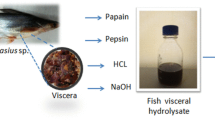
Abstract
Fish protein hydrolysates are digested form of protein with various bioactive properties where, the cleavages of molecular bonds of proteins can be broken by the enzymatic and chemical process. In this study, antioxidant properties of spray dried protein hydrolysate prepared from Pangasius viscera by using enzymatic (papain and pepsin), and chemical methods (hydrochloric acid and sodium hydroxide) were evaluated. Among the different treatments, pepsin-derived visceral protein hydrolysate showed the maximum antioxidant activity when used at higher concentrations. Essential amino acids (EAA) and hydrophobic amino acids are higher in papain-derived visceral protein hydrolysate. In pepsin-derived visceral protein hydrolysate, major proportion was contributed by glycine (Gly), glutamine (Glu), proline (Pro), and asparagine (Asp). Higher amount of aromatic amino acids are found in alkali-derived FVPH. Scanning electron microscopy (SEM) images of pepsin fish visceral protein hydrolysate showed better globular structure than the other treatments. It can be concluded that among the different treatments, the visceral protein hydrolysate prepared with pepsin had better overall quality regarding antioxidant properties and papain in nutritional point of view.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Processing of fish and shellfish generates approximately 50–60% of raw material as waste. Even though it is considered as waste, it can be utilized for the extraction of many valuable components such as fish oil, protein, collagen, gelatin, enzyme, and minerals. Studies in these aspects identified some bioactive compounds from fish and shellfish wastes (Kim and Mendis 2006). Preparation of protein hydrolysate from fish and shellfish wastes and their chemical, functional, and antioxidant properties were reported in several studies (Hassan et al. 2018; Dhanabalan et al. 2017). These protein hydrolysates are an excellent source of amino peptides with antioxidative properties in different oxidative systems. The antioxidative activity of protein and peptide can be due to scavenging of oxygen-containing compounds or metal chelating ability (Kristinsson 1998).
The protein hydrolysate has antioxidant properties which enable the production of protein enriched and oxidatively stable seafood. According to Pihlanto-Leppälä (2000), bioactive peptides have 2–20 amino acid residues per molecule and their activities are based on the original composition and sequence of amino acids. The application of enzyme in the modification of protein is an efficient way to recover potential bioactive peptides (Sarmadi and Ismail 2010). In peptide sequencing, parent proteins are usually inactive but can be liberated during enzymatic digestion or fermentation. Antioxidants play a significant role in human health by protecting the body against various degenerative diseases and in food preservation by retarding oxidation-induced deterioration. Free radical-mediated oxidation and antioxidants have been widely discussed (Chalamaiah et al. 2012).
Food industries are using many synthetic antioxidants for the preservation of food. The most commonly used antioxidants are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butyl hydroquinone (TBHQ), which are added to fat and oil foods to prevent oxidative deterioration. These synthetic antioxidants are slightly carcinogenic to some animals (Shahidi and Zhong 2010). Currently, the natural antioxidants are attracting an increasing interest to use because of their safety and large distribution properties. Besides antioxidative activities, the functional properties of hydrolysate are also crucial to food product formulation (Klompong et al. 2007).
Pangasius is widely cultured fish in many countries with very high production potential. Filleting and mincing operations generate around 60–70% of biomass as waste. Some products are already prepared by some researchers like Pangasius skin for the extraction of gelatin (Jamilah et al. 2011) and frame meat for protein hydrolysate (Tanuja et al. 2012). However, there is no report on evaluation of Pangasius viscera for protein hydrolysate. Therefore, the objective of this study was to evaluate the antioxidant properties and instrumental characteristics of spray dried Pangasius visceral protein hydrolysate prepared by chemical and enzymatic methods.
Materials and methods
Materials
Pangasius viscera were collected from local fish vendor Mumbai, India. This waste was packed inside an insulated container with ice, with waste to ice ratio of 1:1 and brought to the department of post-harvest technology. The visceral parts were washed with potable water and packed in polyethylene bags kept in sealed condition at − 18 °C until further use.
The preparation of an enzymatic hydrolysate from viscera
Protein hydrolysate extraction was carried out according to a method prescribed by Jiang et al. (2014) with slight modifications. The frozen intestine was defrosted at room temperature and homogenized with distilled water with 1:2 (w/v) solid to liquid ratio by using a homogenizer (IKA T18 basic, Germany) and placed in a boiling water bath (Shanti, Scientific industries, Mumbai, India) for 10 min to ensure deactivation of endogenous enzymes (Guerard et al. 2002; Ovissipour et al. 2009). The protein was digested with two kinds of enzymes with an enzyme/substrate ratio of 1:100 (w/w). Temperature and pH were adjusted to optimum values for each enzyme: papain (T1), 55 °C, pH 7.0; pepsin (T2), 37.5 °C, pH 2.0; and the mixture was incubated under continuous stirring up to 5 h and then heated in water bath (Shanti, Scientific industries, Mumbai) at 100 °C for 15 min to ensure enzyme deactivation, and the pH (Eutech tutor pH/°C meter, Eutech Instruments, Singapore) was adjusted to 7.0. The content was then centrifuged (Eltek, RC 41000F, Elektrocraft, India Pvt Ltd, Mumbai, India) at 10,000×g for 30 min at 4 °C. Collected supernatant was then spray dried (SM Scitech, Calcutta, India) at 180 °C inlet temperature to get powder and stored at − 18 °C in deep freezer (Blue star, Mumbai, India).
Preparation of hydrolysate from fish viscera using hydrochloric acid
Acid protein hydrolysate extraction was carried out by Tsugita and Scheffler (1982) method with slight modification. The frozen intestine was defrosted at room temperature and then dissolved in distilled water with the ratio of 1:2 (w/v) and maintained at pH 2.0 with 6 N HCl (T3), at 110 °C for 24 h. After completing hydrolysis process, pH was adjusted to 7.0. The content was centrifuged at 10,000×g for 30 min at 4 °C. Collected supernatant was then spray dried at 180 °C inlet temperature to get powder and stored at − 18 °C in a plastic centrifuge tube.
Preparation of hydrolysate from fish viscera using alkali
Alkali protein hydrolysate extraction was carried out by Tannenbaum et al. (1970) method with slight modification. The frozen intestine was air thawed at room temperature and then dissolved in distilled water with a ratio of 1:2 (w/v) and maintained at pH 12.5 with 10 N NaOH (T4) and kept at 95 °C for 20 min. After completing hydrolysis process, pH was adjusted to 7.0. The content was then centrifuged at 10,000×g for 30 min at 4 °C. Collected supernatant was then spray dried at 180 °C inlet temperature to get powder and stored at − 18 °C in a plastic centrifuge tube.
Antioxidant activities
DPPH radical scavenging activity
The DPPH radical scavenging activity of fish visceral protein hydrolysate (FVPH) was analyzed by the method of Shimada et al. (1992) with slight modification. Proton-donating substances react with DPPH, the free radicals are scavenged, and the absorbance is reduced. 0.5 mL of the sample solution with different concentration (0.4–2.0 mg/mL) was mixed with 2.5 mL of DPPH (0.02 M in methanol) after vortex mixture was incubated at room temperature (25–30 °C) for 30 min in dark place. The absorbance of resulting solution was measured at 517 nm (UV-2450, Shimadzu). Methanol was used for control sampling. The DPPH radical scavenging capacities of samples were measured as decreasing order of absorbance of DPPH radical and calculated by using the following equation:
- A :
-
Absorbance
All determinations were performed in triplicate. A lower absorbance of the reaction mixture indicated a higher DPPH radical-scavenging activity. BHA and Trolox were used as a standard.
ABTS radical scavenging activity
ABTS radical [2, 2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid)] scavenging ability was measured by the method suggested by Hernández-Ledesma et al. (2005) with slight modification. In brief, free-radical scavenging activity of fish visceral protein hydrolysate was based on ABTS radical decolorization assay. The cationic ABTS radical was produced by the reaction of 7 mM aqueous ABTS stock solution and 2.45 mM potassium persulfate (1:1) kept in the dark place for 16 h before the use. ABTS radical solution was diluted with 5.0 mmol/L phosphate buffered saline with a pH of 7.4 to obtain an absorbance of 0.70 ± 0.02 at 734 nm. One milliliter of diluted ABTS radical solution was mixed with 1 mL of different concentrations of samples. Ten minutes later, the absorbance was measured at 734 nm against the corresponding blank. The ABTS scavenging activity of samples was calculated with following equation:
- A :
-
Absorbance
BHA was used as a standard substance.
Reducing power assay
The reducing power was measured according to the procedure described by Wu et al. (2003) with some modifications. Briefly, sample solution (2.0 mL) with various concentrations 0.5, 1, 2, 3, 4, and 5 mg/mL was mixed with 2.0 mL of phosphate buffer (0.2 M, pH 6.6) and 2.0 mL of potassium ferricyanide (1%). 1.0 mL of trichloroacetic acid (10%) was added to the mixture and incubated at 50 °C for 20 min. After vortex, the fluid was centrifuged at 3000×g for 10 min. The upper layer of solution (2.0 mL) was collected and mixed with 2.0 mL of deionized water and 0.3 mL of ferric chloride (0.1%), and the mixture was incubated at room temperature for 10 min, the absorbance of the mixture was measured at 700 nm. Higher absorbance (A700) indicated greater reductive potential. BHA was used as a positive control. All experiments were carried out in triplicate.
Amino acid composition
The amino acid composition was determined following the method of Ishida et al. (1981) with slight modification. The samples were hydrolyzed with 6 N HCl at 110 °C under anaerobic condition for 24 h. The hydrolyzed samples were derivatized using a kit (AccQ-Fluor reagent, WAT052880, Waters Co., USA). The derivatized samples were injected in high-performance liquid chromatography (HPLC, 1525, water) equipped with a C18 RP column with fluorescence detector (2475, waters). The amino acids were identified and quantified by comparing with the retention times and peak area standards (Amino Acid Hydrolysate Standard, P/N WAT088122, Waters Co., USA).
Fourier transform infrared spectroscopy (FTIR)
Change in functional group absorbance in protein hydrolysate was studied using an FTIR spectrometer (Bruker, Ettlingen, Germany) equipped with a micro-attenuated total reflectance (ATR) accessory. One milligram of hydrolysate was mixed with 100 mg of potassium bromide (KBr) and ground gently with an agate pestle and mortar under a lamp to form a very fine powder and compressed to disc using a hydraulic pellet press (Type KP, Kimaya Engineers, Mumbai, India) into a thin disc. The spectrometer was controlled by Opus Software-version 6.5 to collect spectra over the wavenumber range of 4000 to 400 cm-1, by accumulating 32 scans with a resolution of 4 cm-1. Data collection for each sample took less than 2 min.
Scanning electron microscopy (SEM)
The spray dried visceral protein hydrolysate sample was smeared on a small piece of adhesive carbon tape which is fixed on a brass stub. Then samples were subjected to gold coating using the sputtering unit (model: JFC1600) for 10 s at 10 mA of current. The gold-coated sample placed in a chamber of SEM (JEOL, JSM 6390 LV, Tokyo, Japan) and secondary electron/back scattered electron images are recorded.
Results and discussion
Antioxidant activity
DPPH
DPPH free-radical scavenging activity of FVPH prepared with different treatments is depicted in Fig. 1. As the concentration of FVPH increased, DPPH free-radical scavenging activity also increased significantly (p < 0.05). Among the treatments, pepsin-extracted hydrolysate (T2) exhibited higher DPPH free-radical scavenging activity than papain (T1)-, acid-, and alkali-treated hydrolysate when the concentration is increase to 1.2 mg/mL. Radical scavenging activity of pepsin-extracted hydrolysate (T2) was lowest at 0.4 mg/mL, i.e., 33.56 ± 2.99% but papain (T1), acid (T3), and alkali (T4) treated were 43.15 ± 2.99%, 43.84 ± 2.74%, and 41.32 ± 3.38%, respectively. At 2 mg/mL concentration, pepsin-treated hydrolysate (T2) had shown higher antioxidant activity, i.e., 90.87 ± 1.58 as compared with T1, T3, and T4 treatments with values of 65.75 ± 1.19, 63.01 ± 1.81, and 65.75 ± 2.47, respectively. Trolox and BHA showed very high DPPH radical scavenging activity at 0.4 mg/mL (Fig. 1) in comparison to FVPH. DPPH radical scavenging activity is widely used to evaluate the hydrogen donating capacity of an antioxidant. Antioxidant properties of protein hydrolysate depend on several factors such as the degree of hydrolysis, solubility of proteins, presence of free amino acids, and type of enzymes used depends on their target of cleavage on polypeptide chain (Galla et al. 2012; Bougatef et al. 2010). DPPH is a stable free radical that shows maximal absorbance at 517 nm in methanol at different concentration (Bougatef et al. 2009). When DPPH molecule comes in contact with a proton-donating substance (H+), its color is changed from purple to yellow, and the absorbance is reduced. In DPPH test, the protein hydrolysates reduced the DPPH radical to a yellow-colored compound, apparently due to the DPPH radical accepting an electron or hydrogen to become a stable diamagnetic molecule. Proton-donating capability in pepsin-derived FVPH might be responsible for the higher radical scavenging activity. Pepsin mainly acted on the N-terminal aromatic amino acid and several researchers are suggested that pepsin is useful for the production of bioactive peptides from fish protein such as dark tuna muscle, hoki frame protein and smoothhound muscle protein (Bougatef et al. 2009; Je et al. 2008; Kim et al. 2007; Qian et al. 2007). Higher DPPH radical scavenging activity of FVPH indicated that it contained more electrons for donating to free radicals; it is converted into the more stable product and terminated the radical chain reaction (Kittiphattanabawon et al. 2012). Lower molecular weight peptide shows higher antioxidant activity reported by Jun et al. (2004). They found 13 kDa peptides in yellowfin sole hydrolysate produced by pepsin.
DPPH free-radical scavenging activity of FVPH prepared with different treatments. T1, visceral protein hydrolysate with papain; T2, visceral protein hydrolysate with pepsin; T3, visceral protein hydrolysate with hydrochloric acid; T4, visceral protein hydrolysate with NaOH; and BHA, butylated hydroxyl anisole. The bars indicate the standard deviation of means (n = 3), (p < 0.05)
ABTS scavenging activity
Oxidized ABTS develops a colored cation-radical and shows strong absorbance at 734 nm. When an antioxidant sample is added to the solution, the oxidation of ABTS is delayed, and the reaction consumes a greater quantity of electoral current, which is related to the degree of antioxidant activity of the sample added. The results for the ABTS radical scavenging activity of FVPH with different treatments are shown in Fig. 2. From the results, it was observed that the values for ABTS scavenging activities with different treatments were in the lower range of 56.57 ± 2.10 to 62.88 ± 1.01% at 0.5 mg/mL concentration of hydrolysate and higher range of 80.55 ± 0.48 to 88.33 ± 1.37% at 3.5 mg/mL concentration of hydrolysate. ABTS values for different treatments were decreasing in the order of pepsin (T2) > papain (T1) > alkali (T4) > acid (T3) in the case of a Pangasius viscera protein hydrolysate. Generally ABTS radical scavenging activity of a compound was studied at different concentration ranging from 0.04 to 2 mg (Luo et al. 2013). With high ABTS radical-scavenging activity, it was postulated that antioxidative compounds were most likely hydrophilic. All hydrolysates are peptides or proteins which were proton donor and could respond with radical to react and convert into more stable products, thereby terminating the radical chain reaction. ABTS radical assay is an excellent tool for determining the antioxidative activity, in which the radical is quenched to form ABTS radical complex (Binsan et al. 2008). According to Klompong et al. (2009) the ABTS radical is relatively stable and is readily reduced by antioxidants. The amino acid sequence of peptides might affect the antioxidant activity. Also, the free amino acids produced determined the scavenging activity. Among the amino acids, Cys, Trp, Tyr, and His showed better ABTS scavenging activity (Gómez-Ruiz et al. 2008).
ABTS scavenging activity of FVPH prepared with different treatments. T1, visceral protein hydrolysate with papain; T2, visceral protein hydrolysate with pepsin; T3, visceral protein hydrolysate with hydrochloric acid; T4, visceral protein hydrolysate with NaOH; and BHA, butylated hydroxyl anisole. The bars indicate the standard deviation of means (n = 3), (p < 0.05)
Reducing power
Reducing power of FVPH increased significantly (p < 0.05) with increasing the concentration of hydrolysate (Jamdar et al. 2010), result showed in Fig. 3. Pepsin-treated FVPH (T2) had shown the highest reducing power (0.41 ± 0.01) at 0.5 mg/mL as compared with other treatments. With increasing concentration of hydrolysate (at 5 mg/mL) in treatments, pepsin-treated FVPH (T2) had shown the highest activity (0.84 ± 0.01) followed by papain- (T1) (0.47 ± 0.01) and acid-treated FVPH (T3) (0.28 ± 0.00) and lowest in alkali hydrolysate (T4), i.e., 0.19 ± 0.03. Strong reducing power activity of pepsin FVPH (T2) may be ascribed to the presence of hydrogen ion (protons and electrons) generated during peptide cleavages. Therefore, all the treatments showed some antioxidant activities which might be related to their reducing activities. Reducing power of the treatments were lower in comparison to the range of buckwheat protein hydrolysate (Tang et al. 2009) and higher than Spanish mackerel skin hydrolysate (Chi et al. 2014) and 0.08–0.25 of hemoglobin hydrolysate (Chang et al. 2007). Several works also reported that the reducing power increased with increasing the concentration of hydrolysate (Zhu et al. 2006 and Dhanabalan et al. 2017). Bougatef et al. (2010) also found very less reducing power in hydrolysate of sardine (Sardinella aurita) extracted by the crude enzyme. The difference in reducing activity of all treatments may be due to the substrate specificity of enzyme and properties of the chemicals used for extraction of hydrolysate from fish viscera. Tanuja et al. 2012 and Cumby et al. 2008 also reported the significant difference in the reducing capacities of hydrolysate prepared with the two different enzymes might be due to the substrate specificity of enzymes.
Reducing power assay of FVPH prepared with different treatments. T1, visceral protein hydrolysate with papain; T2, visceral protein hydrolysate with pepsin; T3, visceral protein hydrolysate with hydrochloric acid; T4, visceral protein hydrolysate with NaOH; and BHA, butylated hydroxyl anisole. The bars indicate the standard deviation of means (n = 3), (p < 0.05)
Amino acids composition
The protein content of visceral protein hydrolysate (FVPH) obtained with various extraction conditions were reported by Hassan et al. 2018 which were significantly different. Among the treatments, FVPH prepared with enzymatic method had higher protein content than chemical methods. Among the enzymatic process, FVPH prepared with pepsin (T2) had highest protein content (82.55%) followed by papain (T1), i.e., 78.14%. Acid-treated FVPH extracted with hydrochloric acid (T3) had 69.13% crude protein content and the lowest protein value was found in alkali aided FVPH (T4) sample with 57.86% protein content (Hassan et al. 2018).
The amino acid composition of a protein hydrolysate is essential as it provides information on its nutritional value, functional properties, and antioxidative activity as nutraceutical supplement. The amino acid composition of spray dried FVPH expressed as g/100 g is presented in Table 1. It showed that the variation in the amino acid composition of FVPH with different treatments might be due to different enzymes and chemicals used for the extraction. The present investigation revealed that Gly and Thr content ranged between 10.23 to 14.95 and 0.58 to 6.31 respectively and found highest in pepsin-extracted protein hydrolysate (T2). Glutamate (Glu), glycine (Gly), and proline (Pro) were present in high quantity in all the treatments except papain visceral protein hydrolysate (T1) where in the glutamate content was less. The results are in agreement with Nasri et al. (2013) who reported that Gly and Thr were the most abundant amino acids found in fish protein hydrolysate. According to Qian et al. (2008), Gly was also a suitable hydrogen donor and the presence of high amount of Gly in FVPH in the present investigation is an indicator of its higher antioxidant activity. Riisom et al. (1980) reported that several amino acids also showed antioxidant properties, i.e., Trp, His, Tyr, Lys, and Met. Aromatic amino acid tends to donate electrons and convert free radicals to stable molecules while retaining their stability through resonance structures and thus increasing the radical-scavenging properties of amino acids (Sarmadi and Ismail 2010). Variation in amino acid composition of different fish protein hydrolysate mainly depends on several factors like source of an enzyme: the raw material used for hydrolysate preparation and hydrolysis conditions (Klompong et al. 2009; Sathivel et al. 2003, 2005). Total essential amino acids were found highest in papain-treated FVPH (T1) followed by acid (T3), pepsin (T2), and alkali treatment (T4), i.e., 53.16, 35.23, 30.17, and 29.40, respectively. According to Amiza et al. (2012), fish protein hydrolysate had higher essential amino acid values than recommended for the human. Similar observations were also reported by Liceaga-Gesualdo and Li-Chan (1999) and Wasswa et al. (2007). TEAA in T2 was found lower comparative to other treatments due to decomposition and oxidation (Tsugita and Scheffler 1982; Shahidi et al. 1995). Despite the priority of Phe, Tyr, and Trp as hydrogen donors, the hydrophobic amino acids could increase the presence of peptides at the water-lipid interface and then access to scavenge free radicals from the lipid phase (Ranathunga et al. 2006). Among the treatments, pepsin-treated FVPH (T2) contains more histidine units (4.63) and contained imidazole ring which can be attributed to the chelating and lipid radical-trapping ability in the hydrolysate. Uchida and Kawakishi (1992) and Park et al. (2001) were also reported histidine-containing peptides worked as an antioxidant. Lee et al. (2012) reported hydrolysate contained the His in the N-terminus of the sequence, with strong scavenging ability on DPPH, OH−, and superoxide.
Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared (FTIR) spectroscopy is an important technique to study the functional groups of a protein hydrolysate. The FTIR spectra ranging from 4000 to 400 cm−1 wavenumbers of visceral fish protein extracted with different treatments are displayed in Fig. 4. A wide and strong infrared absorption peak from 3600 to 3000 cm−1 was observed in all of the treatments, indicating the –NH stretching which corresponds to the flexural vibration frequencies of the intra-and inter-molecular hydrogen bonds (Tan et al. 2014). The peaks found in all the treatments T1 (2926.04 and 2854.44 cm−1), T2 (2927.87 cm−1), T3 (2926.85 and 2856.28 cm−1), and T4 (2923.57 and 2853.33 cm−1) respectively indicate the CH anti-symmetric and symmetric stretching modes of methyl (CH3) and methylene (CH2) that generally found in aliphatic side chain of proteins (Chen et al. 2013).
There were three characteristic bands in FTIR spectrum for visceral protein hydrolysate, consisted of amide I (1600–1700 cm−1) caused by stretching of CO bonds, Amide II (1530–1550 cm−1) assigned to deformation of NH bonds and stretching of CN bonds and Amide III (1240–1450 cm−1) bands corresponding to C–N stretching, respectively (Al-Jowder et al. 1999). The observed peaks in Amide I region, at 1633.77 cm−1 (T1) represents the β-sheet secondary structure which indicates the disruption of the native structure during hydrolysis by papain. The presence of 1658.39 cm−1 (T2), 1657.21 cm−1 (T3), and 1657.97 cm−1 (T4) respectively indicate the α-helix or disordered structure (Barth 2007). The absence of Amide II band in treatment T1 and T4 and presence of these bands in other treatments is due in-plane NH bending and CN stretching vibration. This condition represents that T2 and T3 vibrated more between in-plane NH and CN groups (Putra et al. 2017). The observed peaks in Amide III region were found to be T1 (1405 cm−1), T2 (1401; 1337.11 (α-helix), 1242.09 cm−1 (β-sheet)), T3 (1403 cm−1), and T4 (1311.52 (α-helix), 1403.016 cm−1) respectively. From the spectra, it was observed that pepsin and alkali hydrolyzed viscera protein hydrolysate has a higher intensity of the Amide III region. The protein hydrolysates might have differences regarding structure and conformation due to differences in amino acid.
The observed peaks 1047.78 cm−1 (T1), 1078.98 cm−1 (T2), 1046.04 cm−1 (T3), and 996.26; 1139.09 cm−1 (T4) respectively correspond to the saccharide bands which indicate that the sample contains aldehyde groups. The aldehyde may have formed due to oxidation of lipids present in the fish muscle during the hydrolysis process, as it was carried out at the temperature of 180 °C and intensity is more in T4 (Elavarasan et al. 2016). The bands observed 663.91 and 772.22 cm−1 (T1), 663.60 cm−1 (T3), and 699.54 cm−1 (T4) respectively represent to Amide V region which corresponds to out-of-plane NH bending and was absent in pepsin-treated protein hydrolysate. The peak corresponding to 541.87 cm−1 in T4 indicates out-of-plane C=O bending (Kong and Yu 2007).
Scanning electron microscopy
Scanning electron microscopy was carried out to study the surface morphology of spray dried FVPH particles. In this study, spray drying has been proved to be the most advantageous to manufacture homogenous spherical globule of hydrolysate as shown in Fig. 5. SEM images of different treatments were showed almost spherical beads like structure except for acid hydrolysate (T3). Pepsin hydrolysate (T2) structure showed nearly spherical beads than papain hydrolysate (T1) and alkali hydrolysate (T4) was an irregular shape but in acid hydrolysis (T3) structure was degraded due to acid medium. Based on the magnifications, there were significant differences among the internal structure of different spray dried FVPH. Rosenberg and Young (1993) explained the predominance of particles fragmentation of milk fat whey protein microencapsulation powders. During spray drying, the thermal expansion of either the air or water vapors inside the drying particles provoke ballooning of particles during the late stage of drying may result in rupturing of a bubble. The lipid-containing retentate bubbles expand and then partially shrink as more water is removed during the late phase of drying. The low lipid retainable expands so much that the thin walls rupture and only dried fragments remain.
Scanning electron microscopic (SEM) images of spray dried FVPH prepared with different treatments. T1, visceral protein hydrolysate with papain; T2, visceral protein hydrolysate with pepsin; T3, visceral protein hydrolysate with hydrochloric acid; T4, visceral protein hydrolysate with NaOH; and BHA, butylated hydroxyl anisole
Conclusions
Fish protein hydrolysates can be successfully extracted from Pangasius viscera in powder form with enzyme and chemical treatment by using spray drying technology. Pangasius visceral protein hydrolysate obtained with pepsin enzyme treatment (T2) in the present investigation had the better antioxidant activity like DPPH, ABTS, and reducing power assays compared to acid (T3), alkali (T4), and papain (T1) digested hydrolysate. This visceral protein hydrolysate had a good amount of total essential amino acids, hydrophobic amino acids, and aromatic amino acids. According to FTIR spectra, there was no significance difference in the functional groups of different hydrolysate while SEM image analysis revealed that spray dried powder gave a spherical bead-like structure of hydrolysate. Based on the various analyses, pepsin (T2) is better for extraction of fish protein hydrolysate among the treatments. The finding of this work can be useful for the better utilization of fish processing waste.
References
Al-Jowder O, Defernez M, Kemsley EK, Wilson RH (1999) Mid-infrared spectroscopy and chemometrics for the authentication of meat products. J Agric Food Chem 47(8):3210–3218
Amiza MA, Kong YL, Faazaz AL (2012) Effects of degree of hydrolysis on physicochemical properties of Cobia (Rachycentron canadum) frame hydrolysate. Int Food Res J 19(1):199–206
Barth A (2007) Infrared spectroscopy of proteins. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1767:1073–1101
Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H (2008) Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem 106(1):185–193
Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M (2009) Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem 114(4):1198–1205
Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M (2010) Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem 118(3):559–565
Chalamaiah M, Hemalatha R, Jyothirmayi T (2012) Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem 135(4):3020–3038
Chang CY, Wu KC, Chiang SH (2007) Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem 100(4):1537–1543
Chen X, Ru Y, Chen F, Wang X, Zhao X, Ao Q (2013) FTIR spectroscopic characterization of soy proteins obtained through AOT reverse micelles. Food Hydrocoll 31(2):435–437
Chi CF, Cao ZH, Wang B, Hu FY, Li ZR, Zhang B (2014) Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules 19(8):11211–11230
Cumby N, Zhong Y, Naczk M, Shahidi F (2008) Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem 109(1):144–148
Dhanabalan V, Xavier M, Kannuchamy N, Asha KK, Singh CB, Balange A (2017) Effect of processing conditions on degree of hydrolysis, ACE inhibition, and antioxidant activities of protein hydrolysate from Acetes indicus. Environ Sci Pollut R 24(26):21222–21232
Elavarasan K, Shamasundar BA, Badii F, Howell N (2016) Angiotensin I-converting enzyme (ACE) inhibitory activity and structural properties of oven-and freeze-dried protein hydrolysate from fresh water fish (Cirrhinus mrigala). Food Chem 206:210–216
Galla NR, Pamidighantam PR, Akula S, Karakala B (2012) Functional properties and in vitro antioxidant activity of roe protein hydrolysates of Channa striatus and Labeo rohita. Food Chem 135(3):1479–1484
Gómez-Ruiz JÁ, López-Expósito I, Pihlanto A, Ramos M, Recio I (2008) Antioxidant activity of ovine casein hydrolysates: identification of active peptides by HPLC–MS/MS. Eur Food Res Technol 227(4):1061–1067
Guerard F, Guimas L, Binet A (2002) Production of tuna waste hydrolysates by a commercial neutral protease preparation. J Mol Catal B Enzym 19:489–498
Hassan MA, Deepitha RP, Xavier KM, Gupta S, Nayak BB, Balange AK (2018) Evaluation of the properties of spray dried visceral protein hydrolysate from Pangasianodon hypophthalmus (Sauvage, 1978) extracted by enzymatic and chemical methods. Waste Biomass Valor:1–12. https://doi.org/10.1007/s12649-018-0302-1
Hernández-Ledesma B, Miralles B, Amigo L, Ramos M, Recio I (2005) Identification of antioxidant and ACE-inhibitory peptides in fermented milk. J Sci Food Agric 85(6):1041–1048
Ishida Y, Fujita T, Asai K (1981) New detection and separation method for amino acid by high-preformance liquid chromatogaphy. J Chromatogr 204:143–148
Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V, Sharma A (2010) Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitor activity of peanut protein hydrolysate. Food Chem 121(1):178–184
Jamilah B, Tan KW, Hartina MU, Azizah A (2011) Gelatins from three cultured freshwater fish skins obtained by liming process. Food Hydrocoll 25(5):1256–1260
Je JY, Qian ZJ, Lee SH, Byun HG, Kim SK (2008) Purification and antioxidant properties of big eye tuna (Thunnus obesus) dark muscle peptide on free radical-mediated oxidative systems. J Med Food 11(4):629–637
Jiang H, Tong T, Sun J, Xu Y, Zhao Z, Liao D (2014) Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem 154:158–163
Jun SY, Park PJ, Jung WK, Kim SK (2004) Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellow fin sole (Limanda aspera) frame protein. Eur Food Res Technol 219(1):20–26
Kim SK, Mendis E (2006) Bioactive compounds from marine processing by-products–a review. Food Res Int 39(4):383–393
Kim SY, Je JY, Kim SK (2007) Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J Nutr Biochem 18(1):31–38
Kittiphattanabawon P, Benjakul S, Visessanguan W, Shahidi F (2012) Gelatin hydrolysate from blacktip shark skin prepared using papaya latex enzyme: antioxidant activity and its potential in model systems. Food Chem 135(3):1118–1126
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 102(4):1317–1327
Klompong V, Benjakul S, Yachai M, Visessanguan W, Shahidi F, Hayes KD (2009) Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe trevally (Selaroides leptolepis). J Food Sci 74(2):126–133
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39(8):549–559
Kristinsson HG (1998) Reaction kinetics, biochemical and functional properties of salmon muscle proteins hydrolyzed by different alkaline proteases (Doctoral dissertation, University of Washington)
Lee SJ, Kim YS, Hwang JW, Kim EK, Moon SH, Jeon BT, Jeon YJ, Kim JM, Park PJ (2012) Purification and characterization of a novel antioxidative peptide from duck skin by-products that protects liver against oxidative damage. Food Res Int 49(1):285–295
Liceaga-Gesualdo AM, Li-Chan ECY (1999) Functional properties of fish protein hydrolysate from herring (Clupea harengus). J Food Sci 64(6):1000–1004
Luo HY, Wang B, Li ZR, Chi CF, Zhang QH, He GY (2013) Preparation and evaluation of antioxidant peptide from papain hydrolysate of Sphyrna lewini muscle protein. Food Sci and Technol 51(1):281–288
Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, Karra-Châabouni M (2013) ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: effect on meat lipid oxidation. Food Res Int 54(1):552–561
Ovissipour M, Abedian A, Motamedzadegan A, Rasco B, Safari R, Shahiri H (2009) The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem 115(1):238–242
Park PJ, Jung WK, Nam KS, Shahidi F, Kim SK (2001) Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J Am Oil Chem Soc 78(6):651–656
Pihlanto-Leppälä A (2000) Bioactive peptides derived from bovine whey proteins: opioid and ace-inhibitory peptides. Trends Food Sci Technol 11(9):347–356
Putra SNKM, Ishak NH, Sarbon NM (2017) Preparation and characterization of physicochemical properties of golden apple snail (Pomacea canaliculata) protein hydrolysate as affected by different proteases. Bio Agri Biotechnol 13:123–128
Qian ZJ, Je JY, Kim SK (2007) Antihypertensive effect of angiotensin I converting enzyme-inhibitory peptide from hydrolysates of bigeye tuna dark muscle, Thunnus obesus. J Agric Food Chem 55(21):8398–8403
Qian J, Tang Q, Cronin B, Markovich R, Rustum A (2008) Development of a high performance size exclusion chromatography method to determine the stability of Human Serum Albumin in a lyophilized formulation of Interferon alfa-2b. J Chromatogr A 1194(1):48–56
Ranathunga S, Rajapakse N, Kim SK (2006) Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur Food Res Technol 222(3-4):310–315
Riisom T, Sims RJ, Fioriti JA (1980) Effect of amino acids on the autoxidation of safflower oil in emulsions. J Am Oil Chem Soc 57(10):354–359
Rosenberg M, Young SL (1993) Whey proteins as microencapsulating agents. Microencapsulation of anhydrous milkfat-structure evaluation. Food Struct 12(1):4
Sarmadi BH, Ismail A (2010) Antioxidative peptides from food proteins: a review. Peptides 31(10):1949–1956
Sathivel S, Prinyawiwatkul W, King JM, Grimm CC, Lloyd S (2003) Oil production from catfish viscera. J Am Oil Chem Soc 80(4):377–382
Sathivel S, Smiley S, Prinyawiwatkul W, Bechtel PJ (2005) Functional and nutritional properties of red salmon (Oncorhynchus nerka) enzymatic hydrolysates. J Food Sci 70(6)
Shahidi F, Zhong Y (2010) Novel antioxidants in food quality preservation and health promotion. Eur J Lipid Sci Technol 112(9):930–940
Shahidi F, Han XQ, Synowiecki J (1995) Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem 53(3):285–293
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40(6):945–948
Tan ES, Ying-Yuan N, Gan CY (2014) A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SPI) after the extraction optimisation. Food Chem 152:447–455
Tang CH, Peng J, Zhen DW, Chen Z (2009) Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem 115(2):672–678
Tannenbaum SR, Ahern M, Bates RP (1970) Solubilization of fish protein concentrate. I. An alkaline process. Food Technol 24(5):604
Tanuja S, Viji P, Zynudheen AA, Joshy C (2012) Composition, functional properties and antioxidative activity of hydrolysates prepared from the frame meat of Striped catfish (Pangasianodon hypophthalmus). Egypt J Biol 14(1):27–35
Tsugita A, Scheffler JJ (1982) A rapid method for acid hydrolysis of protein with a mixture of trifluoroacetic acid and hydrochloric acid. The FEBS J 124(3):585–588
Uchida K, Kawakishi S (1992) Sequence-dependent reactivity of histidine-containing peptides with copper (II)/ascorbate. J Agric Food Chem 40(1):13–16
Wasswa J, Tang J, Gu XH, Yuan XQ (2007) Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chem 104(4):1698–1704
Wu HC, Chen HM, Shiau CY (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res Int 36(9):949–957
Zhu K, Zhou H, Qian H (2006) Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem 41(6):1296–1130
Acknowledgements
The authors thank the Director, ICAR-Central Institute of Fisheries Education, Mumbai. The Maulana Azad National Fellowship given by UGC, India, to the first author is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Pangasius visceral protein hydrolysate prepared by enzymatic and chemical methods.
• Spray dried powder was evaluated for antioxidant instrumental quality traits.
• FVPH prepared with pepsin showed higher antioxidant activity.
• Essential amino acids are higher with papain-derived FVPH.
Rights and permissions
About this article
Cite this article
Hassan, M.A., Xavier, M., Gupta, S. et al. Antioxidant properties and instrumental quality characteristics of spray dried Pangasius visceral protein hydrolysate prepared by chemical and enzymatic methods . Environ Sci Pollut Res 26, 8875–8884 (2019). https://doi.org/10.1007/s11356-019-04144-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04144-y