Abstract
Fish protein hydrolysate was produced from the viscera of yellowfin tuna (Thunnus albacares). Hydrolysis conditions (enzyme activity, temperature, and time) were optimized using response surface methodology. A factorial design was applied to minimize enzyme utilization and modeling of degree of hydrolysis (r 2 = 0.94). Lack-of-fit test revealed a non-significant value for the model, indicating that the regression equation was adequate for predicting the degree of hydrolysis under any combination of the variables (P < 0.05). The optimum conditions to reach the highest degree of hydrolysis were: 60.4 °C, 90.25 min, and a protease (Alcalase 2.4 L) activity of 70.22 AU/kg protein. The spray-dried tuna visceral protein hydrolysates had relatively high protein (72.34%) and low lipid (1.43%) content. The chemical score of the hydrolysate indicated that it fulfils adult human nutritional requirements except for methionine. Lysine and methionine were the first and the second limiting amino acids in that order. Phenylalanine was the predominant amino acid in the hydrolysates with respect to common carp requirement. In addition, the protein efficiency ratio of tuna visceral hydrolysate was 2.85–5.35.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World fish production has almost stagnated and presently stands at 132 mmt (FAO 2006). Fish sources once appeared to be inexhaustible, and by-products arising out of fish processing were looked as worthless materials discarded without an attempt of recovery (Kristinsson and Rasco 2000a).
With a dramatically increasing world population and a world catch of fish of more than 100 million tons per year, there is obviously an increased need to utilize our sea resources with more intelligence and foresight (Kristinsson and Rasco 2000a; Ovissipour et al. 2009a; Ovissipour and Ghomi 2009). By applying enzyme technology for protein recovery in fish processing, it may be possible to produce a broad spectrum of food ingredients and improve and upgrade the functional and nutritional properties of protein (Šližyte et al. 2005a). This would utilize both fisheries byproducts, secondary raw materials, and in addition, underutilized species that would otherwise be discarded or processed to low price (non-value-added products). Fish viscera, one of the most important byproducts, are a rich source of protein and polyunsaturated lipids but with low storage stability if not frozen or otherwise preserved (Raa and Gildberg 1982; Ovissipour and Ghomi 2009).
In the process of hydrolyzation, proteolytic enzymes are used to solubilize the fish protein, resulting in two distinguishable fractions, soluble and insoluble. The insoluble fraction may be used as animal feed (Kristinsson and Rasco 2000a), and the soluble fraction, which contains the hydrolyzed protein, may be converted into a food ingredient, incorporating into food systems, or used as a nitrogen source for bacterial growth (Ovissipour and Ghomi 2009; Safari et al. 2009). Dehydration of the soluble hydrolysate results in a more stable, powder, high in protein content. Such a product is known as fish protein hydrolysate (FPH). Produced under controlled proteolysis, FPH possesses desirable functional properties and high nutritional value (Kristinsson and Rasco 2000a). The variables with the most important roles in this complex enzymatic reaction have been reported to be enzyme concentration, protease specificity of the enzyme, pH and temperature of the reaction, the nature of the protein substrate, and the degree of hydrolysis attained (Adler-Nissen 1986).
Generally, Alcalase® 2.4-L-assisted reactions have been repeatedly favored for fish hydrolysis, due to the high degree of hydrolysis that can be achieved in a relatively short time under moderate pH conditions, compared with the neutral or acidic enzymes (Kristinsson and Rasco 2000a, b; Hoyle and Merritt 1994; Shahidi et al. 1995; Benjakul and Morrissey 1997; Aspmo et al. 2005; Bhaskar et al. 2008; Ovissipour et al. 2009b).
Response surface methodology (RSM) is a useful technique for the investigation of complex processes. It has been successfully applied to optimize seafood processing operations (Shahidi et al. 1995; Diniz and Martin 1997; Gbogouri et al. 2004; Bhaskar and Mahendrakar 2008; Bhaskar et al. 2008). RSM defines the effect of the independent variables alone, and in combinations, in the process. In addition to analyzing the effects of variables, this experimental methodology generates a mathematical model that accurately describes the overall process using a significant estimation (Shankar et al. 2008).
Yellowfin tuna (Thunnus albacares) is one of the most important pelagic species in Iran with an annual catch of 41,000 metric tons (Iranian Fisheries Organization) (IFO 2006). The objective of this study was to optimize reaction conditions (i.e., enzyme activity, temperature, and time) to obtain optimal degree of hydrolysis from visceral waste proteins of yellowfin tuna viscera (T. albacares) using Alcalase® 2.4 L.
Materials and Methods
Materials
Whole yellowfin tuna (T. albacares) caught in the winter in Bandar Abbas, south of Iran, was immediately frozen on board at −20 °C. The fish was delivered to the processing plant (Darya-Khorak Co., Babolsar, Iran) within 2 weeks at −20 °C. The viscera were removed while frozen, using an electric saw, and immediately (1 h) transferred to the laboratory. Once received in the laboratory, fish viscera were minced twice using an industrial mixer at medium speed (5 mm plate size) then pooled, and divided into plastic containers. All raw materials were frozen again at −20 °C until analysis. Compositional analyzing experiments were conducted within 2 days after mince freezing.
Alcalase is a bacterial endoproteinase from a strain of Bacillus licheniformis with a proteolytic activity of 2.4 Anson unit/ml, with activity temperature ranges of 35 to 70 °C (Novozymes 2007). It was provided from the Iranian branch of the Danish company Novozymes (Novozymes, Tehran, Iran) and stored at 4 °C until used. All chemical reagents used for the experiment were of analytical grade.
Preparation of Fish Protein Hydrolysate
Preparation of yellowfin tuna viscera hydrolysates was performed according to our previous study (Ovissipour et al. 2009a, b; Safari et al. 2009). Briefly, the fish viscera were first minced twice using an industrial mixer at medium speed (5 mm plate size), then for each run, a 50 g sample was strewn into the 250 ml glass vessel (Erlenmeyer flask) and cooked at 85 °C in a water bath (W614-B, Fater Rizpardaz, Tehran, Iran) for 20 min to inactivate endogenous enzymes (Guerard et al. 2002; Ovissipour et al. 2009a,b). The cooked viscera were mixed with sodium phosphate buffer 1:2 (w/v) and homogenized in a Moulinex® blender for about 2 min. The pH of the mixture was adjusted to the optimum activity of Alcalase, pH 8.5 by adding 0.2 N NaOH. Enzyme was added according to the experimental runs (Tables 1 and 2). All reactions were performed in a shaking incubator (Ivymen System, Comecta, Spain) with constant agitation (200 rpm). After each sampling, reactions were terminated by heating the solution to 95 °C for 15 min (Guerard et al. 2002; Ovissipour et al. 2009a, b), assuring enzyme inactivation. The hydrolysates were cooled on ice and centrifuged at 8,000 × g at 10 °C for 20 min in Hermle labortechnik GmbH z 206A (Germany) centrifuge, to collect the supernatant. Finally, the soluble phase was spray-dried (inlet air t = 170 °C, outlet air t = 80 °C).
Proximate Composition
Moisture
Moisture content of whole viscera was determined by placing approximately 2 g of sample into a pre-weighted aluminum dish. Samples were then dried in an oven at 105 °C overnight or to constant weight (AOAC 2002).
Crud Protein
Total crude protein (N × 6.25) in raw materials and FPH was determined using the Kjeldahl method (AOAC 2002).
Lipid
Total lipid in samples was determined by Soxhlet extraction (AOAC 2002).
Ash
Ash content was estimated by charring a pre-dried sample in a crucible at 600 °C until a white ash was formed (AOAC 2002).
Liquid Protein
Protein in the supernatant was measured, following centrifugation, by the Biuret method (Layne 1957). Bovine serum albumin was used as a standard protein to determine the standard curve. Absorbance was measured at 540 nm in a UV-vis spectrophotometer (Jenway, 6305, UK).
Optimization Experiments
RSM with a completely randomized factorial design, has been applied to optimize hydrolysis conditions. Different experimental treatments are summarized in Table 1. Three independent variables namely enzyme activity (X 1; Anson Unit/kg protein), temperature (X 2; °C), and time (X 3; minute) were employed at five levels (−α, −1, 0, +1, and +α). Experimental planning was based on a preliminary study of enzymatic hydrolysis suggested by enzyme manufacturer (Novozymes, Bagsvaerd, Denmark) and our previous studies (Ovissipour et al. 2009a, b). In addition, we studied two different RSM experiments with different independent variables ranges, which at least current study RSM has been selected (Table 1). Degree of hydrolysis was measured as a response of the independent variables given in Table 2. The behavior of the system was explained by the following equation:
Where, Y is the dependent variable (degree of hydrolysis in real value), β 0 is constant, β i , β ii and β ij are coefficients estimated by the model. X i and X j are levels of the independent variables which represent the linear, quadratic, and cross-product effects of the X 1, X 2, and X 3 on the response (DH), respectively. The model evaluated the effect of each independent variable to the response (Cao et al. 2008).
In this study, we have used five different enzyme to substrate ratios based on enzyme activity (Anson Unit) (Table 1). One Anson unit (AU) is defined as the amount of enzyme that will release 1.0 mEq of tyrosine from urea–denatured hemoglobin per minute at 25 °C at the pH of 7.5 (Aspmo et al. 2005).
Degree of Hydrolysis
Degree of hydrolysis was estimated according to Hoyle and Merritt (1994) as described previously by Ovissipour et al. (2009a, b). This method is based the enzyme deactivation by lowering the pH. Each run after the specified hydrolysis was terminated by the addition of 20% trichloroacetic acid (TCA) followed by centrifugation to collect the 10% TCA soluble material as the supernatant. Then, degree of hydrolysis was computed as:
Amino Acid Analysis
Sample preparation was conducted by hydrolysis of protein with 6 N HCl at 110 °C for 24 h. Derivatisation was applied using o-phthaldialdehyde prior to HPLC analysis (Antoine et al. 1999). The total amino acids were analyzed by the Knauer (Germany) HPLC set using C18 type column (Knauer, Germany) at the flow rate of 1 ml/min−1 with fluorescence detector (RF-530, Knauer, Germany).
Computation of Chemical Score
The chemical score of the protein hydrolysates was computed to study the nutritional value of tuna protein hydrolysates which is related to the essential amino acid profile in a standard protein as described by FAO/WHO (1990). In brief, the chemical score was calculated using the following equation:
Protein Efficiency Ratio
One of the most important scores for evaluating the nutritional value of proteins is the protein efficiency ratio (PER) which measures protein quality by feeding a diet containing 10% of the test protein to rats and measuring their weight gain. This is an expensive and time-consuming method (Šližyte et al. 2005b). There are mathematical equations, which were developed by Alsmeyer et al. (1974) and Lee et al. (1978) for predicting PER values. These equations have also been applied by some researchers to predict the fish protein hydrolysates (Shahidi et al. 1991, Shahidi 1995; Diniz and Martin 1997; Šližyte et al. 2005b; Ovissipour et al. 2009a).
Statistical Analysis
The optimization experiments were carried out through RSM by using a factorial design (three factors, three levels, and a single block) generated using the experimental design model of the Statistical Analysis System: SAS software release 7 (SAS Institute, Cary, NC, USA) (Little et al. 1991; Nilsang et al. 2005) and MATLAB software release 13.0 (MathWorks Inc., Natick, MA, USA). Significance was determined at a 95% probability level.
Results and Discussion
Proximate Composition
The chemical composition of yellowfin tuna viscera and its protein hydrolysate is shown in Table 3. Fresh yellowfin tuna viscera had a protein content of 21.5% and lipid content of 5.08%. The protein content of the spray dried hydrolysate was 72.34% which is similar to those of other published studies on FPH, which have ranged from 63.4% to 90.8% protein (Shahidi et al. 1995; Onodenalore and Shahidi 1996; Kristinsson and Rasco 2000b; Nilsang et al. 2005; Souissi et al. 2007; Wasswa et al. 2007; Bhaskar et al. 2008; Ovissipour et al. 2009a, b). Lipid content in yellowfin tuna hydrolysate reached to 1.43%. Ovissipour et al. (2009a) found that lipid content of Persian sturgeon viscera hydrolysates after 205 min, with 100 AU/kg crude protein was 0.18%. The lipid content in FPH was greatly reduced when compared with the raw material, because lipids were most likely excluded with the insoluble protein fraction by centrifugal separation (Kristinsson and Rasco 2000b; Nilsang et al. 2005; Ovissipour et al. 2009a) or separated as a thin cream layer at the top of the supernatant. Decreasing the lipid content in the protein hydrolysate might significantly contribute to lipid oxidation stability. This may enhance product stability (Shahidi et al. 1995; Diniz and Martin 1997; Kristinsson and Rasco 2000b; Nilsang et al. 2005).
Optimization of Hydrolysis Parameters for DH
The influence of X 1, X 2, and X 3 on the hydrolysis by Alcalase was determined using factorial design as mentioned in the previous section. The best explanatory model equation for the DH value obtained from Alcalase hydrolysis coded data is described in Eq. 2:
The observed values for DH at different combinations of the independent variables are presented in Table 2. According to the related ANOVA (Table 4), the linear, and quadratic terms were significant (P < 0.01). No cross-product term was significant (P > 0.05). Statistical analysis also indicated that within each term, all three hydrolysis factors had a strong and significant influence on DH (P < 0.05). In fact, Adler-Nissen (1986), investigating the hydrolysis of soy protein by bacterial proteases, pointed out the hydrolyzing conditions markedly influenced the peptide bond cleavage in the protein substrate. The same results were observed by other researchers (Diniz and Martin 1997; Gbogouri et al. 2004; Bhaskar and Mahendrakar 2008; Bhaskar et al. 2008). The results model showed that all linear and quadratic terms contributed to the response which are in agreement with Diniz and Martin (1997). The adjusted coefficient of determination (r 2) implies that 94% of the behavior variation could be explained by the fitted model. Moreover, a lack of fit test, which indicates the fitness of the model obtained, was not significant, indicating that the model is sufficiently accurate for predicting the degree of hydrolysis for any combination of experimental independent variables.
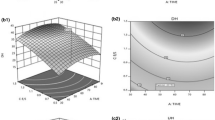
Figure 1 shows the comparison between observed values for the degree of hydrolysis (Y 0) with the predicted values (Y). The plot (Fig. 1) shows an acceptable level of agreement. In addition, coefficient (r 2 = 0.86) reveals a satisfactory mathematical description of the hydrolysis process by the model.
The regression coefficient of DH in this study (r 2 = 0.94) was satisfactory, with a low predicted experimental error (Table 4). High correlations of experimental results with those predicted by RSM models for proteolytic reactions have been reported by several researchers. Bhaskar et al. (2008) reported similar results for DH in hydrolysate produced from Catla viscera (Catla catla) using an alkaline protease.
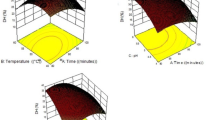
Contour plots and response surface graphs were generated by the predictive model to predict the critical points and the effectiveness of each factor. The combined effects of each pair of variables indicate that in the hydrolysis of yellowfin tuna protein, an increase in DH is achieved by increases in enzyme activity, temperature, and reaction time (Fig. 2), up to certain levels, DH slightly decreases beyond those certain criteria. Such a decrease in hydrolysis rate over higher enzyme activity values, temperatures, and time may be due to denaturation of the protease enzyme and reducing its biological activity (Guerard et al. 2002; Ovissipour et al. 2009a). Similar dependence, between enzyme activity, temperature, and reaction time has been observed for hydrolytic reactions of food proteins using enzymes of microbial origin (Shahidi et al. 1995; Diniz and Martin 1997; Nilsang et al. 2005; Bhaskar and Mahendrakar 2008; Bhaskar et al. 2008). A Decrease in DH% by increasing time, was reported by many researchers (Guerard et al. 2002; Souissi et al. 2007; Wasswa et al. 2007; Ovissipour et al. 2009a, b). Guerard et al. (2002) pointed that reduction in DH% by increasing time may be due to the limitation of enzyme activity by formation of reaction products at high degree of hydrolysis, decrease in concentration of peptide bonds available for hydrolysis, enzyme inhabitation, and enzyme deactivation.
The optimum conditions (enzyme activity, temperature, and time) were predicted using response surface graphs and contour plots for DH (Fig. 2). Figure 2a shows the effect of time and temperature on DH at pH of 8.5. A Quadratic effect of time and temperature was significant. The results indicated that DH increases up to 53% with an increase in temperature (to a maximum of 65 °C), and reaction time up to 90 min. Hydrolysis at higher temperatures, and longer time results in higher DH values, but a decrease in the rate of hydrolysis. Figure 2b shows the effect of time and enzyme activity on DH. Reaction time and enzyme activity had quadratic effect on DH. The highest DH was observed at the enzyme activity of 70.22 AU/kg protein. DH values decreased at higher activities, showing that enzyme to substrate ratio is a critical factor in enzymatic hydrolysis, which may function as a limiting factor at higher values in process. In a study related to hydrolyzing Pacific whiting solid waste with Alcalase, it has been observed that an increase in enzyme concentration, although causing an increase in DH, results in a reduced rate of DH (Benjakul and Morrissey 1997). These results have also been reported by Bhaskar et al. (2008) and Bhaskar and Mahendrakar (2008). In addition, Batista et al. (2010) reported that an increment in enzyme concentration, although causes an increment in DH, but also results in a reduce rate of hydrolysis.
The effect of temperature and enzyme activity on DH is shown in Fig. 2c. There is a quadratic effect of temperature and enzyme activity on DH value. However, higher temperatures (more than 60 °C) result in higher DH values.
These results suggest that a response surface model can be used to predict optimal hydrolysis conditions. The stationary point (maximum) of the fitted model was found by deriving first derivatives of the function in Eq. 3:
Optimum reaction conditions to reach the highest degree of hydrolysis are shown in Table 5. Reaction conditions were 60.4 °C for hydrolyzing temperature, 90.25 min for hydrolyzing time, and 70.22 AU/kg protein for enzyme activity at the stationary point. Gbogouri et al. (2004) assumed that the optimum conditions for hydrolyzing salmon byproducts using Alcalase were 55 °C and an enzyme to substrate ratio of 5%. Bhaskar et al. (2008) found that the optimum conditions for hydrolyzing visceral waste proteins from Indian carp (C. catla) to reach 50% DH, were 135 min, 55 °C, and Alcalase enzyme concentration of 11 AU/l-protein extract at pH 8.5. Benjakul and Morrissey (1997) evaluated different combinations of reaction conditions for hydrolyzing proteinaceous waste materials recovered from processing Pacific whiting (Merluccius productus), however they have reported lower DH in their study. A high degree of hydrolysis may reduce the bitterness of the final product (Adler-Nissen 1984). It has been reported that Alcalase tends to produce less bitter hydrolysates compared with other proteases (Hoyle and Merritt 1994; Benjakul and Morrissey 1997). Furthermore, it is well known that the peptide chain length and DH depends upon the extent of hydrolysis, conditions of hydrolysis, enzyme concentration, and type of the substrate proteins (Kristinsson and Rasco 2000a). Hence, the optimum conditions for hydrolyzing different substrates will be different and will vary depending upon the substrate used, particularly with the content and reactivity of any endogeneous proteases present.
Amino Acid Composition
The amino acid composition of yellowfin tuna visceral protein hydrolysates (n = 2), and chemical scores are presented in Table 6. The amino acids represent more than 80% of the total amino acid profile of yellowfin tuna visceral protein hydrolysate.
The chemical score provides an estimate of the nutritive value of a protein. This parameter compares levels of essential amino acids between the test, and the standard proteins (Ovissipour et al. 2009a). In the current study, computed chemical scores are based on the reference protein of FAO/WHO (1990) for adults and amino acid requirements of juvenile common carp, as listed by NRC (1993). The amino acid composition in this study and comparison with reference proteins indicates that the amino acid profiles of the yellowfin tuna viscera hydrolysates were generally higher in essential amino acids, compared with the suggested amino acid pattern recommended by FAO/WHO for adult humans except in terms of methionine. Similar results are reported by Ovissipour et al. (2009a) for Persian sturgeon viscera hydrolysates. The chemical score of the yellowfin tuna visceral protein hydrolysates shows that lysine, methionine, and phenylalanine are the most limiting amino acids, and that other amino acids are present at levels exceeding the requirements of juvenile common carp (Cyprinus carpio) (NRC 1993) (Table 6). Furthermore, for many fish species including carp, growth rates produced by diets with large amounts of free amino acids are inferior to diets of similar amino acid composition in which the nitrogen component is in the form of protein (Walton et al. 1986; Dabrowski and Guderley 2002). Using hydrolysates with an intermediate chain length and limited amounts of free amino acids would be a valuable ingredient in formulated and nutritionally balanced fish diets (Pigott and Tucker 2002). These results are in agreed with our previous study on Persian sturgeon hydrolysates chemical score. Bhaskar et al. (2008) by studying the chemical score of Catla Alcalase hydrolysates, founded that methionine and phenylalanine are first and second amino acids in compared with common carp requirements, respectively.
The results of PER values are presented in Table 7. PER values in the current study were 2.85–5.38 for Alcalase hydrolysates. PER values of 2.86–3.24 for cod hydrolysates and 2.61–3.11 for capelin hydrolysates were reported by Shahidi et al. (1991, 1995), respectively. Diniz and Martin (1997) reported that, PER for dogfish hydrolysates by Alcalase were 2.9–3.14. Šližyte et al. (2005b) based on their PER results, assumed that, cod visceral hydrolysates have high nutritional value. In addition, Ovissipour et al. (2009a) reported 2.4–6.45 PER for Persian sturgeon visceral protein hydrolysates by Alcalase. The results of PER indicated that, the tuna visceral protein hydrolysate has good potential as feed ingredient.
Conclusion
Yellowfin tuna (T. albacares) is one of the most important pelagic species in Iran with an annual catch of 41,000 metric tons. Hydrolysis of Yelowfin tuna visceral waste protein using Alcalase resulted in DH values of more than 53%. The DH is significantly influenced by enzyme activity, reaction time, and temperature. RSM used for optimizing of hydrolysis conditions resulted in a temperature of 90.25 °C for 60.4 min and on enzyme activity of 70.22 AU/kg protein. The yellowfin tuna viscera hydrolysate has relatively high protein (72.34%) and low lipid content (1.43%). Based on tuna visceral hydrolysates amino acid composition and PER, the hydrolysate prepared from visceral waste has high potential for applications in aquaculture and animal feeds. It is also an effective nitrogen source (as peptone) for microbial growth media.
References
Adler-Nissen, J. (1984). Control of the proteolytic reaction and of the level of bitterness in protein hydrolysis process. Journal of Chemical Technology and Biotechnology, 34B, 215–222.
Adler-Nissen, J. (1986). Enzymatic hydrolysis of food proteins (pp. 57–109). Copenhagen: Elsevier Applied Science Publishers.
Alsmeyer, R. H., Cunningham, A. E., & Happich, M. L. (1974). Equations predicting PER from amino acid analysis. Food Technology, 28(7), 34–40.
Antoine, F. R., Wei, C. I., Littell, R. C., & Marshall, M. R. (1999). HPLC method for analysis of free amino acids in fish using o-Phthaldialdehyde precolumn derivatization. Journal of Agricultural and Food Chemistry, 47, 5100–5107.
AOAC (2002). In: Hortwitz W (ed) Official Methods of Analysis of AOAC International, 17th edn. Gaithersburg: AOAC International
Aspmo, S. I., Horn, S. J., & Eijsink, V. G. H. (2005). Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochemistry, 40, 1957–1966.
Batista, I., Ramos, C., Coutinho, J., Bandarra, N. M., & Nunes, M. L. (2010). Characterization of protein hydrolysates and lipids obtained from black scabbardfish (Aphanopus carbo) by-products and antioxidative activity of the hydrolysates produced. Process Biochemistry, 45, 18–24.
Benjakul, B., & Morrissey, M. T. (1997). Protein hydrolysates from Pacific whiting solid wastes. Journal of Agricultural and Food Chemistry, 45, 3423–3430.
Bhaskar, N., & Mahendrakar, N. S. (2008). Protein hydrolysate from visceral waste proteins of Catla (Catla catla): optimization of hydrolysis conditions for a commercial neutral protease. Bioresource Technology, 99(10), 4105–4111.
Bhaskar, N., Benila, T., Radha, C., & Lalitha, R. G. (2008). Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresource Technology, 99(2), 335–343.
Cao, W., Zhang, C., Hong, P., & Ji, H. (2008). Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chemistry, 109, 176–183.
Dabrowski, K., & Guderley, H. (2002). Intermediary metabolism. In J. E. Halver & R. W. Hardy (Eds.), Fish Nutrition (3rd ed., pp. 309–367). Boston: Elsevier Science.
Diniz, A. M., & Martin, A. M. (1997). Optimization of nitrogen recovery in the enzymatic hydrolysis of dogfish (Squalus acanthias) protein: Composition of the hydrolysates. International Journal of Food Sciences and Nutrition, 48, 191–200.
FAO. (2006). Food and agricultural organisation of the United Nations. Year book of fishery statistics. Rome, 98, 1&2.
FAO/WHO (1990). Energy and protein requirements. Report of joint FAO/WHO/UNU Expert Consultation Technical Report. FAO/WHO and United Nations University, Geneva, Series No. 724, pp. 116–129.
Gbogouri, G. A., Linder, M., Fanni, J., & Parmentier, M. (2004). Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. Journal of Food Science, 69, 615–622.
Guerard, F., Guimas, L., & Binet, A. (2002). Production of tuna waste hydrolysates by a commercial neutral protease preparation. Journal of Molecular Catalysis. B, Enzymatic, 19–20, 489–498.
Hoyle, N. T., & Merritt, J. H. (1994). Quality of fish protein hydrolysate from herring (Clupea harengus). Journal of Food Science, 59, 76–79.
IFO (2006). Iranian Fisheries Organization. www.shilat.com
Kristinsson, H. G., & Rasco, B. A. (2000a). Fish protein hydrolysates: production, biochemical and functional properties. Critical Reviews in Food Science and Nutrition, 40, 43–81.
Kristinsson, H. G., & Rasco, B. A. (2000b). Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. Journal of Agricultural and Food Chemistry, 48, 657–666.
Layne, E. (1957). Spectrophotometric and turbidimetric methods for measuring proteins. Methods in Enzymology, vol. 3 (p. 450). New York: Academic Press.
Lee, Y. B., Elliot, J. G., Rickansrud, D. A., & Mugberg, E. C. (1978). Predicting protein efficiency ratio by the chemical determinations of connective tissue content in meat. Journal of Food Science, 43, 1359–1362.
Little, R. C., Feund, R. J., & Spector, P. C. (1991). SAS system for linear models (3rd ed.). Cary: SAS Institute.
Nilsang, S., Lertsiri, S., Suphantharika, M., & Assavanig, A. (2005). Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. Journal of Food Engineering, 70, 571–578.
Novozymes. (2007). Alcalase® 2.4 L FG. Product data sheet. www.novozymes.com.
NRC (1993). National Research Council. National Academy of Sciences, Nutrient Requirements of Fish (p. 124). Washington: National Academy Press.
Onodenalore, A. C., & Shahidi, F. (1996). Protein dispersions and hydrolysates from shark (Isurus oxyrinchus). Journal of Aquatic Food Product and Technology, 5, 43–59.
Ovissipour, M., & Ghomi, M. R. (2009). Biotechnology in seafood production (1st ed., pp. 1–198). Tehran: Islamic Azad University Publication.
Ovissipour, M., Abedian, A. M., Motamedzadegan, A., Rasco, B., Safari, R., & Shahiri, H. (2009a). The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from the Persian sturgeon (Acipenser persicus) viscera. Food Chemistry, 115, 238–242.
Ovissipour, M., Safari, R., Motamedzadegan, A., & Shabanpour, B. (2009b). Chemical and biochemical hydrolysis of Persian sturgeon (Acipenser persicus) visceral protein. Food and Bioprocess Technology, doi:10.1007/s11947-009-0284-x
Pigott, G., & Tucker, B. (2002). Special feeds. In J. E. Halver & R. W. Hardy (Eds.), Fish nutrition (3rd ed., pp. 651–669). Boston: Elsevier Science.
Raa, J., & Gildberg, A. (1982). Fish silage: a review. CRC Critical Reviews in Food Science and Nutrition, 16, 383–419.
Safari, R., Motamedzadegan, A., Ovissipour, M., Regenstein, J. M., Gildberg, A., & Rasco, B. (2009). Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food and Bioprocess Technology. doi:10.1107/s11947-009-0225-8.
Shahidi, F., Naczk, M., Pegg, R. B., & Synowiecki, J. (1991). Chemical composition and nutritional value of processing discards of cod (Gadus murhua). Food Chemistry, 42, 145–151.
Shahidi, F., Han, X. Q., & Syniwiecki, J. (1995). Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chemistry, 53, 285–293.
Shankar, T. J., Sokhansanj, S., Bandyopadhyay, S., & Bawa, A. S. (2008). A case study on optimization of biomass flow during single-screw extrusion cooking using genetic algorithm (GA) and response surface methodology (RSM). Food and Bioprocess Technology. doi:10.1007/s11947-008-0172-9.
Šližyte, R., Rustad, T., & Storrø, I. (2005a). Enzymatic hydrolysis cod (Gadus morhua) by-products, optimization of yield and properties of lipid and protein fractions. Process Biochemistry, 40, 3680–3692.
Šližyte, R., Daukšas, E., Falch, E., Storrø, I., & Rustad, T. (2005b). Characteristics of protein fractions generated from cod (Gadus morhua) by-products. Process Biochemistry, 40, 2021–2033.
Souissi, N., Bougatef, A., Triki-Ellouz, Y., & Nasri, M. (2007). Biochemical and functional properties of Sardinella (Sardinella aurita) by-product hydrolysates. Food Technology and Biotechnology, 45, 187–194.
Walton, M. J., Cowey, C. B., Coloso, R. M., & Adron, J. W. (1986). Dietary requirements of rainbow trout for tryptophan, lysine and arginine determined by growth and biochemical measurements. Fish Physiology and Biochemistry, 2, 161–169.
Wasswa, J., Tang, J., Gu, X. H., & Yuan, X. Q. (2007). Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chemistry, 104, 1698–1704.
Acknowledgments
We express our thanks to the Ministry of Science and Tarbiat Modares University (TMU, Iran) for financial and technical supports. We would like to appreciate Prof. Turid Rustad, Prof. Barbara Rasco, Prof. David W. Levine and Mr. Ali Taheri for their scientific supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ovissipour, M., Abedian Kenari, A., Motamedzadegan, A. et al. Optimization of Enzymatic Hydrolysis of Visceral Waste Proteins of Yellowfin Tuna (Thunnus albacares). Food Bioprocess Technol 5, 696–705 (2012). https://doi.org/10.1007/s11947-010-0357-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0357-x