Abstract
Protein hydrolysates prepared from visceral parts of fish contains many peptides with better nutritional and functional properties. In this study quality of spray dried visceral protein hydrolysates extracted from Pangasius processing waste using enzymatic and chemical treatments were studied and its effects on the yield, chemical composition, color and functional properties were evaluated. Among the fish visceral protein hydrolysates extracted with different treatments, i.e. by using papain, pepsin, acid and alkali, it was observed that the treatment with pepsin demonstrated the better results followed by papain, acid and alkali treatments. Pepsin significantly (p < 0.05) had the higher degree of hydrolysis (65.16 ± 3.45%), protein content (78.55 ± 1.81%), L* value (78.62 ± 0.05), hue angle (93.02 ± 0.12), water holding capacity (0.84 ± 0.03 mL/g) oil absorption capacity (1.57 ± 0.04 mL/g) emulsion stability index (87.98 ± 2.13 min) and second highest yield of 5.6%. The maximum yield of 5.8% of hydrolysate was observed with papain treatment. The color of hydrolysates varied from lighter to less yellow color with better emulsification properties depending upon the treatment. The spray dried and enzymatically extracted hydrolysate had lower turbidity with increasing pH, lowest solubility, foaming capacity and stability were observed at pH 5.0. Present study indicated the possibility of extracting peptides with good functional properties by enzymatic treatments. The content of bioactive peptides could make the Pangasius visceral hydrolysate prepared with pepsin enzyme suitable for protein extraction and use for incorporation in food for increasing its functional properties.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish can serve as a source of several functional materials, such as protein, polyunsaturated fatty acids, minerals, vitamins, antioxidants, enzymes and bioactive peptides. The fish processing operations such as stunning of fish, grading, removal of slime, descaling, washing, beheading, gutting, cutting of fins, slicing into steaks, filleting, meat bone separation, packaging, labeling and distribution leads to generation of by-products such as scales, heads, skin, viscera and roe in large quantities [1]. Gutting of fish involves the removal of internal organs and cleaning the body cavity of peritoneum, kidney tissue and blood which contribute around 5–8% of the fish weight [2]. Most of the time, these waste are thrown in to nearby water bodies, land fillings sites and creating the environmental problems [3]. Reorganization of the limited biological resources and increasing environmental pollution has emphasized the need for better and proper utilization of the underutilized fish and the by-product from the fishing industries through value addition [4]. These protein rich products have a range of dynamic properties that can potentially be used in food system as binder, emulsifiers and gelling agents [5]. Improving the functional properties of these proteins, including solubility, water holding capacity, oil binding capacity, emulsifying and foaming characteristics are major challenges in food science. One alternative is to produce a form of concentrated protein product that may be used as food ingredients due to the capability of their functional properties [6]. Acid and enzymatic hydrolysis is an alternative approach to recover biomass from marine origin which results in a soluble product known as fish protein hydrolysate (FPH). The soluble hydrolysate is subjected to dehydration resulting in a more stable powdered form with high protein content [4].
For the preparation of protein hydrolysates chemical and biological method can be used. Chemical hydrolysis of protein was considered as a conventional method for producing protein hydrolysate by using either acid or alkali for breaking peptide bonds under high pressure and temperature conditions. Chemical extraction methods are cheaper and consume lesser time but extent of hydrolysis and the quality of product are difficult to control due to its harsh unspecific peptide bond cleaving [7]. Enzymatic hydrolysis of proteins allows preparation of bioactive peptides and these can be obtained by in-vitro hydrolysis of protein sources using appropriate proteolytic enzymes. The physico-chemical conditions of the reaction media, such as temperature and pH of the protein solution, must be adjusted in order to optimize the activity of the enzyme used. Proteolytic enzyme from microbes, plants and animals can be used for the hydrolysis process of marine proteins to develop bioactive peptides [8]. These bioactive peptides generally contain 2–20 amino acid units and inactive within the sequences of their parent protein but can be released by enzymatic hydrolysis [9]. Preparation of protein hydrolysate from fish and shellfish waste among the use of different chemicals, endogenous enzyme alone or in combination with, enzyme substrate ratio, optimization of hydrolysis condition and studied on their function properties were reported by different studies. Guerard et al. [4], Liceaga-Gesualdo and Li-Chan [6], Aspmo et al. [10], Bhaskar and Mahendrakar [11], Ovissipour et al. [12], Ovissipour et al. [13] and Nazeer and Kumar [14].
Pangasius become a candidate species for culture as it grows fast and is a hardy species. The production of Pangasius has increased tremendously in India and as a result its proper utilization has become need of hour. Preparation of hydrolysate from Pangasius viscera would provide a better utilization of the by-products traditionally used for pet food or fish feed industries. Protein hydrolysis with strong chemical and solvent is performed at extreme temperature and pH, generally yields a produce with reduced nutritional qualities, poor functionally and hence restricted in use as flavor enhancers [15]. Thus enzymatic modification of protein using selected proteolytic enzyme to cleave specific peptide bonds is more frequently used in food industries to produce hydrolysate with excellent functional properties [16]. Digestion parameters such as time, temperature and pH are tightly controlled to produce FPH with the desired functional and nutritional properties, balanced amino acid composition and high digestibility. Protein hydrolysate is produced for a wide variety of uses in the food industries including milk replacers and flavor enhancers in confectionary products. Therefore the investigation has been undertaken to study the effect of both the chemical and enzymatic hydrolysis on the quality of FPH from Pangasius viscera.
Materials and Methods
Materials
Pangasius viscera were collected from local fish seller of Four Bungalows fish market, Mumbai, India. This waste was packed in a polyethylene bag, with ice 1:1 ratio and brought to the laboratory where it was washed, packed in polyethylene bags and stored at − 18 °C until further use.
Preparation of Enzymatic Hydrolysate from Viscera
Protein hydrolysate extraction was carried out according to Jiang et al. [17] with slight modification. Frozen viscera was defrosted at room temperature, grinded and mixed in distilled water at a ratio of 1:2 (w/v), and placed in boiling water for 10 min to ensure deactivation of endogenous enzymes [4, 12]. The protein was digested with two kinds of enzymes at an enzyme/substrate ratio of 1:100 (w/w). Temperature and pH were adjusted to optimum values for each enzyme [papain (T1) − 55 °C, pH 7.0 and pepsin (T2) − 37.5 °C, pH 2.0] and the mixture was incubated under continuous stirring for 5 h and then heated in water bath (Shanti, Scientific industries, Mumbai, India) at 90 °C for 15 min to ensure enzyme deactivation, and then pH (Eutech tutor pH/°C meter, Eutech Instruments, Singapore) was adjusted to 7.0. The content was then centrifuged (Eltek, RC 41000F, Elektrocraft, Mumbai, India) at 10,000 × g for 30 min at 4 °C. Collected supernatant was then spray dried (SM Scitech, Calcutta, India) at 180 °C inlet temperature to get powder and stored at − 18 °C (Blue star, Mumbai, India) till further use.
Preparation of Hydrolysate from Viscera Using Hydrochloric Acid
Acid protein hydrolysate extraction was carried out by Tsugita and Scheffler [18] method with slight modification (T3). Frozen viscera was defrosted at room temperature and then mixed thoroughly in distilled water with the ratio of 1:2 (w/v) and a pH of 2.0 was maintained with the addition of 6 N HCl, at 110 °C for 24 h. After completing hydrolysis process, pH was adjusted to 7.0. The content was then centrifuged at 10,000 × g for 30 min at 4 °C. Collected Supernatant was then spray dried at 180 °C inlet temperature to get powder and stored at − 18 °C till further use.
Preparation of Hydrolysate from Viscera Using Alkali
Alkali protein hydrolysate extraction was carried out by Tannenbaum et al. [19] method with slight modification (T4). Frozen viscera was thawed at room temperature and further homogenized in distilled water at 1:2 (w/v) ratio and maintained at pH 12.5 with 10 N NaOH and kept at 95 °C for 20 min. After completing hydrolysis process, pH was adjusted to 7.0. The content was then centrifuged at 10,000 × g for 30 min at 4 °C. Collected supernatant was then spray dried at 180 °C inlet temperature to get powder and stored at − 18 °C till further use.
Degree of Hydrolysis
Degree of hydrolysis (DH) is defined as the percentage of free amino groups cleaved from protein, which was calculated from the percent ratio of α-amino nitrogen (AN) and the total protein nitrogen (TPN). The AN was determined by a formal titration method [20]. The spray dried hydrolysate was dissolved in 50 mL deionized water with a concentration of 0.08 mg/mL and titrated against 0.04 M NaOH till its pH value reached 8.2. Then 10 mL of formaldehyde aqueous solution (20% v/v) was added to the solution which was fully mixed and titrated again with 0.04 M NaOH till its pH value reached 9.2. The volume (mL) of 0.04 M NaOH consumed was recorded as V1. Similarly a blank experiment was done with deionized water instead of sample and the volume (mL) of 0.04 M NaOH consumed was recorded as V0. Alpha amino nitrogen content was calculted with the following formula:
where C is the concentration (mol/L) of NaOH used, M is the mass (g) of sample used, 0.014 represents the mass (g) of nitrogen which is equivalent to 1 mL of 1 M NaOH. Protein was determined by Biuret method [21]. The degree of hydrolysis was calculated as per Cao et al. [22].
Yield Percentage
After solublization of viscera by enzyme and chemicals, protein hydrolysates were extracted by centrifugation and then spray dried to obtain visceral protein hydrolysate in powder form. The protein powder was weighed and the amount of protein recovered as yield. Hydrolysate yield was calculated as g of protein hydrolysate per 100 g of viscera. The yield of protein hydrolysate was calculated based on wet weight of viscera of fish using the following formula.
Proximate Composition
The moisture, ash and fat content of fish viscera and extracted hydrolysate were determined according to the method of AOAC 2005 [23]. The moisture content was determined by drying samples at 102 ± 2 °C for 16–18 h until constant weight was obtained. The nitrogen content of the samples was determined using the Kjeldahl method with a distillation unit (Pelican, Kelplus, India). A factor of 6.25 was used to convert the nitrogen value to crude protein content. Crude lipid content was determined by Soxhlet method using petroleum ether as solvent. Ash content was determined by incinerating samples at 600 °C for 6 h.
Color Measurements
The color of the hydrolysate powder was measured by using Lab scan XE–Colorimeter (ColorFlex, Hunter Associates Laboratory, Reston, VA, USA) which gives acceptable level based on L*, a* and b* values. It was standardized under “C” illuminant condition according to the Commission International de I’ Eclairage (CIE) using a standard reference tiles. From the values the chroma and hue angle were calculated as follows [24]:
Turbidity Measurements
The turbidity of hydrolysate was measured by using procedure of Aspmo et al. [10].The pH of the hydrolysate was adjusted in the range of 3–10 using NaOH or HCl and the solutions were then diluted to 1% (w/v) in MilliQ-purified water. All solutions were allowed to react for 30 min at room temperature. Aggregation in the different solutions was estimated by turbidity measurements at 410 nm in Spectrophotometer (UV-PLUS Shimadzu, Kyoto, Japan). All measurements were done in triplicate and samples containing only MilliQ water were used as blanks.
where A is the Absorbance and l is the path length of the cuvette.
Functional Properties
Water Holding Capacity (WHC)
The water holding capacity (WHC) was determined using the method described by Rodriguez-Ambriz et al. [25]. Protein samples (100 mg) were mixed with 1000 µL of distilled water using a stirrer. The protein suspension was then centrifuged at 1800 × g for 20 min at 22 °C. The supernatant was decanted, and the tube was drained at a 45°angle for 10 min.
Oil Absorption Capacity (OAC)
OAC was determined using the method described by Lin and Zayas [26] 100 mg of protein sample was vortexed with 1000 µL of sunflower oil for 30 s. The resulting emulsion was incubated at room temperature for 30 min, and then centrifuged at 13,600 × g for 10 min at 25 °C. The supernatant was decanted and drained at a 45° angle for 20 min. The volume of oil absorbed equals the sample’s oil absorption capacity.
Emulsifying Properties
Emulsifying properties were determined according to the method of Pearce and Kinsella [27]. Vegetable oil and 1% protein solution were mixed in 1:3 ratio and homogenized at 20,000 rpm for 1 min (Polytron, PT-MR 2100, Switzerland). An aliquot of the emulsion (50 µL) was pipette at 0 and 10 min from the bottom and mixed with 5 mL of 0.1% sodium dodecyl sulphate (SDS) solution. The absorbance of the diluted solution was measured for 0 (A0) and 10 min (A10) at 500 nm using a Spectrophotometer (Meu, Quant Biotek, Winoski, USA). The emulsifying activity index (EAI) and the emulsion stability index (ESI) where calculated as follows [27]:
where ΔA = A0 − A10 and Δt = 10 min
Solubility
Solubility of FVPH was determined according to the method of Taheri et al. [28]. To determine protein solubility, 200 mg of protein hydrolysate sample were dispersed in 20 mL of deionized water and pH of the mixture was adjusted to 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 with 1 or 6 N HCl and 1 or 6 N NaOH. The mixture was stirred at room temperature for 30 min and centrifuged at 7500 × g for 15 min. Protein contents in the supernatant were determined using the Biuret method [21]. Total protein content in the sample was determined after solubilization of the sample in 0.5 N NaOH. Protein solubility was calculated as follows:
Foaming Properties
Foaming capacity and stability of protein hydrolysate were determined according to the method of Sathe and Salunkhe [29]. 20 mL of 0.5% sample solution were adjusted to pH 2, 4, 6, 8 and 10, followed by homogenization at a speed of 1600 rpm, using a homogenizer (Polytron PT2100, Kinematica AG, Switzerland) to incorporate the air for 2 min at room temperature. The whipped sample was immediately transferred into a 25 mL cylinder and the total volume was read after 30 s. The foaming capacity was calculated according to the following Equation [29]:
where A is the volume after whipping (mL), B is the volume before whipping (mL).
The whipped sample was allowed to stand at 20 °C for 3 min and the volume of whipped sample was then recorded. Foam stability was calculated as follows:
where A is the volume after standing (mL), B is the volume before whipping (mL).
Statistical Analysis
All assays for chemical, physical and functional properties were conducted in triplicates and the data were presented as the mean ± standard deviation. Statistical analysis was performed with SPSS-16. Difference was considered significant at p < 0.05.
Results and Discussion
Degree of Hydrolysis (DH)
Degree of hydrolysis of Pangasius visceral protein hydrolysate is given in Table 1. Maximum DH was found with pepsin treated sample (65.16%) followed by papain (61%), acid (60.84%) and least in alkali hydrolysate (15.38%) respectively. Soussi et al. [30] observed that hydrolysate prepared from sardine (Sardinella aurita) head and viscera with spray drying had increased DH values of 6.92, 9.31 and 10.16% with increased time of hydrolysis in the presence of Alcalase. According to Mackie [31], the enzyme will be absorbed into the suspended particles, where the hydrolysis of enzyme-susceptible peptide linkages will take place simultaneously. Wishuthiphaet and Kongruang [32] reported on papain and acid hydrolysis of fish with 30–35% degree of hydrolysis in acid and 20–24% in papain whereas Abdulazeez et al. [33] reported 22.2% DH with 1% papain digestion from king fish of Arabian Gulf coast. Tanuja et al. [15] reported the use of papain and Bromelain with 0.5% (w/w) concentration for digestion of frame meat of Pangasianodon hypophthalmus and found the DH value 10% in papain and 8% in bromelain induced hydrolysate. According to Blenford [34] acid hydrolysis of protein is used more commonly than hydrolysis under alkaline condition. Wisuthiphaet et al. [32] hydrolyzed low cost marine fish with 4, 6 and 8 M HCl and 2, 4 and 6% papain and reported DH as 30–35% in acid and 20–24% in papain.
Yield of Protein Hydrolysate
The extraction of FPH from Pangasius viscera was carried out using two different enzymes and compared with acid and alkali hydrolysis. Yield of protein hydrolysate is shown in Table 1. Degree of hydrolysis is related with the yield of protein hydrolysate. It was observed that Pangasius viscera hydrolyzed more in papain with the yield of 5.8% followed by pepsin (5.6%), acid (4.76%) and alkali (3.3%). Souissi et al. [30] observed the yield of 4.23% for protein hydrolysate obtained from sardine fish head and viscera which is lower than the yield of hydrolysate obtained with enzyme and acid treatment in the present investigation. Papain enzyme gave better yield possibly due to better degree of hydrolysis. Similarly Tanuja et al. [15] studied on striped catfish hydrolysate and found 6% yield in papain and 5.5% with bromelian. Hoyle and Merrit [35] also reported yield ranging from 3.6 to 5.5% for the hydrolysate obtained by alcalase treatment of raw material of mince hearing fillets and hearing defatted by ethanol extraction, cooking and pressing. Typical yields of FPH have been reported to be 10–15%, based on fresh fish substrates [16].
Proximate Composition of Viscera and Hydrolysate
Proximate composition of Pangasius viscera and its respective spray dried hydrolysate are given in Table 2. Viscera had moisture content of 73.30 ± 0.60%; protein 15.61 ± 1.88%; fat 8.48 ± 1.21% and ash 1.77 ± 0.54% respectively. Protein is the main component in the preparation of hydrolysate and as Pangasius viscera contained 15.61% protein, it was used for extraction of protein hydrolysate with different treatments in the present investigation. Pepsin hydrolysate gave the highest protein (82.55 ± 1.81%) followed by papain (78.14 ± 1.18%) and acid treatment (69.13 ± 0.00%) and lowest in alkali hydrolysate with 57.86 ± 0.97% of protein. The results of protein content found in the present investigation for papain and pepsin hydrolysate were similar to other researchers [12, 30, 33, 35,36,37]. Pangasius visceral protein hydrolysate had maximum fat i.e, 13.64 ± 0.27% in alkali protein hydrolysate followed by 10.61 ± 1.11% in acid protein hydrolysate, 7.51 ± 0.17% in papain and 5.12 ± 0.16% in pepsin protein hydrolysate. Moisture was 3–4% in spray dried visceral hydrolysate and highest ash content i.e 23.69 ± 0.16% was reported in alkali visceral protein hydrolysate followed by 15.91 ± 0.31% in acid visceral protein hydrolysate and 11.37 ± 1.52 in papain visceral protein hydrolysate. The lowest ash of 8.86 ± 0.31 was reported in pepsin visceral protein hydrolysate. 70.0 ± 1.9% protein, 14.95 ± 0.5 fat, 8.2 ± 0.6% moisture and 6.9 ± 0.2% ash got in papain treated Cirrhinus mrigala egg protein hydrolysate [38]. Mukin et al. [39] found 81.3% protein, 0.56% fat, 5.55% moisture and 12.6% ash in processing waste hydrolysate of Salmon salar muscle. High protein content in FPH is due to removal of insoluble material during hydrolysis and solubilization of proteins during hydrolysis. High quantity of Lipid in protein hydrolysate may be on account of the nature of substrate with varying content of lipid in the sample and removal of lipids with insoluble protein fraction by centrifuge, low moisture content, high temperature used during process of evaporation and spray drying. Ash content in protein hydrolysate was reported by many researcher which ranged between 0.45 and 27% of total composition. High ash content of FPH is due to addition of acid and base for adjusting the pH of protein slurry [38].
Color of Spray Dried Powder
Color parameters of hydrolysate are important aesthetic and physical properties depending on the application for which the hydrolysate is intended. Instrumental color measurements of different hydrolysates are depicted in Table 3. The color of hydrolysate depends on the raw materials used and method of extraction [37]. Visceral hydrolysates were significantly yellowish to darker in appearance with minimal fishy odor and taste. It was observed that the different treatments of spray dried hydrolysates resulted in different lightness (L*) values varied from 78.62 ± 0.05 to 57.62 ± 1.26, redness (a*) − 1.27 ± 0.05 to 5.24 ± 0.13 and yellowness (b*) 23.66 ± 0.74 to 31.91 ± 0.09, chroma, 23.83 ± 0.75 to 32.22 ± 0.09 and H° 86.51 ± 0.21 to 93.02 ± 0.12. Pangasius visceral hydrolysates had significantly (p < 0.05) different coloration. According to Bueno-Solano et al. [40] color influences the overall acceptability of food products and is affected by several factors such as species, processing, fat content, moisture, light, temperature, hemoglobin, myoglobin and new protein ingredients in food formulation. Color parameter of cobia frame spray dried protein hydrolysate for L*, a* and b* were in the range of 59.7–61.7, − 0.42 to − 0.58 and 4.57–5.72 respectively [37]. Color data showed that visceral protein hydrolysate of spray dried have more yellowish color and a pronounced fishy odor and test. Pangasius viscera have different section of digestive tract that contains high colorant pigments. More light and yellowish color of visceral protein hydrolysate may be due to the higher levels of hemoglobin, myoglobin and other pigments that are found in the digestive tract that remained soluble after centrifugation [41].
Turbidity
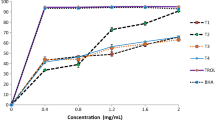
The results of turbidity as effect of pH on the different chemically and enzymaticaly extracted hydrolysate are shown in the Fig. 1. From the results, it can be seen that turbidity of protein hydrolysate is pH dependent and hydrolysate prepared from different treatments apparently had quite similar trend for the turbidity values. It was observed from the results that, with increasing pH from 3.0 to 10 of the samples, turbidity was decreasing. This result supports the protein solubility of hydrolysate which has inverse relationship with turbidity. Turbidity and pH had inverse relation between them and also inverse relation between the turbidity of hydrolysate and degree of solubilization of dry matter in the supernatant of the hydrolysate [10]. They also found lowest overall turbidity, with highest solubilization and endogenous activity gives vise-versa or opposite result. Turbidity is one of the ways of detecting possible formations of peptide aggregation in hydrolysate [42].
Functional Properties
Functional properties defined as “those functional and chemical properties which affect the behavior of proteins in food systems during processing storage and preparation and consumption” [43]. The functional properties of FPHs are important, particularly if they are used as ingredients in food products. One of the major advantages and goals of enzymatically hydrolyzing fish proteins is to modify and improve their functional properties. The functional properties of protein in a food system depend in part on the water holding capacity which refers to the ability of proteins to imbibe water and retain it against a gravitational force within protein matrix [44]. Enzymatic hydrolysis of fish proteins generate a mixture of free amino acids di-, tri-, and oligopeptides, increases the number of polar groups and the solubility of the hydrolysate, and therefore modifies functional characteristics of the proteins improving their functionality and bioavailability [45]. Chemical and enzymatic hydrolysis of food proteins results in protein hydrolysate which produces water holding capacity, oil binding capacity, emulsifying properties, solubility and foaming properties at different pH.
Water Holding Capacity (WHC)
In this study, it was observed that water holding capacity of Pangasius visceral hydrolysates prepared with different treatments were significantly different (p < 0.05). Water holding capacity values of papain, pepsin, acid and alkali hydrolysate were 0.8 ± 0.08, 0.84 ± 0.03, 0.71 ± 0.09 and 0.89 ± 0.8 (mL/g hydrolysate) respectively (Table 4). The values for water holding capacity for different hydrolysate in the present investigation are comparatively lower than the WHC reported by other researchers [28, 46, 47]. Functional properties of proteins in a food system depend in part on the water–protein interaction and the final outcome greatly depends on how well the protein binds and holds water in a food system. Water holding capacity refers to the ability of protein to imbibe water and retain it against gravitational force within a protein matrix, such as protein gels beef or fish muscle, and it is positively correlated with water binding capacity [48]. Diniz and Martin [46] also reported that, there are no any correlation in between DH and WHC.
Oil Absorption Capacity (OAC)
Oil absorption capacity is an important functional properties of an ingredients used in meat and confectionary product [49]. The mechanism for this is attributed to the combination of physical entrapment of oil and the hydrophobicity of material. Hydrophobicity of fish processing co-product protein hydrolysate develops because hydrolysis cleaves the protein chain so more internal hydrophobic groups are exposed [44]. Oil absorption capacity depends on several factors and may affect the ability of hydrolysate to bind fat, such as bulk density of protein, degree of hydrolysis, enzyme substrate specificity. An OAC of 1.08 ± 0.12, 1.03 ± 0.04, 1.20 ± 0.12 and 1.39 ± 0.05 (mL/g hydrolysate) were observed for of papain, pepsin, acid and alkali hydrolysates respectively. Oil absorption capacities of chemically and enzymatically hydrolyzed visceral protein were significantly different (p < 0.05). Alkali hydrolysate had the highest 1.39 ± 0.05 (mg/g) oil absorption capacity among the other hydolysate and it had lower degree of hydrolysis (Table 4). Similar results i.e., 0.91 ± 0.03, 2.19 ± 0.5 and 1.4 ± 0.2 (mL/g) at different degree of hydrolysis were also found in sardine protein hydrolysate [30]. These results could be explained by the fact that hydrolysis can liberate some peptides from the native protein, which would enhance the flexibility of peptides and also fat increase the OAC which is present in the hydrolysate. The OAC values observed in this study are lower to those found for grass carp skin hydrolysate which was from 2.4 to 33.6 mg oil/g hydrolysate [47] and hydrolysate of Atlantic salmon had more OAC value of 3.22–5.90 mg oil/g of protein [44].
Emulsifying Properties
Proteins have the ability to stabilize food emulsion. The results of emulsifying activity index (EAI) and the emulsion stability index (ESI) are depicted in Table 4. From the results it was observed that the emulsifying properties of Pangasius visceral protein hydrolysate varied significantly (p < 0.05) with different treatments. Alkali extracted hydrolysate had an EAI value of 61.70 ± 0.76 (m2/g) which was higher as compared with other treatments but pepsin and acid extracted spray dried protein hydrolysate had the greater ESI values i.e 87.98 ± 2.13 and 71.76 ± 3.20 respectively. The emulsifying properties of Pangasius visceral hydrolysate can be explained based on their surface properties or how effectively the hydrolysate lowers the interfacial tension between the hydrophobic and hydrophilic components in food. The mechanism of the emulsification process is the absorption of proteins to the surface of freshly formed oil droplets during homogenization and form a protective membrane that prevents droplets from coalescing. Hydrolysate is surface active material and promotes oil-in-water emulsions because they are water soluble and contain hydrophilic and hydrophobic functional groups [50].
Solubility
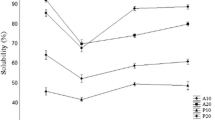
Solubility is one of the most important functional properties of protein and protein hydrolysate. Solubility fraction of different protein hydrolysate as effect of pH is showed in Fig. 2. All hydrolysates were soluble over a wide range of pH with different solubility percentage. Minimum solubility of different protein hydrolysate found at 5 pH i.e. 74.99 ± 0.00, 61.47 ± 0.00, 21.46 ± 1.40 and 14.78 ± 1.08 values for papain, pepsin, acid and alkali extracted hydrolysates respectively. These results of solubility are very well coincided with the lowest foaming capacity and foaming stability at pH 5.0 as shown in Figs. 3 and 4 respectively. This may be due to fact peptide could not move rapidly to the interface at this condition. Amiza et al. [37] reported higher solubility with higher DH including hydrolysate from silver salmon by-products [50] and yellow stripe trevally [51]. The high peptide solubility of protein hydrolysate indicates potential applications in food industry. Many of the other functional properties such as emulsification and foaming are affected by solubility [52] and therefore it is an excellent indicator of the protein hydrolysate functionality and its potential application [43]. Hydrophobic interactions promote protein–protein interactions and result in decreased solubility, whereas ionic interactions promote protein–water interactions and result in increased solubility. High solubility of protein hydrolysate is due to cleavage of protein into smaller peptides that usually have increased solubility [53].
Foaming Properties
Foam formation is governed by three factors, including, penetration, transportation and reorganization of molecules at the air–water interface. To exhibit good foaming, a protein must be capable of migrating rapidly to the air–water interface, unfolding and rearranging at the interface [54]. Foaming properties of the enzymatic and chemically extracted hydrolysates were determined by measuring their whippability at pH values from 3.0 to 8.0. In this study foaming properties were found highest in chemically extracted hydrolysate. Acid extracted protein hydrolysate had the highest foaming capacity ranging from 87.50 ± 2.50 to 137.50 ± 2.50% and stability characteristics 25.00 ± 0.00 to 126.67 ± 2.89% under different pH. Lowest Foaming capacity and stability of extracted hydrolysate were found at pH 5.0 which coincided with minimal protein solubility and results were showed in Figs. 3 and 4. The effect of pH on foaming properties of porcine plasma protein hydrolysate was lowest at pH 5.0 [55]. These results are supporting to the foaming properties of Pangasius visceral hydrolysate found in the present investigation. Poor foaming properties of enzymatically extracted hydrolysate in the present investigation can be due to the small size of peptides. Weak foams are commonly observed when food proteins are hydrolyzed. However, the advantage of using hydrolyzed proteins as foaming agents is their insensitivity to change in pH. The pH of the dispersing medium markedly affects foaming, particularly foam stability, with foaming properties being highest close to the isoelectric point of the protein [43]. Shahidi and Amarowicz [56] reported good foaming properties of capelin protein hydrolysates at low DH. Further hydrolysis could reduce the foaming stability since the more microscopic peptides do not have strength needed to maintain stable foam.
Conclusions
FPH can be successfully extracted from Pangasius viscera using enzyme and chemical treatment. Pangasius visceral protein hydrolysate obtained in the present investigation had the better yield, degree of hydrolysis, were light in color with slight fishy odor. This visceral protein hydrolysate had the ability to enhance the quality and functional properties of the food product. Functional properties of extracted hydrolysate resulted in a considerable higher solubility and lower turbidity, better foaming properties over a wide pH range with enzymatically treated hydrolysate. Pepsin visceral protein hydrolysate had higher degree of hydrolysis, yield%, color, proximate and functional properties because pepsin has better protein digestibility properties. Based on the different analysis, pepsin is better for extraction of FPH among the treatments. The finding of this work will contribute the utilization of fish processing waste.
References
Galla, N.R., Pamidighantam, P.R., Akula, S., Karakala, B.: Functional properties and in vitro antioxidant activity of roe protein hydrolysates of Channa striatus and Labeo rohita. Food Chem. 135(3), 1479–1484 (2012)
Waterman, J.J.: Measures, stowage rates and yields of fishery products. Torry Research Station, Aberdeen (1979)
Xavier, K.A.M., Geethalekshmi, V., Senapati, S.R., Mathew, P.T., Joseph, A.C., Nair, K.R.: Valorization of squid processing waste as animal feed ingredient by acid ensilaging process. Waste Biomass Valoriz. 8(6), 2009–2015 (2017)
Guerard, F., Guimas, L., Binet, A.: Production of tuna waste hydrolysates by a commercial neutral protease preparation. J. Mol. Catal. B 19, 489–498 (2002)
Balti, R., Bougatef, A., El-Hadj Ali, N., Zekri, D., Barkia, A., Nasri, M.: Influence of degree of hydrolysis on functional properties and angiotensin I-converting enzyme-inhibitory activity of protein hydrolysates from cuttlefish (Sepia officinalis) by-products. J. Sci. Food Agric. 90(12), 2006–2014 (2010)
Liceaga-Gesualdo, A.M., Li-Chan, E.C.Y.: Functional properties of fish protein hydrolysate from herring (Clupea harengus). J. Food Sci. 64(6), 1000–1004 (1999)
Hall, G.M., Ahmad, N.H.: Surimi and fish-mince products. Fish Process. Technol. 2, 74–92 (1997)
Simpson, B.K., Nayeri, G., Yaylayan, V., Ashie, I.N.A.: Enzymatic hydrolysis of shrimp meat. Food Chem. 61(1), 131–138 (1998)
Shahidi, F., Zhong, Y.: Bioactive peptides. J. AOAC Int. 91(4), 914–931 (2008)
Aspmo, S.I., Horn, S.J., Eijsink, V.G.: Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem. 40(5), 1957–1966 (2005)
Bhaskar, N., Mahendrakar, N.S.: Protein hydrolysate from visceral waste proteins of Catla (Catla catla): optimization of hydrolysis conditions for a commercial neutral protease. Bioresour. Technol. 99(10), 4105–4111 (2008)
Ovissipour, M., Abedian, A., Motamedzadegan, A., Rasco, B., Safari, R., Shahiri, H.: The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem. 115(1), 238–242 (2009)
Ovissipour, M., Kenari, A.A., Motamedzadegan, A., Nazari, R.M.: Optimization of enzymatic hydrolysis of visceral waste proteins of yellowfin tuna (Thunnus albacares). Food Bioprocess Technol. 5(2), 696–705 (2012)
Nazeer, R.A., Kumar, N.S.: Purification and identification of antioxidant peptide from black pomfret, Parastromateus niger (Bloch, 1975) viscera protein hydrolysate. Food Sci. Biotechnol. 20(4), 1087 (2011)
Tanuja, S., Viji, P., Zynudheen, A.A., Joshy, C.: Composition, functional properties and antioxidative activity of hydrolysates prepared from the frame meat of Striped catfish (Pangasianodon hypophthalmus). Egypt. J. Biol. 14(1), 27–35 (2012)
Quaglia, G.B., Orban, E.: Influence of enzymatic hydrolysis on structure and emulsifying properties of sardine (Sardina pilchardus) protein hydrolysates. J. Food Sci. 55(6), 1571–1573 (1990)
Jiang, H., Tong, T., Sun, J., Xu, Y., Zhao, Z., Liao, D.: Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 154, 158 –163 (2014)
Tsugita, A., Scheffler, J.J.: A rapid method for acid hydrolysis of protein with a mixture of trifluoroacetic acid and hydrochloric acid. FEBS J. 124(3), 585–588 (1982)
Tannenbaum, S.R., Ahern, M., Bates, R.P.: Solubilization of fish protein concentrate. I. An alkaline process. Food Technol. 24(5), 604 (1970)
Wu, H.C., Chen, H.M., Shiau, C.Y.: 1: Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 36(9), 949–957 (2003)
Robinson, H.W., Hogden, C.G.: The biuret reaction in the determination of serum proteins. 1. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J. Biol. Chem. 135, 707–725 (1940)
Cao, W., Zhang, C., Hong, P., Ji, H.: Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem. 109(1), 176–183 (2008)
AOAC: Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Washington, DC (2005)
Hunt, R.W.G.: The specification of colour appearance. I. Concepts and terms. Color Res. Appl. 2(2), 55–68 (1977)
Rodríguez-Ambriz, S.L., Martínez-Ayala, A.L., Millán, F., Davila-Ortiz, G.: Composition and functional properties of Lupinus campestris protein isolates. Plant Food Hum. Nutr. 60(3), 99–107 (2005)
Lin, C.S., Zayas, J.F.: Functionality of defatted corn germ proteins in a model system: fat binding capacity and water retention. J. Food Sci. 52(5), 1308–1311 (1987)
Pearce, K.N., Kinsella, J.E.: Emulsifying properties of proteins: evaluation of a turbidimetric technique. J. Agric. Food Chem. 26, 716–723 (1978)
Taheri, A., Anvar, S.A.A., Ahari, H., Fogliano, V.: Comparison the functional properties of protein hydrolysates from poultry byproducts and rainbow trout (Onchorhynchus mykiss) viscera. Iran. J. Fish. Sci. 12(1), 154–169 (2013)
Sathe, S.K., Salunkhe, D.K.: Functional properties of the great northern bean (Phaseolus vulgaris L.) proteins: emulsion, foaming, viscosity, and gelation properties. J. Food Sci. 46(1), 71–81 (1981)
Souissi, N., Bougatef, A., Triki-Ellouz, Y., Nasri, M.: Biochemical and functional properties of sardinella (Sardinella aurita) by-product hydrolysates. Food Technol. Biotechnol. 45(2), 187 (2007)
Mackie, I.M.: General review of fish protein hydrolysates. Anim. Feed Sci. Technol. 7(2), 113–124 (1982)
Wisuthiphaet, N., Kongruang, S., Chamcheun, C.: Production of fish protein hydrolysates by acid and enzymatic hydrolysis. J. Med. Bioeng. 4, 6 (2015)
Abdulazeez, S.S., Ramamoorthy, B., Ponnusamy, P.: Proximate analysis and production of protein hydrolysate from king fish of Arabian Gulf Coast-Saudi Arabia. Int. J. Pharm. Biol. Sci. 3(1), 138–144 (2013)
Blenford, D.E.: Protein hydrolysates: functionalities and uses in nutritional products. Int. Food Integr. 3, 45 (1994)
Hoyle, N.T., Merritt, J.O.H.N.: Quality of fish protein hydrolysates from herring (Clupea harengus). J. Food Sci. 59(1), 76–79 (1994)
Motamedzadegan, A., Davarniam, B., Asadi, G., Abedian, A., Ovissipour, M.: Optimization of enzymatic hydrolysis of yellowfin tuna Thunnus albacares viscera using Neutrase. Int Aquatic Res 2(3), 173–181 (2010)
Amiza, M.A., Kong, Y.L., Faazaz, A.L.: Effects of degree of hydrolysis on physicochemical properties of Cobia (Rachycentron canadum) frame hydrolysate. Int. Food Res. J. 19(1), 199–206 (2012)
Chalamaiah, M., Rao, G.N., Rao, D.G., Jyothirmayi, T.: Protein hydrolysates from meriga (Cirrhinus mrigala) egg and evaluation of their functional properties. Food Chem. 120(3), 652–657 (2010)
Mukhin, V.A., Novikov, V.Y., Ryzhikova, L.: S.A protein hydrolysate enzymatically produced from the industrial waste of processing Icelandic scallop Chlamys islandica. Appl. Biochem. Microbiol. 37(3), 292–296 (2001)
Bueno-Solano, C., López-Cervantes, J., Campas-Baypoli, O.N., Lauterio-García, R., Adan-Bante, N.P., Sánchez-Machado, D.I.: Chemical and biological characteristics of protein hydrolysates from fermented shrimp by-products. Food Chem. 112(3), 671–675 (2009)
Šližytė, R., Mozuraitytė, R., Martínez-Alvarez, O., Falch, E., Fouchereau-Peron, M., Rustad, T.: Functional, bioactive and antioxidative properties of hydrolysates obtained from cod (Gadus morhua) backbones. Process Biochem. 44(6), 668–677 (2009)
Groleau, P.E., Morin, P., Gauthier, S.F., Pouliot, Y.: Effect of physicochemical conditions on peptide–peptide interactions in a tryptic hydrolysate of β-lactoglobulin and identification of aggregating peptides. J Agri Food chem. 51(15), 4370–4375 (2003)
Kinsella, J.E., Melachouris, N.: Functional properties of proteins in foods: a survey. Critical Reviews in Food Sci. Nutr. 7(3), 219–280 (1976)
Kristinsson, H.G., Rasco, B.A.: Fish protein hydrolysates: production, biochemical, and functional properties. Critical reviews in Food Sci. Nutr. 40(1), 43–81 (2000)
Mullally, M.M., O’callaghan, D.M., Fitzgerald, R.J., Donnelly, W.J., Dalton, J.P.: Zymogen activation in pancreatic endoproteolytic preparations and influence on some whey protein hydrolysate characteristics. J. Food Sci. 60(2), 227–233 (1995)
Diniz, F.M., Martin, A.M.: Effects of the extent of enzymatic hydrolysis on functional properties of shark protein hydrolysate. LWT-Food Sci. Technol 30(3), 266–272 (1997)
Wasswa, J., Tang, J., Gu, X.H., Yuan, X.Q.: Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chem. 104(4), 1698–1704 (2007)
Damodaran, S.: Amino acids, peptides and proteins. In: Fennema, O.R. (ed.) Food chemistry, pp. 321–429. Marcel Dekker, Inc., New York (1996)
Sathivel, S., Bechtel, P.J., Babbitt, J., Prinyawiwatkul, W., Negulescu, I.I., Reppond, K.D.: Properties of protein powders from arrowtooth flounder (Atheresthes stomias) and herring (Clupea harengus) byproducts. J. Agric. Food Chem. 52(16), 5040–5046 (2004)
Gbogouri, G.A., Linder, M., Fanni, J., Parmentier, M.: Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 69(8), 615–622 (2004)
Klompong, V., Benjakul, S., Kantachote, D., Shahidi, F.: Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 102(4), 1317–1327 (2007)
Wilding, P., Lillford, P.J., Regenstein, J.M.: Functional properties of proteins in foods. Chem. Technol. Biotechnol. 34(3), 182–189 (1984)
Shahidi, F.: 16 Seafood processing by-products. In: Seafoods: chemistry, processing technology and quality, Springer, Boston (1994)
Halling, R.E.: Notes on Collybia. II. Additional taxa that are green in alkaline solution. Mycology. 1, 634–642 (1981)
Liu, Q., Kong, B., Xiong, Y.L., Xia, X.: Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 118(2), 403–410 (2010)
Shahidi, F., Amarowicz, R.: Antioxidant activity of protein hydrolyzates from aquatic species. J. Am. Oil Chem. Soc. 73(9), 1197–1199 (1996)
Acknowledgements
The authors wish to thank the Director, ICAR-Central Institute of Fisheries Education, Mumbai for providing the facilities to conduct the research. The Maulana Azad National Fellowship given by UGC, India to the first author is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, M.A., Deepitha, R.P., Xavier, K.A.M. et al. Evaluation of the Properties of Spray Dried Visceral Protein Hydrolysate from Pangasianodon hypophthalmus (Sauvage, 1978) Extracted by Enzymatic and Chemical Methods. Waste Biomass Valor 10, 2547–2558 (2019). https://doi.org/10.1007/s12649-018-0302-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0302-1