Abstract
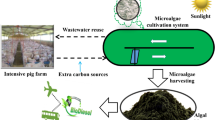
Treatment of slaughterhouse wastewater is a huge industrial problem. The use of an algae biorefinery platform could be a sustainable technological alternative that produces value-added compounds instead of dumping the wastewater. For this reason, this research aimed to evaluate squalene production from the microalgae Phormidium autumnale cultivated using agroindustrial wastewater. A derivatization method was performed to determine the squalene and fatty acids content, evaluated by gas chromatography with flame ionization and mass spectrometry detectors. A total of 0.18 g/kg of squalene were found in the biomass, with a high content of unsaturated fatty acids (52%). Sensitivity analysis estimated production of 727–72,750 kg/year in industries with different capacities. In this sense, P. autumnale in agroindustrial wastewater could offer a potential alternative method of squalene production.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Squalene, a natural triterpene, is a putative head-to-head condensation product of farnesyl diphosphate, catalyzed by squalene synthase enzymes [1]. Squalene biosynthesis in prokaryotic microorganisms occurs until the building blocks isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), a pathway that is also responsible for the formation of many terpenoids [2]. Squalene can be found in diverse types of cells, playing an important role as an intermediate in sterol biosynthesis, and also impacts human health [3]. Various positive effects of this triterpene on health have been reported, including for use in the treatment of cancer, in lipid-based anticancer prodrugs for chemotherapy, as an antiviral treatment against the hepatitis C virus, as a means of cardiovascular protection, and for its antioxidant activity [4,5,6,7,8,9].
Shark liver oil, traditionally, is the richest source of squalene, but use of this resource conflicts with marine wildlife preservation. In this sense, the biotechnological route of squalene production has been increasing, especially given the higher productivity and yield obtained from this process [10].
In this sense, microalgae biotechnology offers an alternative for squalene production, as shown by the microalgae Aurantiochytrium mangrovei, which presents high squalene content that ranges from 0.2 to 0.4 g/kg of cellular dry mass [11, 12]. The cyanobacteria Synechocystis sp. PCC 6803 was used for the same purpose, genetically engineered to optimize yield, with accumulation of 0.80 g/kg [13]. There are some differences among cell structures of microalgae. Aurantiochytrium mangrovei is a eukaryote algae, while Synechocystis sp. PCC 6803 is a prokaryote microorganism. The largest difference is the metabolic pathway involved in squalene production. In eukaryotic cells, two pathways are responsible for squalene production, the mevalonate pathway (MVA) and the methylerythritol pathway (MEP). For prokaryotic cells, by contrast, MEP is responsible for producing this secondary metabolite [14]. Cyanobacteria are today a potential source of many bioactive compounds, mostly secondary metabolites, and the production of these compounds is strongly related to environmental conditions [15]. In this sense, genetic engineering is not always necessary to produce squalene; it may be accomplished through environmental modification to activate a specific metabolic route.
Cyanobacteria are photosynthetic, prokaryotic microorganisms and can produce numerous metabolites in diverse environments because they are robust and grow in different temperatures support high nutrient concentrations, and have metabolic versatility, belong to a group of diazotrophs, which allows some strains, to perform nitrogen fixation [16]. Therefore, some strains can also achieve respiration using an exogenous source of carbon [17]. These particularities allow wastewater to be used as a carbon source providing tertiary treatment, using substrates with higher concentrations of nitrogen and phosphorous. The Phormidium autumnale cyanobacteria is a filamentous cyanobacterium, that possesses these abilities [18]. Also, another study observed that this strain demonstrates high capacity for the removal of organic matter [19] alongside the simultaneous production of high-value, unsaponifiable compounds, such as carotenoids [15, 20, 21].
In fact, agroindustrial wastewater is a great alternative for use as a carbon source, offering new technological routes to produce valuable compounds from biomass [22]. In this context, another study reported using living organisms to produce fine chemicals, such as polyhydroxyalkanoates (PHAs), from slaughterhouse wastes, proving that these processes emerge as a “white biotechnology” process [23]. Also, use of industrial slaughterhouse waste reduces process costs while simultaneously minimizing hazardous environmental emissions [24].
For reasons of environmental policy, then, and in light of economic, sustainability, and practical perspectives, it is important to demonstrate the feasibility of such bio-products [25]. These bio-compounds could emerge from algae biorefinery platforms, thus reducing the environmental implications of their waste precursors [26]. In this regard, this work aims to evaluate a sustainable route to produce squalene from biomass of the cyanobacteria Phormidium autumnale.
Materials and Methods
Microorganisms and Culture Media
Stock axenic cultures of P. autumnale, originally isolated from the Cuatro Cienegas desert (26°59′N, 102°03′W, in Mexico), were propagated and maintained in solidified agar–agar (20 g/L) containing synthetic BG11 medium [27]. The incubation conditions used were 25 °C, a photon flux density of 15 µmol/(m s), and a photoperiod of 12/12 h light/dark.
Wastewater
Slaughterhouse wastewater was acquired from an industrial facility located in Santa Catarina, Brazil (27º14′02″S, 52º01′40″W). This wastewater, obtained from the discharge point of an equalization tank over a period of 1 year, was carefully studied for hydrogenionic potential (pH), chemical oxygen demand (COD), total phosphorus (P–PO43−), Total Kjeldahl Nitrogen (N-TKN), volatile solids (VS), fixed solids (FS), total solids (TS), and suspended solids (SS), following the standard methods for the examination of water and wastewater [28]. The average composition of the wastewater was pH 5.9 ± 0.05, COD 4100 ± 874 mg/L, P–PO43− 2.84 ± 0.2 mg/L, N-TKN 128.5 ± 12.1 mg/L, VS 2.9 ± 1.4 mg/L, FS 0.9 ± 0.3 mg/L, TS 3.8 ± 2.7 mg/L, SS 1.9 ± 0.8 mg/L.
Production of Microalgal Biomass
Biomass was produced in heterotrophic conditions, using slaughterhouse wastewater as the culture medium. Cultivations were performed in a bubble column bioreactor [29] operating under a batch regime and fed on 2.0 L of wastewater. The experimental conditions were as follows: initial concentration of inoculum 100 mg/L, temperature 26 °C, pH adjusted to 7.6, carbon-to-nitrogen ratio of 30 (adjusted when necessary with glucose), aeration of one unit volume of air per unit volume of wastewater per min, absence of light, and residence time of 168 h [17]. The biomass was separated from the wastewater by centrifugation and subsequently freeze-dried for 24 h at − 50 °C under − 175 mmHg. The cultivations were performed twice and in duplicate, so experimental data refer to the mean value of the four repetitions.
Sampling and Data Analysis
The samples were collected aseptically every 24 h during the microorganism growth phase, and biomass data were used to calculate the biomass [PX = (Xi − Xi − 1)⋅(ti − ti − 1)−1, mg/(L h)] and lipid [PL = PX ⋅ L, mg/(L h)] productivities, in which Xi is the biomass concentration at time ti (mg/L), Xi − 1 is the biomass concentration at time ti − 1 (mg/L), t is the residence time (h), and L is the lipid content of the Phormidium autumnale biomass (%). Total concentrations of organic carbon were used to calculate the substrate consumption rate rS = dS/dt, mg/(L h), and the biomass yield coefficient YX/S = dX/dS (g cell/g) substrate, where S0 is the initial substrate concentration (mg/L), S is the substrate concentration (mg/L), and t is the time (h).
Experiment
Reagents
The following analytical grade reagents were used: methanol and chloroform from Vetec (São Paulo, SP, Brazil); anhydrous sodium sulfate, sodium methoxide, methanolic solution (1 M), methyl acetate, and diethyl ether from Sigma-Aldrich (St. Louis, MO, USA); and oxalic acid from Synth (São Paulo, SP, Brazil). Hydrochloric acid and 0.05% butyl hydroxyl toluene (BHT) from Dinâmica (São Paulo, SP, Brazil) were used in the chloroform extractor. Hexane was also from Dinâmica (São Paulo, SP, Brazil). A squalene standard (98.9%) and a mixture of fatty acid methyl esters (FAME) Mix-37 (P/N 47885-U) were obtained from Sigma-Aldrich. The squalene stock solution, with 1 mg/mL concentration, was prepared by weighing 10 mg of the standard in a volumetric flask of 10 mL and completing with hexane.
Lipid Extraction
The total lipid fraction from the dry biomass was extracted by a modified version of the method of Bligh and Dyer [30]. In this method, around 0.5 g of cyanobacteria samples were pre-treated with 5 mL hydrochloric acid (2 M) solution to allows the cells rupture. Afterward, extraction was performed in the absence of light, and the lipid content was determined gravimetrically. After each extraction procedure, the chloroform–lipid extracts were evaporated at 50 °C under vacuum (− 760 mmHg) and submitted to the transesterification method.
Derivatization
Fatty acid methyl esters were obtained following Christie [31]. Around 50 mg of microalgal lipid extract was inserted into a flask tube, to which 2 mL of hexane plus 40 µL of methyl acetate were added. Homogenization by vortex followed for 30 s. Then, 60 µL of sodium methoxide methanolic solution (1 M; methylation solution) was added, with shaking for 2 min. A solution of oxalic acid in diethyl ether (0.4 M; termination solution) solubilized the polar lipids. Then, hexane was added, standing alone for 1 h at ambient temperature, and the extract was centrifuged at 1775×g for 5 min. The supernatant was transferred into a 1.5 mL vial for further chromatographic analysis.
Fatty Acid Profile
The methylated samples were analyzed using a gas chromatographic instrument equipped with a flame ionization detector (GC-FID), Varian 3400 (Palo Alto, CA, USA), and an autosampler, Varian 8200 (Palo Alto, CA, USA). An aliquot of 1 µL of the sample was injected into a split/splitless injector, operating in split mode, with a 50:1 ratio at 240 °C. The carrier gas was hydrogen under constant pressure of 20 psi. The FAME were separated using a capillary column, SP-2560 Supelco (Bellefonte, PA, USA, 100 m × 0.25 mm × 0.20 µm). The temperature of the oven was initially 80 °C (hold time of 5 min). Afterward, the temperature was increased to 175 °C at the rate of 15 °C/min, to 190 °C at the rate of 5 °C/min, and finally up to 240 °C at the rate of 8 °C/min, maintaining isothermal conditions for 15 min. The FID temperature was held steady at 280 °C.
The FAME were identified using the authentic standard, FAME Mix-37. The fatty acid methyl ester standard was evaluated under the same conditions; consequently, its retention times were used to identify the fatty acids, expressed as percentages of the total chromatographic area.
Squalene Determination
The same FAME extract was used for squalene analysis. The injection port of the GC-FID was operated in splitless mode (splitter valve off by 0.8 min; 30:1) at 280 °C. Hydrogen at constant pressure of 15 psi was used as the carrier gas. Separation was performed in a non-polar column, RTX-5MS Restek (Bellefonte, PA, USA, 30 m × 0.25 mm i.d. × 0.25 μm). The temperature was initially 200 °C, with an increase to 280 °C at a rate of 15 °C/min and then to 330 °C at a rate of 5 °C/min, maintaining isothermal conditions for 10 min. The temperature of the detector was maintained at 280 °C.
Quantification was acquired using a five-point analytical curve (10–50 mg/L), and some validation parameters were studied, such as linearity. For this purpose, a linear regression equation was used to determine the linear correlation coefficient (R2) of the calibration curve, with precision expressed as relative standard deviation (RSD). The limit of detection (LOD) was estimated according to the concentration of the compound at a signal-to-noise ratio of three. The limit of quantification (LOQ) was achieved by injecting sequential dilutions of the standards, with the LOQ calculated as the concentration that results in a signal-to-noise ratio greater than or equal to 10. Accuracy was determined by a recovery assay from samples spiked with a known (20 mg/L) amount of the standard, with accuracy expressed as percentage recovered of the standard.
Positive identification of squalene in the samples was performed by comparing the retention time and mass spectra obtained experimentally from an authentic standard solution. Identification was performed using gas chromatography coupled to a mass spectrometer (GC/MS), Shimadzu QP-2010 Plus (Tokyo, Japan), under the same chromatographic conditions as described for GC-FID, except with helium as the carrier gas. The GC/MS interface and ion source (+ 70 eV) were held at 280 °C, and the single, quadrupole mass analyzer was operated in scan mode (35–350 m/z).
Sensitivity Analysis: Estimate of Squalene Production
The annual production of squalene by the biomass was estimated for industries with different capacities (100; 1000; and 10,000 m3/d) operating 24 h per day, 336 days per year. Biomass and squalene concentration data were used to calculate squalene productivity [PS = PX⋅S, mg/(L h)], where PX is the biomass productivity and S is the squalene concentration in the biomass (mg/g).
Results and Discussion
Squalene Determination in Cyanobacterial Biomass
Squalene was positively identified in the biomass lipid extract, and suitable chromatographic selectivity (Fig. 1). In the evaluated linear range (10–50 mg/L), a correlation coefficient of 0.998 was observed, indicating satisfactory linearity under this method. The calibration curve was constructed by plotting the peak area versus the squalene concentration, with parameters of 3822.1 for the slope and 14,216 for the intercept. This method shows a LOD and LOQ of 0.3 and 1.0 ng/L, respectively. The precision of this extraction method, expressed as relative standard deviation (RSD), was 12.0%. Accuracy was acquired in triplicate, with a spike of 20 mg/L standard recovered and an average result of 101%. to European Commission [32], acceptable values of recovery range from 70 to 120%; hence, the result in this instance can be considered acceptable.
Estimate of Squalene Production in Heterotrophic Cultivation
Squalene production by the cyanobacteria Phormidium autumnale was observed, with the values shown in Table 1. Squalene was quantitatively evaluated in dry biomass to have a concentration of 0.18 g/kg. The total lipid fraction extracted was 103.0 g/kg. Regarding growth kinetics, this study showed high biomass productivity of 15 mg/(L h). Also, considering that this bioactive compound is lipophilic and intracellular, squalene productivity is expressed as squalene content multiplied by biomass productivity, giving squalene productivity of 5.6 × 10−3 mg/(L h) in parallel with 1.5 mg/(L h) of lipid productivity. Additionally, the cultivation system is related to conversion of organic carbon alongside the production of compounds with metabolic activity. Observed carbon conversion yields amounted to 5.8 × 10−2 (mgsqualene/gcarbon), lipid conversion yields were 33.0 (mglipids/gcarbon), and biomass yields were 320 (mgbiomass/gcarbon) with the cultivation system used.
In this sense, considering that today’s global market has increased demand for bioactive compounds, exploration of cyanobacterial biomass for industrial-scale production, has a great future, because such biomass is established as a commercial source of high-value chemicals and commercial products, such as β-carotene, docosahexaenoic, eicosapentenoic acid, and phycobilin pigments [33,34,35,36].
Distinct studies have not detected squalene in wild types microalgae cell of the species Chlamydomonas reinhardtii and Phaeodactylum tricornutum [37, 38]. Therefore, some strains are squalene accumulators, such as Schizochytrium mangrovei and Botryococcus braunnii, for which studies found concentrations of 0.16 and 1.80 g/kg, respectively [39, 40].
Likewise, distinct biotechnological routes for the microbial production of squalene have been explored, including using the Saccharomyces cerevisiae strain, which presents a concentration of 0.04 g/kg, lower than the result acquired from the present work. On the other hand, in one study, Saccharomyces cerevisiae presented a concentration of 1.30 g/kg at the end of the fermentation process [41]. Also, the yeast Torulaspora delbrueckii showed elevated production of 0.43 g/kg [42].
Unfortunately, deep-sea shark liver oil is the most common form of squalene isolated for supplemental use by the pharmaceutical industry. Squalene is commonly found in deep-sea sharks due to its role in neutral buoyancy. The squalene concentration is species-dependent. For example the Portuguese dogfish presents with 37.37%, Leafscale gueper with 49.89% and Black dogfish with 35.38% of the liver oil [43], while Centrophorus scalpratus, at 82.00%, presents substantially higher [44].
However the cyanobacteria can stand out as squalene producer, because demonstrate higher production rates during entire year and do not require a long period of time to acquire the compound, representing a renewable source. In addition, squalene’s several demonstrated applications in the food industry [45]. Also, considering that, the Phormidium autumnale possess ability to grow in diverse environments, as shown by the high biomass conversion efficiency of manipueira (cassava wastewater) under heterotrophic cultivation, which results in biofuel production [46]. Likewise, this cyanobacteria have been reported as potential producer of carotenoids; when biomass is cultivated in agroindustrial wastewater, pronounced production of 107,902 kg/year was obtained [21]. Therefore, a techno-economic analysis using the Phormidium autumnale strain showed that, using agroindustrial wastewater and estimating commercial biomass value at USD 480/ton, a profit margin of 94% is possible to obtain [47].
Given this possibility, a sensitivity analysis was performed to express the production of squalene from cyanobacterial biomass. The calculations assumed different industrial capacities. Given a small industry with 100 m3/days of capacity it is possible to produce 727 kg/year of squalene. In turn, a midsize industry of 1000 m3/days, can be produced 7275 kg/year of squalene, and a large industry of 10,000 m3/days, can produce 72,750 kg/year of squalene.
Further, in comparison with deep-sea sharks. For an adult Echinorhinus brucus shark species, the average squalene concentration in the liver oil on average is 38.5% [48], the liver from male sharks weights about 4.25 kg [49], and it is well known that the oil content can range from 10 to 70% in shark livers [50]. Given 10% squalene concentration in shark liver oil, one animal on average reaches 0.16 kg of squalene, while 70% squalene concentration in shark liver oil would reach 1.15 kg of squalene per animal. These concentrations promote intense shark hunting. From this traditional source, in order to obtain an amount similar to that which could be acquired from a small industry, it is necessary to slaughter at least 635 of sharks containing 70% of liver oil and 4446 animals with livers containing 10% of the oil. Squalene extraction from cyanobacteria by using food wastewater as a substrate proved to be an alternative to shark slaughter. For this reason, extracting squalene from cyanobacteria using food wastewater as a substrate proved to be an alternative to shark slaughter.
Finally, the squalene content of a microorganism is located in the cells, more specifically, the configuration of squalene determines its location, for example, if the structure of squalene is similar to a sterol configuration, it probably stays into the cellular membrane [2]. Thus, the squalene in cyanobacteria should be considered an intracellular component, situated in the lipid fraction, the extraction of which occurs simultaneously with the extraction of lipid content from the biomass.
Generally, the oil composition of single cell lipid presents metabolites with high nutritional value beyond squalene, such as fatty acids, particularly those with an unsaturated profile. Ten fatty acids were identified in the cyanobacterial biomass, as shown in Table 2. Altogether, 48% were saturated (SFA) and 52% were unsaturated fatty acids; 26.4% were monounsaturated (MUFA) and 25.5% were polyunsaturated fatty acids (PUFA). Also, a representative amount of palmitic acid (C16:0), oleic acid (18:1n9), and stearic acid (C18:0) were found in this biomass. Similarly, in the same strain, although in another study, shared C18:0, C16:0 and C18:1n9 fatty acids were demonstrated as major compounds [51]. Besides, cyanobacterial oil contains a composition of all lipid, including a fatty acid profile similar to that of shark liver oil [52].
Conclusion
This study investigated cyanobacteria as a powerful, sustainable, novel route to obtain squalene and compares this method with non-renewable sources. Also, the results demonstrated effective kinetic parameters. Indeed, the sensitivity analysis demonstrates the production of bioactive compounds in the amounts of kilograms per year given diverse industrial capacities and quantities of slaughterhouse wastewater emissions.
References
Xu, W., Ma, X., Wang, Y.: Production of squalene by microbes: an update. World J. Microbiol. Biotechnol. 32, 195 (2016)
Spanova, M., Daum, G.M.: Squalene–biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 113, 1299–1320 (2011)
Mura, S., Bui, D.T., Couvreur, P., Nicolas, J.: Lipid prodrug nanocarriers in cancer therapy. J. Control. Release. 208, 25–41 (2015)
Xu, R., Fazio, G.C., Matsuda, S.P.: On the origins of triterpenoid skeletal diversity. Phytochemistry. 65(3), 261–291 (2004)
Narayan Bhilwade, H., Tatewaki, N., Nishida, H., Konishi, T.: Squalene as novel food factor. Curr. Pharm. Biotechnol. 11, 875–880 (2010)
Reddy, L.H., Couvreur, P.: Squalene: a natural triterpene for use in disease management and therapy. Adv. Drug Deliver. Rev. 61, 1412–1426 (2009)
Roselló-Soto, E., Koubaa, M., Moubarik, A., Lopes, P.R., Saraiva, A.J., Boussetta, N., Grimi, N., Barba, F.J.: Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Tech. 45, 296–310 (2015)
Saito, K., Shirasagoa, Y., Suzukic, T., Aizakid, H., Hanadaa, K., Wakitad, T., Nishijimae, M., Fukasawa, M.: Targeting cellular squalene synthase, an enzyme essential for cholesterol biosynthesis, is a potential antiviral strategy against hepatitis C virus. J. virol. 89, 2220–2232 (2015)
Wolosik, K., Knas, M., Zalewska, A., Niczyporuk, M., Przystupa, A.W.: The importance and perspective of plant-based squalene in cosmetology. J. Cosmet. Sci. 64(1), 59–66 (2013)
Ghimire, G.P., Thuan, N.H., Koirala, N., Sohng, J.K.: Advances in biochemistry and microbial production of squalene and its derivatives. J. Microbiol. Biotechnol. 26, 441–451 (2016)
Nakazawa, A., Matsuura, H., Kose, R., Kato, S., Honda, D., Inouye, I., Watanabe, M.M.: Optimization of culture conditions of the thraustochytrid Aurantiochytrium sp. strain 18W-13a for squalene production. Bioresour. Technol. 109(Supplement C), 287–291 (2012). https://doi.org/10.1016/j.biortech.2011.09.127
Fan, K.W., Aki, T., Chen, F., Jiang, Y.: Enhanced production of squalene in the Thraustochytrid aurantiochytrium mangrovei by medium optimization and treatment with terbinafine. World J. Microb. Biot. 26, 1303–1309 (2010)
Englund, E., Pattanaik, B., Ubhayasekera, S.J.K., Stensjö, K., Bergquist, J., Lindberg, P.: Production of squalene in Synechocystis sp. PCC 6803. PLoS ONE. 9(3), e90270 (2014). https://doi.org/10.1371/journal.pone.0090270
Siedenburg, G., Jendrossek, D.: Squalene-hopene cyclases. Appl. Environ. Microbiol. 77, 3905–3915 (2011)
Dangi, S.K., Dubey, S., Bhargava, S.: Cyanobacterial diversity: a potential source of bioactive compounds. Adv. Biol. Microbiol. 4(2474–7617), 001–003 (2017)
Koller, M., Marsalek, L.: Cyanobacterial polyhydroxyalkanoate production: status quo and quo vadis? Curr. Biotechnol. 4(4), 464–480 (2015)
Chew, K.W., Yap, J.Y., Show, P.L., Suan, N.H., Juan, J.C., Ling, T.C., Chang, J.S.: Microalgae biorefinery: high value products perspectives. Bioresour. Technol. 229, 53–62 (2017)
Santos, A.M., Roso, G.R., Menezes, C.R., Queiroz, M.I., Zepka, L.Q., Jacob-Lopes, E.: The bioeconomy of microalgal heterotrophic bioreactors applied to agroindustrial wastewater treatment. Desalt. Water Treat. 64, 12–20 (2017)
Santos, A.M., Depra, M.C., Santos, A.M., Zepka, L.Q., Jacob-Lopes, E.: Aeration energy requirements in microalgal heterotrophic bioreactors applied to agroindustrial wastewater treatment. Curr. Biotechnol. 5, 249–254 (2015)
Rodrigues, D.B., Menezes, C.R., Mercadante, A.Z., Jacob-Lopes, E., Zepka, L.Q.: Bioactive pigments from microalgae Phormidium autumnale. Food Res. Int. 77(Part 2), 273–279 (2015)
Rodrigues, D.B., Flores, É.M.M., Barin, J.S., Mercadante, A.Z., Jacob-Lopes, E., Zepka, L.Q.: Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 65(Part B), 144–148 (2014). https://doi.org/10.1016/j.foodres.2014.06.037
Freitas, A.C., Rodrigues, D., Rocha-Santos, T.A.P., Gomes, A.M.P., Duarte, A.C.: Marine biotechnology advances towards applications in new functional foods. Biotechnol. Adv. 30, 1506–1515 (2012)
Titz, M., Kettl, K.-H., Shahzad, K., Koller, M., Schnitzer, H., Narodoslawsky, M.: Process optimization for efficient biomediated PHA production from animal-based waste streams. Clean Technol. Environ. Policy. 14(3), 495–503 (2012)
Shahzad, K., Narodoslawsky, M., Sagir, M., Ali, N., Ali, S., Rashid, M.I., Ismail, I.M.I., Koller, M.: Techno-economic feasibility of waste biorefinery: using slaughtering waste streams as starting material for biopolyester production. Waste Manag. (New York, N.Y.). 67, 73–85 (2017). https://doi.org/10.1016/j.wasman.2017.05.047
Luque, R., Clark, J.H.: Valorisation of food residues: waste to wealth using green chemical technologies. Sustain. Chem. Process. 1, 1–3 (2013)
Rodrigues, D.B., Menezes, C.R., Mercadante, A.Z., Jacob-Lopes, E., Zepka, L.Q.: Bioactive pigments from microalgae Phormidium autumnale. Food Res Int. 77(Part 2), 273–279 (2015). https://doi.org/10.1016/j.foodres.2015.04.027
Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M., Stanier, R.Y.: Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 111, 1–61 (1979)
Water Pollution Control Federation American water works association.: Standard Methods for the Examination of Water and Wastewater, t.e.E.A., APHA, WPCF. Water Pollution Control Federation American water works association, Washington, DC (2005)
Francisco, E.C., Franco, T.T., Wagner, R., Jacob-Lopes, E.: Assessment of different carbohydrates as exogenous carbono source in cultivation of cyanobacteria. Bioproc. Biosyst. Eng. 1, 2–11 (2014)
Bligh, E.G., Dyer, W.J.: A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37(8), 911–917 (1959). https://doi.org/10.1139/o59-099
Christie, W.W.: A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 23, 1072–1075 (1982)
European Commission: Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed (2007)
Romari, K., Godart, F., Calleja, P.: Production of Docosahexaenoic Acid and/or Eicosapentaenoic Acid and/or Carotenoids in Mixotrophic Mode by Nitzschia. Google Patents (2017)
Meixner, K., Kovalcik, A., Sykacek, E., Gruber-Brunhumer, M., Zeilinger, W., Markl, K., Haas, C., Fritz, I., Mundigler, N., Stelzer, F., Neureiter, M., Fuchs, W., Drosg, B.: Cyanobacteria biorefinery—production of poly(3-hydroxybutyrate) with Synechocystis salina and utilisation of residual biomass. J. Biotechnol. 265(Supplement C), 46–53 (2018). https://doi.org/10.1016/j.jbiotec.2017.10.020
Sekar, S., Chandramohan, M.: Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J. Appl. Phycol. 20(2), 113–136 (2008). https://doi.org/10.1007/s10811-007-9188-1
Gong, M., Bassi, A.: Carotenoids from microalgae: a review of recent developments. Biotechnol Adv. 34(8), 1396–1412 (2016). https://doi.org/10.1016/j.biotechadv.2016.10.005
Kajikawa, M., Kinohira, S., Ando, A., Shimoyama, M., Kato, M., Fukuzawa, H.: Accumulation of Squalene in a microalga Chlamydomonas reinhardtii by genetic modification of squalene synthase and squalene epoxidase genes. PLoS ONE. 10(3), e0120446 (2015). https://doi.org/10.1371/journal.pone.0120446
Fabris, M., Matthijs, M., Carbonelle, S., Moses, T., Pollier, J., Dasseville, R., Baart, G.J., Vyverman, W., Goossens, A.: Tracking the sterol biosynthesis pathway of the diatom Phaeodactylum tricornutum. New Phytol. 204(3), 521–535 (2014). https://doi.org/10.1111/nph.12917
Achitouv, E., Metzger, P., Rager, M.N., Largeau, C.: C31-C34 methylated squalenes from a Bolivian strain of Botryococcus braunii. Phytochemistry. 65(23), 3159–3165 (2004). https://doi.org/10.1016/j.phytochem.2004.09.015
Jiang, Y., Fan, K.-W., Tsz-Yeung Wong, R., Chen, F.: Fatty acid composition and squalene content of the marine microalga Schizochytrium mangrovei. J. Agric. Food Chem. 52(5), 1196–1200 (2004). https://doi.org/10.1021/jf035004c
Bhattacharjee, P., Shukla, V.B., Singhal, R.S., Kulkarni, P.R.: Studies on fermentative production of squalene. World J. Microbiol. Biotechnol. 17(8), 811–816 (2001)
Bhattacharjee, P., Singhal, R.S.: Extraction of squalene from yeast by supercritical carbon dioxide. World J. Microb. Biotechnol. 19, 605–608 (2003)
Remme, J.F., Larssen, W.E., Bruheim, I., Sæbø, P.C., Sæbø, A., Stoknes, I.S.: Lipid content and fatty acid distribution in tissues from Portuguese dogfish, leafscale gulper shark and black dogfish. Comp. Biochem. Physiol. Part B. 143(4), 459–464 (2006). https://doi.org/10.1016/j.cbpb.2005.12.018
Papiol, V., Fanelli, E., Cartes, J.E., Rumolo, P., López-Pérez, C.: A multi-tissue approach to assess the effects of lipid extraction on the isotopic composition of deep-sea fauna. J. Exp. Mar. Biol. Ecol. 497(Supplement C), 230–242 (2017). https://doi.org/10.1016/j.jembe.2017.10.001
Popa, O., Băbeanu, N.E., Popa, I., Niță, S., Dinu-Pârvu, C.E.: Methods for obtaining and determination of squalene from natural sources. Biomed. Res. Int. (2015). https://doi.org/10.1155/2015/367202
Francisco, É.C., Franco, T.T., Maroneze, M.M., Zepka, L.Q., Jacob-Lopes, E.: Produção de biodiesel de terceira geração a partir de microalgas. Ciência Rural. 45, 349–355 (2015)
Roso, G.R., dos Santos, A.M., Queiroz, M.I., Barin, J.S., Zepka, L.Q., Jacob-Lopes, E.: The econometrics of production of bulk oil and lipid extracted algae in an agroindustrial biorefinery. Curr. Biotechnol. 4(4), 547–553 (2015)
Venugopal, V., Kumaran, A.K., Sekhar Chatterjee, N., Kumar, S., Kavilakath, S., Nair, J.R., Mathew, S.: Biochemical characterization of liver oil of Echinorhinus brucus (bramble shark) and its cytotoxic evaluation on neuroblastoma cell lines (SHSY-5Y). Scientifica. (2016). https://doi.org/10.1155/2016/6294030b
Silas, E.G., Selvaraj, G.S.D.: Descriptions of the adult and embryo of the bramble shark Echinorhinus brucus (Bonnaterre) obtained from the continental slope of índia. J. Mar. Biol. Assoc. India. 14, 395–401 (1972)
Nichols, P., Rayner, M.S., Stevens, J.D.: A Pilot Investigation of Northern Australian Shark Liver Oils: Characterization and Value-adding. CSIRO Marine Research, Hobart (2001)
Martineau, E., Wood, S.A., Miller, R.M., Jungblut, A.D., Hawes, I., Webster-Brown, J., Packer, M.A.: Characterisation of Antarctic cyanobacteria and comparison with New Zealand strains. Hydrobiologia. 711, 139–154 (2013)
Clarke, M.W., Connolly, P.L., Bracken, J.J.: Catch, discarding, age estimation, growth and maturity of the squalid shark Deania calceus west and north of Ireland. Fish. Res. 56, 139–153 (2002)
Acknowledgements
The present study was carried out with the financial support of the National Council for Scientific and Technological Development (CNPq)- Brazil and funding from FAPERGS - Foundation for Research Support of the State of Rio Grande do Sul, Porto Alegre, RS, Brazil and CAPES improving coordination of Higher Education Personnel for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mariane Bittencourt Fagundes declares that she has no conflict of interest and all the other authors: Raquel Guidetti Vendruscolo, Mariana Manzone Maroneze, Cristiano Ragagnin Menezes, Leila Queiroz Zepka, Juliano Smanioto Barin, Eduardo Jacob-Lopes and Roger Wagner also declares that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fagundes, M.B., Vendruscolo, R.G., Maroneze, M.M. et al. Towards a Sustainable Route for the Production of Squalene Using Cyanobacteria. Waste Biomass Valor 10, 1295–1302 (2019). https://doi.org/10.1007/s12649-017-0191-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0191-8