries (Guan et al. 2011), and 94 has promising antidiabetic effects (Sivakumar et al. 2009).
P. tomentosa glycerides and their biological activity
Thirty acylglycerols (98–127) belonging to 1,3-di-O-acetyl-2-O-(acyl)-glycerols, 1-O-acetyl-2-O-(acyl)-sn-glycerols and 2-O-(acyl)-glycerols, including only five known compounds (99, 106, 108, 114, and 119), have been identified in the exudates secreted from the glandular trichomes on the leaves: 1,3-di-O-acetyl-2-O-[(3R,6S)-3-(acetyloxy)-6-hydroxyeicosanoyl]-glycerol (98), 1-O-acetyl-2-O-[(3R)-3-(acetyloxy)octa-decanoyl]-sn-glycerol (99), 1-O-acetyl-2-O-[(3R,7R)-3,7-bis(acetyloxy)octadecanoyl]-sn-glycerol (100), 1-O-acetyl-2-O-[(3R,6S)-3-(acetyloxy)-6-hydroxyoctadecanoyl]-sn-glycerol (101), 2-O-[(3R,7R)-3,7-dihydroxy-eicosanoyl]-glycerol (102), 2-O-[(3R,6S)-3,6-dihydroxy-eicosanoyl]-glycerol (103), 2-O-[(3R,8R)-3,8-dihydroxy-eicosanoyl]-glycerol (104), 2-O-[(3R,9R)-3,9-dihydroxy-eicosanoyl]-glycerol (105), 1-O-acetyl-2-O-[(3R)-3-(acetyloxy) eicosanoyl]-sn-glycerol (106), 1-O-acetyl-2-O-[(3R,6S)-3,6-bis(acetyloxy)eicosanoyl]-sn-glycerol (107), 1-O-acetyl-2-O-[(3R,7R)-3,7-bis(acetyloxy)eicosanoyl]-sn-glycerol (108), 1-O-acetyl-2-O-[(3R,8R)-3,8-bis(acetyloxy)eicosanoyl]-sn-glycerol (109), 1-O-acetyl-2-O-[(3R,9R)-3,9-bis(acetyloxy)eicosanoyl]-sn-glycerol (110), 1-O-acetyl-2-O-[(3R,6S)-3-(acetyloxy)-6-hydroxyeicosanoyl]-sn-glycerol (111), 1-O-acetyl-2-O-[(3R,8R)-3-(acetyloxy)-8-hydroxyeicosanoyl]-sn-glycerol (112), 1-O-acetyl-2-O-[(3R,9R)-3-(acetyloxy)-9-hydroxyeicosanoyl]-sn-glycerol (113), 2-O-[(3R)-3-(acetyloxy)octadecanoyl]-glycerol (114), 2-O-[(3R,6S)-3-(acetyloxy)-6-hydroxy-octadecanoyl]-glycerol) (115), 2-O-[(3R,7R)-3-(acetyloxy)-7-hydroxy-octadecanoyl]-glycerol (116), 2-O-[(3R,8R)-3-(acetyloxy)-8-hydroxy-octadecanoyl]-glycerol (117), 2-O-[(3R,9R)-3-(acetyloxy)-9-hydroxy-octadecanoyl]-glycerol (118), 2-O-[(3R,7R)-3,7-bis(acetyloxy) octadecanoyl]-glycerol (119), 2-O-[(3R,8R)-3,8-bis(acetyloxy)octadecanoyl]-glycerol (120), 2-O-[(3R)-3-(acetyloxy)eicosanoyl]-glycerol (121), 2-O-[(3R,6S)-3-(acetyloxy)-6-hydroxy-eicosanoyl]-glycerol (122), 2-O-[(3R,7R)-3-(acetyloxy)-7-hydroxy-eicosanoyl]-glycerol (123), 2-O-[(3R,8R)-3-(acetyloxy)-8-hydroxy-eicosanoyl]-glycerol (124), 2-O-[(3R,9R)-3-(acetyloxy)-9-hydroxy-eicosanoyl]-glycerol (125), 2-O-[(3R,8R)-3,8-bis(acetyloxy)eicosanoyl]-glycerol (126), 2-O-[(3R,9R)-3,9-bis(acetyloxy)eicosanoyl]-glycerol (127). The relatively most abundant constituents were 111 (20 % of the total glycerides), 126 (14 %), and 120 and 127 (12 %) (Asai et al. 2009). Another analysis of the secretions of the glandular hairs on the leaves of both bud flushes and adult trees as well as the flowers of adult trees showed that these secretions contain ten glycerides (106, 108–110, 118, 122, 124–127). These compounds showed sticky character, but they were not toxic to several insects. Therefore, the glandular hairs on the leaves and flowers may serve only to physically deter herbivores (Kobayashi et al. 2008) (Fig. 6).
Miscellaneous P. tomentosa compounds and their biological activity
Six known phenolic acids, namely p-hydroxybenzoic acid (128), vanillic acid (129), gallic acid (130), cinnamic acid (131), p-coumaric acid (132), and caffeic acid (133) have been identified in the leaves of P. tomentosa (128–133), bark (130 and 131) and wood (133) (Ota et al. 1993; Si et al. 2008c, 2011b), and p-ethoxybenzaldehyde (134) is present in flowers (Yuan et al. 2009). The 5,7-dihydroxy-6-geranylchromone (135) isolated from fruits shows only moderate cytotoxic activity against a suspension culture of Nicotiana tabacum cv. Bright Yellow (BY-2), which confirms that ring B of the flavonoid skeleton is important for this activity (Šmejkal et al. 2008a). A large group of essential oil substances found in the flowers of P. tomentosa have also been identified (Oprea et al. 2004; Ibrahim et al. 2013) (Fig. 7).
Conclusions
Plants are still highly esteemed all over the world as rich sources of therapeutic agents. In this context, traditional Chinese herbal medicines, such as Paulownia continue to influence a modern healthcare. It has been estimated that approximately 420,000 plant species exist on Earth, but little is known about the phytochemical or therapeutic qualities of most of them. Thanks to the development of the spectral and other analytical methods used in modern phytochemistry, many of the principal physiologically active secondary metabolites have been identified and researched in detail. The accumulated knowledge of traditional medicine is therefore, playing an important role in enhancing the success of drug discovery in herbal medicine. Approximately 80 % of the currently known antimicrobial, cardiovascular, immunosuppressive, and anticancer drugs are of plant origin (Pan et al. 2013).
As mentioned in this review, P. tomentosa is a rich source of multifarious secondary metabolites, mainly prenylated flavonoids. As of today 135 compounds, including flavonoids, lignans, phenolic glycosides, quinones, terpenoids, glycerides, phenolic acids, and other miscellaneous compounds have been isolated from various extracts of this plant.
Of increasing interest are the isolation and identification of P. tomentosa prenylated flavonoids, as they have shown promising pharmacological effects. In the first experiments, their antioxidant, antibacterial, antiphlogistic, cytotoxic, and SARS-CoV PL activities have been discovered along with inhibitory effects on human acetylcholinesterase, butyrylcholinesterase, and bacterial neuraminidases. More than 40 compounds with modified prenyl or geranyl side-chains attached at C-6 of the flavonoid skeleton have been isolated from the flowers, fruit, and leaves of P. tomentosa. Only two of these compounds have shown the presence of a five-carbon side-chain; for the others a ten-carbon side-chain is typical. Further, only a few compounds have shown a geranyl moiety modified by hydroxylation at C-6″ or C-7″. Some compounds have a geranyl group modified by formating a heterocyclic moiety, which is also unusual. Most of them have never been isolated from any other plant species. However, further in vivo pharmacology studies are needed to precisely elucidate biological mechanism of action, efficacy, and toxicity of these promising therapeutic agents. Elucidation of the structure–activity relationships is also crucial for their further total syntheses and introduction into medical practice. For these reasons, the study of P. tomentosa as a source of biologically active metabolites is significant and future interest in this plant is ensured.
References
Adriani C, Bonini C, Iavarone C et al (1981) Isolation and characterization of paulownioside, a new highly oxygenated iridoid glucoside from Paulownia tomentosa. J Nat Prod 44:739–744
Ahmad M, Rizwani GH (1995) Acteoside: a new antihypertensive drug. Phytother Res 9:525–527
Akbay P, Calis I, Űndeger Ű et al (2002) In vitro immunomodulatory activity of verbascoside from Nepeta ucrainica L. Phytother Res 16:593–595
Andersen OM, Markham KR (2006) Flavonoids: chemistry, biochemistry, applications. CRC Press, Boca Raton
Aniol M, Swiderska A, Stompor M et al (2012) Antiproliferative activity and synthesis of 8-prenylnaringenin derivatives by demethylation of 7-O- and 4′-O-substituted isoxanthohumols. Med Chem Res 21:4230–4238
Arutyunyan TV, Korystova AF, Kublik LN et al (2012) Effects of taxifolin on the activity of angiotensin-converting enzyme and reactive oxygen and nitrogen species in the aorta of aging rats and rats treated with nitric oxide synthase inhibitor and dexamethasone. Age 35:2089–2097
Asai T, Hara N, Kobayashi S et al (2008) Geranylated flavanones from the secretion on the surface of the immature fruits of Paulownia tomentosa. Phytochemistry 69:1234–1241
Asai T, Hara N, Kobayashi S et al (2009) Acylglycerols (=glycerides) from the glandular trichome exudate on the leaves of Paulownia tomentosa. Helv Chim Acta 92:1473–1494
Babula P, Mikelová R, Adam V et al (2006) Chromatografické stanovení naftochinonů v rostlinách (Chromatographic evaluation of naphtochinones in plants). Chem Listy 100:271–276
Bai Y, Tohda C, Zhu S et al (2011) Active components from Siberian ginseng (Eleutherococcus senticosus) for protection of amyloid β(25-35)-induced neuritic atrophy in cultured rat cortical neurons. J Nat Med 65:417–423
Bansal S, Vyas S, Bhattacharya S et al (2013) Catechin prodrugs and analogs: a new array of chemical entities with improved pharmacological and pharmacokinetic properties. Nat Prod Rep 30(11):1438–1454
Bero J, Hannaert V, Chataigné G et al (2011) In vitro antitrypanosomal and antileishmanial activity of plants used in Benin in traditional medicine and bio-guided fractionation of the most active extract. J Ethnopharmacol 137:998–1002
Betts JW, Wareham DW, Haswell SJ et al (2013) Antifungal synergy of theaflavin and epicatechin combinations against Candida albicans. J Microbiol Biotechnol 23:1322–1326
Botta B, Vitali A, Menendez P (2005) Prenylated flavonoids: pharmacology and biotechnology. Curr Med Chem 12:713–739
Bragança de Moraes CM, Melo DA, Santos RC et al (2012) Antiproliferative effect of catechin in GRX cells. Biochem Cell Biol 90:575–584
Bulzomi P, Bolli A, Galluzzo P et al (2010) Naringenin and 17β-estradiol coadministration prevents hormone-induced human cancer cell growth. IUBMB Life 62:51–60
Bulzomi P, Bolli A, Galluzzo P et al (2012) The naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high bisphenol a background. IUBMB Life 64:690–696
Cai X, Zhang H, Tong D et al (2011) Corosolic acid triggers mitochondria and caspase-dependent apoptotic cell death in osteosarcoma MG-63 cells. Phytother Res 25:1354–1361
Calderon-Montano JM, Burgos-Moron E et al (2011) A review on the dietary flavonoid kaempferol. Mini-Rev Med Chem 11(4):298–344
Carey AN, Fisher DR, Rimando AM et al (2013) Stilbenes and anthocyanins reduce stress signaling in BV-2 mouse microglia. J Agric Food Chem 61:5979–5986
Chai X-Y, Ren H-Y, Xu Z-R et al (2009) Investigation of two Flacourtiaceae plants: Bennettiodendron leprosipes and Flacourtia ramontchi. Planta Med 75:1246–1252
Chang CL, Wang GJ, Zhang LJ et al (2010) Cardiovascular protective flavonolignans and flavonoids from Calamus quiquesetinervius. Phytochemistry 71:271–279
Chatrattanakunchai S, Fraser T, Stobart K (2000) Sesamin inhibits lysophosphatidylcholine acyltransferase in Mortierella alpine. Biochem Soc Trans 28:718–721
Chen AY, Chen YC (2013) A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem 138(4):2099–2107
Chen J-W, Zhu Z-Q, Hu T-X et al (2002) Structure–activity relationship of natural flavonoids in hydroxyl radical-scavenging effects. Acta Pharmacol Sin 23(7):667–672
Chen CN, Wu CL, Lin JK (2004) Propolin C from propolis induces apoptosis through activating caspases, Bid and cytochrome c release in human melanoma cells. Biochem Pharmacol 67:53–66
Chen J, Liu Y, Shi YP et al (2009) Determination of flavonoids in the flowers of Paulownia tomentosa by high-performance liquid chromatography. J Anal Chem 64:282–288
Chen C-N, Hsiao C-J, Lee S-S et al (2012) Chemical modification and anticancer effect of prenylated flavanones from Taiwanese propolis. Nat Prod Res 26(2):116–124
Chen C, Chen Z, Xu F et al (2013) Radio-protective effect of catalpol in cultured cells and mice. J Radiat Res 54:76–82
Chen X, Mukwaya E, Wong M-S et al (2014) A systematic review on biological activities of prenylated flavonoids. Pharm Biol (Lond, UK) 52(5):655–660
Cho JK, Ryu YB, Curtis-Long MJ et al (2012) Cholinesterase inhibitory effects of geranylated flavonoids from Paulownia tomentosa fruits. Bioorg Med Chem 20:2595–2602
Cho JK, Curtis-Long MJ, Lee KH et al (2013) Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg Med Chem 21:3051–3057
Choi J, Shin KM, Park HJ et al (2004) Anti-inflammatory and antinociceptive effects of sinapyl alcohol and its glucoside syringin. Planta Med 70:1027–1032
Chung BH, Lee JJ, Kim JD et al (2010) Angiogenic activity of sesamin through the activation of multiple signal pathways. Biochem Biophys Res Commun 391:254–260
Cotin S, Calliste CA, Mazeron MC et al (2012) Eight flavonoids and their potential as inhibitors of human cytomegalovirus replication. Antivir Res 96:181–186
Cushnie TP, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26:343–356
Damtoft S, Jensen SR (1993) Tomentoside and 7-hydroxytomentoside, two new iridoid glucosides from Paulownia tomentosa. Phytochemistry 34:1636–1638
de Aguiar SC, Zeoula LM, Franco SL et al (2013) Antimicrobial activity of Brazilian propolis extracts against rumen bacteria in vitro. World J Microbiol Biotechnol 29:1951–1959
Deepak M, Handa SS (2000) Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother Res 14:463–465
Deliorman D, Calis I, Ergun F et al (2000) Studies on the vascular effects of the fractions and phenolic compounds isolated from Viscum album ssp. album. J Ethnopharmacol 72:323–329
Deurbina AVO, Martini ML, Fernandez B et al (1994) In vitro antispasmodic activity of peracetylated penstemoniside, aucubin and catalpol. Planta Med 60:512–515
Diaz Lanza AM, Abad Martinez MJA, Matellano L et al (2001) Lignan and phenylpropanoid glycosides from Phillyrea latifolia and their in vitro anti-inflammatory activity. Planta Med 67:219–223
Ding Y, Liang C, Yang SY et al (2010) Phenolic compounds from Artemisia iwayomogi and their effects on osteoblastic MC3T3-E1 cells. Biol Pharm Bull 33:1448–1453
Epifano F, Genovese S, Menghini L et al (2007) Chemistry and pharmacology of oxyprenylated secondary plant metabolites. Phytochemistry 68:939–953
Erbar C, Gűlden C (2011) Ontogeny of the flowers in Paulownia tomentosa—a contribution to the recognition of the resurrected monogeneric family Paulowniaceae. Flora 206:205–218
Estrada O, González-Guzmán JM, Salazar-Bookaman M et al (2011) Pomolic acid of Licania pittieri elicits endothelium-dependent relaxation in rat aortic rings. Phytomedicine 18:464–469
Fraga CG, Oteiza PI (2011) Dietary flavonoids: role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic Biol Med 51(4):813–823
Franzyk H, Jensen SR, Thale Z et al (1999) Halohydrins of antirrhinoside—the correct structures of muralioside and epimuralioside. J Nat Prod 62:275–278
Fu G, Pang H, Wong YH (2008) Naturally occurring phenylethanoid glycosides: potential leads for new therapeutics. Curr Med Chem 15:2592–2613
Fu Z, Yuskavage J, Liu D (2013) Dietary flavonol epicatechin prevents the onset of type 1 diabetes in nonobese diabetic mice. J Agric Food Chem 61:4303–4309
Funke I, Melzig MF (2005) Effect of different phenolic compounds on α-amylase activity: Screening by microplate-reader based kinetic assay. Pharmazie 60:796–797
Galvez M, Martin-Cordero C, Ayuso MJ (2006) Pharmacological activities of phenylpropanoids glycosides. Stud Nat Prod Chem 33:675–718
Ghosh A, Sarkar S, Mandal AK, Das N (2013) Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS One 8(4):1–12
Gong X, Zhang L, Jiang R et al (2014) Hepatoprotective effects of syringin on fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide in mice. J Appl Toxicol 34:265–271
Grael CFF, Vichnewski W, De Souza GEP et al (2000) A study of the trypanocidal and analgesic properties [of substances] from Lychnophora granmongolense (Duarte) Semir & Leitao Filho. Phytother Res 14(3):203–206
Grecco Sdos S, Reimão JQ, Tempone AG et al (2012) In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae). Exp Parasitol 130:141–145
Guan T, Qian Q, Tang X et al (2011) Maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycaemic rats by GLT-1 up-regulation. J Neurosci Res 89:1829–1839
Hac-Wydro K (2013) Studies on β-sitosterol and ceramide-induced alterations in the properties of cholesterol/sphingomyelin/ganglioside monolayers. Biochim Biophys Acta 1828:2460–2469
Hakim EH, Fahriyati A, Kau MS et al (1999) Artoindonesianins A and B, two new prenylated flavones from the root of Artocarpus champeden. J Nat Prod 62:613–615
Hakim EH, Asnizar Y, Aimi N et al (2002) Artoindonesianin P, a new prenylated flavone with cytotoxic activity from Artocarpus lanceifolius. Fitoterapia 73:668–673
Hakim EH, Achmad SA, Juliawaty LD et al (2006) Prenylated flavonoids and related compounds of the Indonesian Artocarpus (Moraceae). J Nat Med 60:161–184
Havsteen BH (2002) The biochemistry and medical significance of the flavonoids. Pharmacol Ther 96:67–202
He J, Hu XP, Zeng Y et al (2011) Advanced research on acteoside for chemistry and bioactivities. J Asian Nat Prod Res 13:449–464
Hidalgo M, Martin-Santamaria S, Recio I et al (2012) Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr 7:295–306
Hirano T, Higa S, Arimitsu J et al (2006) Luteolin, a flavonoid, inhibits AP-1 activation by basophils. Biochem Biophys Res Commun 340(1):1–7
Holubová P, Šmejkal K (2011) Changes in the level of bioactive compounds in Paulownia tomentosa fruits. J Liq Chromatogr Relat Technol 34:276–288
Hong D, Yang H, Jin C et al (1998) Scrophulariaceae through Gesneriaceae. Flora China 18:8–10
Horland H, Fujiwara Y, Takemura K et al (2013) Corosolic acid impairs tumor development and lung metastasis by inhibiting the immunosuppressive activity of myeloid-derived suppressor cells. Mol Nutr Food Res 57(6):1046–1054
Hošek J, Závalová V, Šmejkal K et al (2010) Effect of diplacone on LPS-induced inflammatory gene expression in macrophages. Folia Biol (Praha) 56:124–130
Hošek J, Toniolo A, Neuwirth O et al (2013) Prenylated and geranylated flavonoids increase production of reactive oxygen species in mouse macrophages but inhibit the inflammatory response. J Nat Prod 76(9):1586–1591
Huang C, Cui Y, Ji L et al (2013a) Catalpol decreases peroxynitrite formation and consequently exerts cardioprotective effects against ischemia/reperfusion insult. Pharm Biol 51:463–473
Huang D, Hu Z, Yu Z (2013b) Eleutheroside B or E enhances learning and memory in experimentally aged rats. Neural Regen Res 8:1103–1112
Huang YB, Lin MW, Chao Y et al (2014) Anti-oxidant activity and attenuation of bladder hyperactivity by the flavonoid compound kaempferol. Int J Urol 21(1):94–98
Ibrahim NA, El-Hawary SS, Mohammed MMD et al (2013) Chemical composition, antimicrobial activity of the essential oil of the flowers of Paulownia tomentosa (Thunb.) Steud. growing in Egypt. J Appl Sci Res 9(4):3228–3232
Iizuka T, Nagai M, Moriyama H et al (2005) Antiplatelet aggregatory effects of the constituents isolated from the flower of Carthamus tinctorius. Nat Med (Tokyo, Jpn) 59:241–244
Ina H, Ono M, Sashida Y et al (1987) (+)-Piperitol from Paulownia tomentosa. Planta Med 53:504
Jayaraman J, Jesudoss VA, Menon VP et al (2012) Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods 22:568–576
Jiang TF, Du X, Shi YP et al (2004) Determination of flavonoids from Paulownia tomentosa (Thunb) Steud. by micellar electrokinetic capillary electrophoresis. Chromatographia 59:255–258
Jimenéz C, Riguera R (1994) Phenylethanoid glycosides in plants: structure and biological activity. Nat Prod Rep 11:591–606
Jin L, Xue HY, Jin LJ et al (2008) Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin-induced diabetes. Eur J Pharmacol 582:162–167
Jordao CO, Vichnewski W, Petto de Souza GE et al (2004) Trypanocidal activity of chemical constituents from Lychnophora salicifolia Mart. Phytother Res 18(4):332–334
Jung S, Moon HI, Ohk J et al (2012) Inhibitory effect and mechanism on antiproliferation of isoatriplicolide tiglate (PCAC) from Paulownia coreana. Molecules 17:5945–5951
Kadota S, Basnet P, Hase K et al (1994) Matteuorienate A and B, two new and potent aldose reductase inhibitors from Matteuccia orientalis (Hook.) Trev. Chem Pharm Bull 42(8):1712–1714
Kang KH, Jang SJ, Kim BK et al (1994) Antibacterial phenylpropanoid glycosides from Paulownia tomentosa Steud. Arch Pharm Res 17:470–475
Kang KH, Huh H, Kim BK et al (1999) An antiviral furanoquinone from Paulownia tomentosa Steud. Phytother Res 13:624–626
Kang DG, Lee YS, Kim HJ et al (2003) Angiotensin converting enzyme inhibitory phenylpropanoid glycosides from Clerodendron trichotomum. J Ethnopharmacol 89:151–154
Kawamura F, Ohara S, Nishida A (2004) Antifungal activity of constituents from the heartwood of Gmelina arborea: part 1. Sensitive antifungal assay against Basidiomycetes. Holzforschung 58:189–192
Khachatoorian R, Arumugaswami V, Raychaudhuri S et al (2012) Divergent antiviral effects of bioflavonoids on the hepatitis C virus life cycle. Virology 433:346–355
Khan MK, Zill-E-Huma DO (2014) A comprehensive review on flavanones, the major citrus polyphenols. J Food Compos Anal 33(1):85–104
Khan MM, Ishrat T, Ahmad A et al (2010) Sesamin, attenuates behavioural, biochemical and histological alterations induced by reversible middle cerebral artery occlusion in the rats. Chem Biol Interact 183:255–263
Kim HJ, Woo ER, Shin CG et al (2001) HIV-1 integrase inhibitory phenylpropanoid glycosides from Clerodendron trichotomum. Arch Pharm Res 24:286–291
Kim SJ, Kwon DY, Kim YS et al (2010a) Peroxyl radical scavenging capacity of extracts and isolated components from selected medicinal plants. Arch Pharm Res 33:867–873
Kim SK, Cho SB, Moon HI et al (2010b) Neuroprotective effects of a sesquiterpene lactone and flavanones from Paulownia tomentosa Steud against glutamate-induced neurotoxicity in primary cultured rat cortical cells. Phytother Res 24:1898–1900
Kim JK, Lee YS, Kim SH et al (2011) Inhibition of aldose reductase by phenylethanoid glycoside isolated from the seeds of Paulownia coreana. Biol Pharm Bull 34:160–163
Kobayashi S, Asai T, Fujimoto Y et al (2008) Anti-herbivore structures of Paulownia tomentosa: morphology, distribution, chemical constituents and changes during shoot and leaf development. Ann Bot 101:1035–1047
Koch CE, Ganjam GK, Steger J et al (2013) The dietary flavonoids naringenin and quercetin acutely impair glucose metabolism in rodents possibly via inhibition of hypothalamic insulin signalling. Br J Nutr 109:1040–1051
Kollár P, Bárta T, Závalová V et al (2011) Geranylated flavanone tomentodiplacone B inhibits proliferation of human monocytic leukaemia (THP-1) cells. Br J Pharmacol 162(7):1534–1541
Kong LD, Wolfender JL, Cheng CH et al (1999) Xanthine oxidase inhibitors from Brandisia hancei. Planta Med 65:744–746
Koo KA, Sung SH, Park JH et al (2005) In vitro neuroprotective activities of phenylethanoid glycosides from Callicarpa dichotoma. Planta Med 71:778–780
Krishnaswamy M, Purushothaman KK (1980) Plumbagin: a study of its anticancer, antibacterial and antifungal properties. Indian J Exp Biol 18:876–877
Kumar M, Rawat P, Khan MF et al (2010) Phenolic glycosides from Dodecadenia grandiflora and their glucose-6-phosphatase inhibitory activity. Fitoterapia 81:475–479
Kumazawa S, Ueda R, Hamasaka T et al (2007) Antioxidant prenylated flavonoids from propolis collected in Okinawa, Japan. J Agric Food Chem 55(19):7722–7725
Kurkin VA (2003) Phenylpropanoids from medicinal plants: distribution, classification, structural analysis, and biological activity. Chem Nat Compd 39:123–153
Kurkin VA, Dubishchev AV, Ezhkov VN et al (2006) Antidepressant activity of some phytopharmaceuticals and phenylpropanoids. Pharm Chem J 40:614–619
Kurkina AV, Khusainova AI, Daeva ED et al (2011) Flavonoids from Tanacetum vulgare flowers. Chem Nat Compd 47:284–285
Kuzuyama T, Noel JP, Richard SB (2005) Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 435:983–987
Lee JH, Lee JY, Park JH et al (2007) Immunoregulatory activity of daucosterol, a beta-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine 25:3834–3840
Lee JS, Miyashiro H, Nakamura N et al (2008) Two new triterpenes from the rhizome of Dryopteris crassirhizoma, and inhibitory activities of its constituents on human immunodeficiency virus-1 protease. Chem Pharm Bull 56:711–714
Lee S-H, Jung MJ, Heo S-I et al (2009) Anti-inflammatory effect and HPLC analysis of extract from edible Cirsium setidens. J Korean Soc Appl Biol Chem 52:437–442
Lee JH, Lee HJ, Choung MG (2011) Anthocyanin compositions and biological activities from the red petals of Korean edible rose (Rosa hybrid cv. Noblered). Food Chem 129:272–278
Lee Y, Ryu YB, Youn H-S et al (2014) Structural basis of sialidase in complex with geranylated flavonoids as potent natural inhibitors. Acta Cryst D70:1357–1365
Li DQ, Duan YL, Bao YM et al (2004) Neuroprotection of catalpol in transient global ischemia in gerbils. Neurosci Res 50:169–177
Li R, Cai L, Xie X-f et al (2010) 7,3′-Dimethoxy hesperetin induces apoptosis of fibroblast-like synoviocytes in rats with adjuvant arthritis through caspase 3 activation. Phytother Res 24(12):1850–1856
Li Y-L, Wu L, Ouyang D-W et al (2011) Phenolic Compounds of Abies nephrolepis and their NO production inhibitory activities. Chem Biodivers 8(12):2299–2309
Li R, Cai L, Ren D-y et al (2012) Therapeutic effect of 7,3′-dimethoxy hesperetin on adjuvant arthritis in rats through inhibiting JAK2-STAT3 signal pathway. Int Immunopharmacol 14(2):157–163
Li R, Cai L, Xie X-f et al (2013) 7,3′-dimethoxy hesperetin inhibits inflammation by inducing synovial apoptosis in rats with adjuvant-induced arthritis. Immunopharmacol Immunotoxicol 35(1):139–146
Lin LC, Wang YH, Hou YC et al (2006) The inhibitory effect of phenylpropanoid glycosides and iridoid glucosides on free radical production and β2-integrin expression in human leucocytes. J Pharm Pharmacol 58:129–135
Lin J-A, Fang S-C, Wu C-H et al (2011) Anti-inflammatory effect of the 5,7,4′-Trihydroxy-6-geranylflavanone isolated from the fruit of Artocarpus communis in S100B-induced human monocytes. J Agric Food Chem 59(1):105–111
Liu KY, Wu Y-C, Liu I-M et al (2008) Release of acetylcholine by syringin, an active principle of Eleutherococcus senticosus, to raise insulin secretion in Wistar rats. Neurosci Lett 434:195–199
Loke WM, Proudfoot JM, Hodgson JM et al (2010) Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol 30:749–757
Lopez-Lazaro M (2009) Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem 9(1):31–59
Lopez-Lazaro M, Martin-Cordero C, Cortes F et al (2000) Cytotoxic activity of flavonoids and extracts from Retama sphaerocarpa Boissier. Z Naturforsch C (J Biosci) 55(1/2):40–43
Losi G, Puia G, Garzon G et al (2004) Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur J Pharmacol 502:41–46
Lu XY, Li YH, Xiao XW, Li XB (2013) Inhibitory effects of luteolin on human gastric carcinoma xenografts in nude mice and its mechanism. Zhonghua Yi Xue Za Zhi 93:142–146
Mankovskaia A, Lévesque CM, Prakki A (2013) Catechin-incorporated dental copolymers inhibit growth of Streptococcus mutans. J Appl Oral Sci 21:203–207
Martini ND, Katerere DRP, Eloff JN (2004) Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol 93(2–3):207–212
Marzocchella L, Fantini M et al (2011) Dietary flavonoids: molecular mechanisms of action as anti-inflammatory agents. Recent Pat Inflamm Allergy Drug Discov 5(3):200–220
Mastuda H, Morikawa T, Ueda K et al (2002) Structural requirements of flavonoids for inhibition of antigen-induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorg Med Chem 10(10):3123–3128
Matsubara Y, Yusa T, Sawab A et al (1991) Studies on physiologically active substances in citrus fruit peel. Part XX. Structure and physiological activity of phenyl propanoid glycosides in lemon (Citrus limon Burm. f.) peel. Agric Biol Chem 55(3):647–650
Matsuda H, Morikawa T, Ando S et al (2003) Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg Med Chem 11(9):1995–2000
Milligan SR, Kalita JC, Pocock V et al (2000) The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J Clin Endocrinol Metab 85(12):4912–4915
Min YS, Yim SH, Bail KL et al (2005) The effects of apigenin-7-O-β-D-glucuronopyranoside on reflux oesophagitis and gastritis in rats. Auton Autacoid Pharmacol 25:85–91
Mishra N, Rizvi SI (2012) Quercetin modulates Na+/K+ ATPase and sodium hydrogen exchanger in type 2 diabetic erythrocytes. Cell Mol Biol 58(1):148–152
Miyazawa M, Okuno Y, Nakamura S-I et al (2000) Antimutagenic activity of flavonoids from Pogostemon cablin. J Agric Food Chem 48(3):642–647
Moneriz G, Mestres J, Bautista JM et al (2011) Multi-targeted activity of maslinic acid as an antimalarial natural compound. FEBS J 278:2951–2961
Moon HI, Zee OP (2001) Anticancer compound of Paulownia tomentosa. Nat Prod Sci 7:21–22
Murphy BT, Cao S, Norris A et al (2005) Cytotoxic flavanones of Schizolaena hystrix from the Madagascar rainforest. J Nat Prod 68:417–419
Murphy BT, Cao S, Norris A et al (2006) Cytotoxic compounds of Schizolaena hystrix from the Madagascar rainforest. Planta Med 72(13):1235–1238
Nakano D, Kwak CJ, Fujii K et al (2006) Sesamin metabolites induce an endothelial nitric oxide-dependent vasorelaxation through their antioxidative property-independent mechanisms: possible involvement of the metabolites in the antihypertensive effect of sesamin. J Pharmacol Exp Ther 318:328–335
Nascimento IR, Murata AT, Bortoli SA et al (2004) Insecticidal activity of chemical constituents from Aristolochia pubescens against Anticarsia gemmatalis larvae. Pest Manag Sci 60:413–416
Navrátilová A, Schneiderová K, Veselá D et al (2013) Minor C-geranylated flavanones from Paulownia tomentosa fruits with MRSA antibacterial activity. Phytochemistry 89:104–113
Niu H-S, Hsu F-L, Liu I-M et al (2007) Increase of β-endorphin secretion by syringin, an active principle of Eleutherococcus senticosus, to produce antihyperglycemic action in type 1-like diabetic rats. Hormone Metab Res 39:894–898
Niu H-S, Hsu F-L, Liu I-M (2008a) Role of sympathetic tone in the loss of syringin-induced plasma glucose lowering action in conscious Wistar rats. Neurosci Lett 445:113–116
Niu H-S, Liu I-M, Cheng J-T et al (2008b) Hypoglycemic effect of syringin from Eleutherococcus senticosus in streptozotocin-induced diabetic rats. Planta Med 74:109–113
Ogungbe IV, Erwin WR, Setzer WN (2014) Antileishmanial phytochemical phenolics: molecular docking to potential protein targets. J Mol Graph Model 48:105–117
Omosa L, Amugune B, Ndunda B et al (2014) Antimicrobial flavonoids and diterpenoids from Dodonaea angustifolia. S Afr J Bot 91:58–62
Oprea E, Radulescu V, Chiliment S (2004) The analysis of the volatile and semi-volatile compounds of the Paulownia tomentosa flowers by gas chromatography coupled with mass spectrometry. Revista de chimi 55:410–412
Ota M, Azuma T, Onodera S et al (1993) The chemistry of color changes in kiri wood (Paulownia tomentosa Steud.) III. A new caffeic acid sugar ester from Kiri wood. Mozukai Gakkaishi 39:479–485
Pan J, Yuan C, Lin C et al (2003) Pharmacological activities and mechanisms of natural phenylpropanoid glycosides. Pharmazie 58:767–775
Pan J-Y, Chen S-L, Yang M-H et al (2009) An update on lignans: natural products and synthesis. Nat Prod Rep 26(10):1251–1292
Pan Y, Morikawa T, Ninomiya K et al (2010) Bioactive constituents from Chinese natural medicines. XXXVI. Four new acylated phenylethanoid oligoglycosides, Kankanosides J1, J2, K1, and K2, from stems of Cistanche tubulosa. Chem Pharm Bull 58:575–578
Pan SY, Zhou SF, Gao SH et al (2013) New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complement Altern Med 2013:1–25, doi:10.1155/2013/627375
Paneerselvam M, Kawaraguchi Y, Horikawa YT et al (2010) Effect of epicatechin and naloxone on cardioprotective phenotype. FASEB J 24 (Meeting Abstract Supplement) 1029.8
Panossian A, Wagner H (2005) Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res 19:819–838
Panossian A, Kocharian A, Matinian K et al (1998) Pharmacological activity of phenylpropanoids of the mistletoe, Viscum album, host. Pyrus caucasica. Phytomedicine 5:11–17
Papoutsi Z, Kassi E, Mitakou S et al (2006) Acteoside and martynoside exhibit estrogenic/antiestrogenic properties. J Steroid Biochem Mol Biol 98:63–71
Park KS (2013) Aucubin, a naturally occurring iridoid glycoside inhibits TNF-α-induced inflammatory responses through suppression of NF-κB activation in 3T3-L1 adipocytes. Cytokine 62:407–412
Park Y, Kong JY, Cho H (2009) A Furanquinone from Paulownia tomentosa stem for a new cathepsin K inhibitor. Phytother Res 23:1485–1488
Patel D, Shukla S et al (2007) Apigenin and cancer chemoprevention: progress, potential and promise (review). Int J Oncol 30(1):233–245
Peluso MR, Miranda CL, Hobbs DJ et al (2010) Xanthohumol and related prenylated flavonoids inhibit inflammatory cytokine production in LPS-activated THP-1 monocytes: structure-activity relationships and in Silico binding to myeloid differentiation protein-2 (MD-2). Planta Med 76(14):1536–1543
Pelzer LE, Guardia T, Juarez AO et al (1998) Acute and chronic antiinflammatory effects of plant flavonoids. Farmaco 53(6):421–424
Persson IA, Persson K, Andersson RG (2009) Effect of Vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J Agric Food Chem 57:4626–4629
Phommart S, Sutthivaiyakit P, Chimnoi N et al (2005) Constituents of the leaves of Macaranga tanarius. J Nat Prod 68:927–930
Pile JE, Navalta JW, Davis CD et al (2013) Interventional effects of plumbagin on experimental ulcerative colitis in mice. J Nat Prod 76:1001–1006
Plaza M, Pozzo T, Liu J et al (2014) Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J Agric Food Chem 62(15):3321–3333
Plouvier V (1971) The heterosides of Catalpa bignonioides Walt. (Bignoniaceae). Comp Rend Acad Sci 272(D):1443–1446
Prince Vijeya Singh J, Selvendiran K, Mumtaz Banu S et al (2004) Protective role of apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine 11:309–314
Psotová J, Chlopcíková S, Miketová P et al (2004) Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part III. Apigenin, baicalelin, kaempferol, luteolin and quercetin. Phytother Res 18:516–521
Raghukumar R, Vali L, Watson D et al (2010) Antimethicillin-resistant Staphylococcus aureus (MRSA) activity of “pacific propolis” and isolated prenylflavanones. Phytother Res 24:1181–1187
Rao YK, Lee MJ, Chen K et al (2011) Insulin-mimetic action of rhoifolin and cosmosiin isolated from Citrus grandis (L.) osbeck leaves: enhanced adiponectin secretion and insulin receptor phosphorylation in 3T3-L1 cells. Evid Based Complement Altern Med 2011:1–9, doi:10.1093/ecam/nep204
Remya C, Dileep KV, Tintu I et al (2012) Design of potent inhibitors of acetylcholinesterase using morin as the starting compound. Front Life Sci 6(3–4):107–117
Reyes-Zurita FJ, Pachón-Peña G, Lizárraga D et al (2011) The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 11:154
Rodriguez J, Yanez J, Vicente V et al (2002) Effects of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma cell lines: relationship between structure and activity. Melanoma Res 12(2):99–107
Romano B, Pagano E et al (2013) Novel insights into the pharmacology of flavonoids. Phytother Res 27(11):1588–1596
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 22:19–34
Rosselli S, Bruno M, Maggio A et al (2011) Cytotoxic geranylflavonoids from Bonannia graeca. Phytochemistry 72:942–945
Russo M, Spagnuolo C et al (2012) The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol 83(1):6–15
Salem MM, Capers J, Rito S et al (2011) Antiparasitic activity of C-geranyl flavonoids from Mimulus bigelovii. Phytother Res 25(8):1246–1249
Sánchez-Tena S, Alcarraz-Vizán G, Marín S et al (2013) Epicatechin gallate impairs colon cancer cell metabolic productivity. J Agric Food Chem 61:4310–4317
Santhakumari G, Rathinam K, Seshadri C (1978) Angicoagulant activity of plumbagin. Indian J Exp Biol 16:485–487
Sato M, Murakami K, Uno M et al (2013) Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J Biol Chem 288(32):23212–23224
Scambia G, Ranelletti FO, Panici P et al (1994) Quercetin potentiates the effect of Adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother Pharmacol 34(6):459–464
Schilling G, Hügel M, Mayer W (1982) Verbascoside and isoverbascoside from Paulownia tomentosa Steud. Zeit Naturforsch 37(B):1633–1635
Schinella G, Aquila S, Dade M et al (2008) Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya. Planta Med 74:215–220
Schneiderová K, Šlapetová T, Hrabal R et al (2013) Tomentomimulol and mimulone B: two new C-geranylated flavonoids from Paulownia tomentosa fruits. Nat Prod Res 27:613–618
Scogin R (1980) Anthocyanins of the Bignoniaceae. Biochem Syst Ecol 8:273–276
Shan JZ, Xuan YY, Ruan SQ et al (2011) Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin J Integr Med 17:607–611
Sharma U, Bala M, Kumar N et al (2012) Immunomodulatory active compounds from Tinospora cordifolia. J Ethnopharmacol 141:918–926
She G-M, Zhang Y-J, Yang C-R (2013) A new phenolic constituent and a cyanogenic glycoside from Balanophora involucrata (Balanophoraceae). Chem Biodivers 10:1081–1087
Shi Z-H, Li N-G, Tang Y-P et al (2012) Metabolism-based synthesis, biologic evaluation and SARs analysis of O-methylated analogs of quercetin as thrombin inhibitors. Eur J Med Chem 54:210–222
Shieh JP, Cheng KC, Chung HH et al (2011) Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J Agric Food Chem 59:3747–3753
Shukla S, Gupta S (2009) Role of apigenin in human health and disease. In: Preedy VR (ed) Beer in health and disease prevention, Academic Press, San Diego, pp e202–e216
Si C, Deng X, Liu Z (2008a) Structure and activity relationship of antioxidant flavonoids from leaves of Paulownia tomentosa var. tomentosa. In: 2nd international papermaking and environment conference, Tianjin University of Science and Technology, Tianjin, pp 263–266
Si CL, Deng XJ, Liu Z et al (2008b) Studies on the phenylethanoid glycosides with anti-complement activity from Paulownia tomentosa var. tomentosa wood. J Asian Nat Prod Res 10:1003–1008
Si C, Deng X, Xu Q et al (2008c) Characterization of phenolic acids and antioxidant activities of Paulownia tomentosa var. tomentosa leaves. In: Proceedings of the international conference on pulping, papermaking and biotechnology, pp 31–33
Si CL, Liu Z, Kim JK et al (2008d) Structure elucidation of phenylethanoid glycosides from Paulownia tomentosa Steud. var. tomentosa wood. Holzforschung 62:197–200
Si CL, Wu L, Zhu ZY et al (2009) Apigenin derivates from Paulownia tomentosa Steud. var. tomentosa stem barks. Holzforschung 63:440–442
Si CL, Lu YY, Hu HY et al (2011a) Evaluation of total phenolics, flavonoids and anti-inflammatory property of ethanolic extracts of Paulownia tomentosa var. tomentosa bark. Planta Med 77:SL53
Si CL, Lu YY, Qin PP et al (2011b) Phenolic extractives with chemotaxonomic significance from the bark of Paulownia tomentosa var. tomentosa. BioResources 6:5086–5098
Si CL, Shen T, Jiang YY et al (2013) Antioxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cells. Food Chem Toxicol 59:145–152
Sivakumar G, Vail DR, Nair V et al (2009) Plant-based corosolic acid: future anti-diabetic drug? Biotechnol J 4:1704–1711
Šmejkal K (2014) Cytotoxic potential of C-prenylated flavonoids. Phytochem Rev 13:245–275
Šmejkal K, Grycová L, Marek R et al (2007a) C-geranyl compounds from Paulownia tomentosa Fruits. J Nat Prod 70:1244–1248
Šmejkal K, Holubová P, Zima A et al (2007b) Antiradical activity of Paulownia tomentosa (Scrophulariaceae) extracts. Molecules 12:1210–1219
Šmejkal K, Babula P, Šlapetová T et al (2008a) Cytotoxic activity of C-geranyl compounds from Paulownia tomentosa fruits. Planta Med 74:1488–1491
Šmejkal K, Chudík S, Klouček P et al (2008b) Antibacterial C-geranylflavonoids from Paulownia tomentosa (Scrophulariaceae) fruits. J Nat Prod 71:706–709
Šmejkal K, Svačinová J, Šlapetová T et al (2010) Cytotoxic activities of several geranyl-substituted flavanones. J Nat Prod 73:568–572
Sticher O, Lahloub MF (1982) Phenolic glycosides of Paulownia tomentosa bark. Planta Med 46:145–148
Sudsai T, Wattanapiromsakul C, Tewtrakul S (2013) Inhibition of nitric oxide production by compounds from Boesenbergia longiflora using lipopolysaccharide-stimulated RAW264.7 macrophage cells. Songklanakarin J Sci Technol 35(3):317–323
Sumsakul W, Plenqsuriyakarn T, Chaijaroenkul W et al (2014) Antimalarial activity of plumbagin in vitro and in animal models. BMC Complement Altern Med 14:15
Sun Y, Zang Z, Zhong L et al (2013) Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay. PLos One 8(5):e63354
Suolinna EM, Buchsbaum RN, Racker E (1975) Effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res 35(7):1865–1872
Takahashi K, Nakagawa T (1966) Studies on constituents of medicinal plants. VII. The stereochemistry of paulownin and isopaulownin. Chem Pharm Bull 14:641–647
Takamatsu S, Galal AM, Ross SA et al (2003) Antioxidant effect of flavonoids on DCF production in HL-60 cells. Phytother Res 17(8):963–966
Tanaka J, Kadekaru T, Ogawa K et al (2013) Maqui berry (Aristotelia chilensis) and the constituent delphinidin glycoside inhibit photoreceptor cell death induced by visible light. Food Chem 139:129–137
Tang R, Chen K, Cosentino M et al (1994) Apigenin-7-O-β-D-glucopyranoside, an anti-HIV principle from Kummerowia striata. Bioorg Med Chem Lett 4:455–458
Taub PR, Ramirez-Sanchez I, Ciaraldi TP et al (2012) Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: effects of epicatechin rich cocoa. Clin Transl Sci 5(1):43–47
Teixeira MD, Souza CM, Menezes AP et al (2013) Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol Biochem Behav 110:1–7
Tian L-W, Pei Y, Zhang Y-J et al (2009) 7-O-methylkaempferol and -quercetin glycosides from the whole plant of Nervilia fordii. J Nat Prod 72(6):1057–1060
Tohda C, Ichimura M, Bai Y et al (2008) Inhibitory effects of Eleutherococcus senticosus extracts on amyloid β(25–35)-induced neuritic atrophy and synaptic loss. J Pharmacol Sci 107:329–339
Tozuka H, Ota M, Kofujita H et al (2005) Synthesis of dihydroxyphenacyl glycosides for biological and medicinal study: β-oxoacteoside from Paulownia tomentosa. J Wood Sci 51:48–59
Trusheva B, Popova M, Koendhori EB et al (2011) Indonesian propolis: chemical composition, biological activity and botanical origin. Nat Prod Res 25:606–613
Ullevig SL, Zhao Q, Zamora D et al (2011) Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis 219:409–416
Vareed SK, Reddy MK, Schutzki RE (2006) Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with healt benefits. Life Sci 78:777–784
Vasconcelos FC, Gattass CR, Rumjanek VM et al (2007) Pomolic acid-induced apoptosis in cells from patients with chronic myeloid leukemia exhibiting different drug resistance profile. Invest New Drugs 25:525–533
Wang J, Yang Z, Lin L et al (2012) Protective effect of naringenin against lead-inuced oxidative stress in rats. Biol Trace Elem Res 146:354–359
Wang Y-M, Xu M, Wang D et al (2013) Anti-inflammatory compounds of “Qin-Jiao”, the roots of Gentiana dahurica (Gentianaceae). J Ethnopharmacol 147(2):341–348
Wei YJ, Tsai KS, Lin LC et al (2011) Catechin stimulates osteogenesis by enhancing PP2A activity in human mesenchymal stem cells. Osteoporos Int 22(5):1469–1479
Weidmann AE (2012) Dihydroquercetin: more than just an impurity? Eur J Pharmacol 684(1–3):19–26
Wilkinson K, Boyd JD, Glicksman M et al (2011) A high-content drug screen identifies ursolic acid as an inhibitor of amyloid-β interactions with its receptor CD36. J Biol Chem 286:34914–34922
Wollenweber E, Wehde R, Christ M et al (2008) Surface flavonoids in Catalpa ovata, Greyia sutherlandii and Paulownia tomentosa. Nat Prod Commun 3:1285–1287
Wong KC, Haq Ali DM, Boey PL (2012) Chemical constituents and antibacterial activity of Melastoma malabathricum L. Nat Prod Res 26:609–618
Wu A, Lin C, Zhao X et al (2012) Spectroscopic study on interaction between cistanoside F and bovine serum albumin. Zhongguo Zhong Yao Za Zhi 37:1392–1398
Xiong Q, Kadota S, Tani T et al (1996) Antioxidative effects of phenylethanoids from Cistanche deserticola. Biol Pharm Bull 19:1580–1585
Xiong QB, Hase K, Tezuka Y et al (1998) Hepatoprotective activity of phenylethanoids from Cistanche deserticola. Planta Med 64:120–125
Xue HY, Lu YN, Fang XM et al (2012) Neuroprotective properties of aucubin in diabetic rats and diabetic encephalopathy rats. Mol Biol Rep 39:9311–9318
Yang Y-L, Hsu H-T, Wang K-H et al (2011) Hesperetin-7,3′-O-dimethylether selectively inhibits phosphodiesterase 4 and effectively suppresses ovalbumin-induced airway hyperresponsiveness with a high therapeutic ratio. J Biomed Sci (Lond, UK) 18:84
Yang X, Yuan J, Wan J (2012) Cytotoxic phenolic glycosides from Boschniakia himalaica. Chem Nat Comp 48(4):555–558
Yasuda K, Sakaki T (2012) How is sesamin metabolised in the human liver to show its biological effects? Expert Opin Drug Metab Toxicol 8:93–102
Yazaki K, Sasaki K, Tsurumaru Y (2009) Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 70:1739–1745
Yoder BJ, Cao S, Norris A et al (2007) Antiproliferative prenylated stilbenes and flavonoids from Macaranga alnifolia from the Madagascar rainforest. J Nat Prod 70:342–346
Yoo DY, Choi JH, Kim W, Nam SM, Jung HY, Kim JH, Won M-H, Yoon YS, Hwang IK (2013) Effects of luteolin on spatial memory, cell proliferation, and neuroblast differentiation in the hippocampal dentate gyrus in a scopolamine-induced amnesia model. Neurol Res 35:813–820
Yoon JS, Chae MK, Jang SY, Lee SY, Lee EJ (2012) Antifibrotic effects of quercetin in primary orbital fibroblasts and orbital fat tissue cultures of graves' orbitopathy. Invest Ophth Vis Sci 53:5921–5929
Yoshikawa M, Matsuda H, Morikawa T et al (2006) Phenylethanoid oligoglycosides and acylated oligosugars with vasorelaxant activity from Cistanche tubulosa. Bioorg Med Chem 14:7468–7475
Yuan ZL, Luo L, Zang AM et al (2009) Isolation and bioassay of herbicidal active ingredient from Paulownia tomentosa. Chin J Pestic Sci 2:239–243
Yun B-S, Lee I-K, Kim J-P et al (2000) Lipid peroxidation inhibitory activity of some constituents isolated from the stem bark of Eucalyptus globulus. Arch Pharm Res 23(2):147–150
Yun J, Bae H, Choi SE et al (2013) Taxifolin glycoside blocks human ether–a–go–go related gene K(+) channels. Korean J Physiol Pharmacol 17(1):37–42
Zhang SM, Coultas KA (2013) Identification of plumbagin and sanquinarine as effective chemotherapeutic agents for treatment of schistosomiasis. Int J Parasitol Drugs Drug Resist 3:28–34
Zhang DL, Li XQ (2011) Studies on the chemical constituents from the leave of Paulownia tomentosa. Zhong Yao Cai 34:232–234
Zhang W, Zhang W-D, Zhang C et al (2007) Antitumor activities of extracts and compounds from the roots of Daphne tangutica Maxim. Phytother Res 21(11):1113–1115
Zhang J, Chen J, Liang Z et al (2014) New lignans and their biological activities. Chem Biodivers 11(1):1–54
Zhao J, Zhou X-W, Chen X-B et al (2009) α-Glucosidase inhibitory constituents from Toona sinensis. Chem Nat Compd 45:244–246
Zhao J, Ding HX, Wang CM (2012) Isolation, modification and cytotoxic evaluation of flavonoids from Rhododendron hainanense. J Pharm Pharmacol 64:1785–1792
Zheng J, Liu D, Zhao SQ et al (2012) Enzymatic extraction and antibacterial activity from Eucommia ulmoides leaves. Zhong Yao Cai 35:304–306
Zhu ZH, Chao CJ, Lu XY et al (1986) Paulownia in China: cultivation and utilization. Asian Network for Biological Science and International Development Research Centre, Chinese Academy of Forestry, Beijing. http://idl-bnc.idrc.ca/dspace/bitstream/10625/8226/1/71235.pdf. (Cited 13 Mar 2013)
Zima A, Hošek J, Treml J et al (2010) Antiradical and cytoprotective activities of several C-geranyl-substituted flavanones from Paulownia tomentosa Fruit. Molecules 15:6035–6049
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneiderová, K., Šmejkal, K. Phytochemical profile of Paulownia tomentosa (Thunb). Steud.. Phytochem Rev 14, 799–833 (2015). https://doi.org/10.1007/s11101-014-9376-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-014-9376-y