Abstract
Low strength sodium hydroxide pre-treated rice straw was hydrolysed at two different solid loadings i.e. 10 and 15 % (w/v) with in-house cellulases (IC) produced by Aspergillus terreus along with commercial cellulases (CC) by using batch and fed batch hybrid simultaneous saccharification and fermentation process. Optimization of process parameters enhanced the crude cellulase activities i.e. filter paper, β-glucosidase and endoglucanase by 1.6, 2.7 and 2.2 fold in 6 days as compared to activities obtained on a single substrate i.e. rice straw. Out of four fed batch approaches, approach III (A-III) at 10 % solid loading yielded higher ethanol concentrations of 30.55 and 28.66 g L−1 by using thermo tolerant in-house yeast strain Kluyveromyces marxianus at 42 °C. Combination of 9 FPU g−1 substrate and CC plus 30 CBU of commercial β-glucosidase (Cβ) yielded slightly higher theoretical ethanol yields of 92.24 % as compared to 86.54 % obtained from IC+Cβ. Dunnett’s Post Hoc Annova test also proved that CC+Cβ were found to be significant in batch as well fed batch experiments as compared to other combinations tried. Thus, fed batch A-III seems to be an optimistic one to overcome the problems associated with batch mode to achieve higher ethanol yields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass has been found to be a potential feed-stock for novel 2G biofuel i.e. cellulosic ethanol. It can be produced using a wide variety of lignocellulosic crop residues. Large quantities of this valuable resource are being currently burnt in the fields with a significant environmental impact in the region. Cellulases are considered to be an important industrial enzyme due to its higher quantity of contribution in the global enzyme market [1, 2]. The increase in the demand of cellulases in the ever rising biofuel industry resulted in the larger scale production of cellulase enzyme by most economical and effective ways. Cellulase production can be enhanced by using biomass with low lignin content. Recent review of literature revealed that sugarcane bagasse (SB) and wheat bran (WB) are used as the efficient growth substrate for fungal strain due to the advantages like high porosity, presence of growth nutrients, particle size suitability and uniformity required for fungal growth and ease of extraction of cellulase enzymes. The substrate surface remains movable in humid conditions, which increase the surface area for fungal growth and water retention capacity [3].
Fermentation of lower concentration of sugar obtained during enzymatic hydrolysis yields low concentrations of ethanol, which is one of the limitations in cellulosic ethanol technology. High solid loads of pre-treated biomass during enzymatic hydrolysis results in higher concentration of ethanol, but it makes the process most difficult in mixing and heat transfer [4, 5]. There are several modifications of cellulose ethanol technology have been reported to improve the process and to eliminate the problems. Simultaneous saccharification and fermentation (SSF) is one process which combines both biomass hydrolysis and glucose fermentation in a single reactor, which reduces the investment cost as well as the conversion of biomass to ethanol compared to separate hydrolysis and fermentation. At the same time the problems associated with SSF can be reduced by integration of pre-hydrolysis step [6, 7]. Our earlier reports on pre-hydrolysis and fermentation with IC and CC at 42 °C for 72 h were found to be superior over SSF and separate hydrolysis and fermentation, while, few researchers did not find any improvement [8, 9]. There are many other factors which effects the process efficiency i.e. length of pre-hydrolysis period, solid load, enzyme loading, type of pre-treatment method, toxic compounds associated with it, sensitivity of yeast strain towards toxic compounds etc. Fed-batch mode can be adopted to carry out the hydrolysis to solve these problems by gradual increase in enzyme and/or biomass to maintain the desired level of viscosity [10].
Considering the above advantages, the present study was majorly focused on two main important aspects: (i) production of cellulases by in-house fungal strain Aspergillus terreus using mixed substrates (WB and SB) (ii) evaluation of batch and hybrid fed batch SSF of rice straw pre-treated with mild alkali by employing different substrate and enzyme approaches using in-house thermotolerant yeast strain Kluyveromyces marxianus J1.
Materials and Methods
Microorganism and Substrates for Fungal Growth
The fungal strain Aspergillus terreus-D34 (GenBank Accession Number: KF971363, NCBI) used in the present study for bulk crude cellulase production was isolated from a decayed rice straw.11 Two cheap and abundantly available wastes i.e. WB and SB were collected from a local market and nearby sugar factory, Anand, Gujarat (388 001), India, respectively. The substrates were washed to remove the dirt, sun dried and preserved in air tight bags. Analytical grade chemicals, reagents and media were used in the current experiments.
Large Scale Enzyme Production and Extraction
WB and SB were used as solid support material for crude cellulase production under solid state fermentation by Aspergillus terreus. A total 250 g of different proportions of WB and SB namely 20:80; 35:65; 50:50; 65:35 were loaded in each of the tray. Modified Mandels and Weber medium was used as a wetting agent as reported earlier [11]. Substrate to moisture ratio and inoculum size tried were 1:4, 1:5 and 1:6 and 1, 2, 3 and 4 % (v/v), respectively. The contents were autoclaved for 15 min at 121 °C and the trays were exposed to ultra violet light individually for 1 h. Mycelial growth of 2 days old grown fungal strain was inoculated into potato dextrose broth and incubated at 45 °C in an orbital shaker at 150 rpm for 48 h and used as inoculum. The trays were placed in a temperature controlled humidity chamber for 6 days at 45 °C with relative moisture of 75 %. The total capacity of the chamber was 20.8 cu m and having a provision to accommodate 40 trays each of 25 × 15 × 21 cm size. Crude cellulases were extracted by adding 1500 mL of 0.05 M sodium acetate buffer (pH 4.8) to each of the tray and the contents were squeezed using a double layered muslin cloth. The cellulases were pre-clarified by centrifugation at 10000 × g for 10 min at 4 °C. The pre-clarified crude cellulases were kept at 4 °C till they used for enzymatic hydrolysis studies.
Pre-treatment of Rice Straw by Sodium Hydroxide
Rice straw was chopped to 2–3 cm size, passed through 5-mm mesh in a hammer mill (Finex, India) and used for mild alkali pre-treatment (0.5 % NaOH at room temperature for 24 h) as described earlier [11]. The chemically pre-treated solid biomass was washed with tap water till the pH reached to neutral and used for batch and fed batch hybrid SSF studies.
Cellulase Enzyme Source and Yeast Strain
Crude cellulases used for batch and fed batch hybrid SSF contained filter paper activity (FPA) (1.14 ± 0.37 U mL−1), β-glucosidase (14.6 ± 0.45 U mL−1), endoglucanase (28.90 ± 0.33 U mL−1), xylanase (163.4 ± 0.49 U mL−1), respectively and were produced as mentioned in above section. Commercial enzymes were procured from Sigma Aldrich (Celluloclast 1.5 L −25 FPU mL−1; Novozyme 188-266 CBU mL−1). The yeast strains used in the present study was a thermotolerant in-house strain Kluyveromyces marxianus J1 (GenBank Accession Number: KP231175) grows at 42 °C as describer earlier [5]. Fermentation was carried out without adding any extra inorganic salt and organic nutrients to the medium.
Yeast Inoculum Preparation
The Kluyveromyces marxianus J1 inoculum was prepared in a fermentation broth having (g−1); glucose, 30.0; yeast extract, 3.0; peptone, 5.0; (NH4)2HPO4, 0.25 at pH 6.0 ± 0.2 for 12 h at 42 ± 2 °C [5]. Each of the experimental flasks was inoculated with 12 h grown aseptically collected and centrifuged seed culture of Kluyveromyces marxianus J1 at the rate 1 % (v/v).
Batch and Fed Batch Hybrid SSF Process
Batch and fed batch SSF studies were done with 10 and 15 % solids loading using the combination of CC+Cβ and IC+Cβ at 50 °C in 75 mL polypropylene centrifuge bottles (Tarson Make). The enzyme load was chosen (9 FPU g−1 substrate) based on the earlier results [5, 11]. Likewise, β-glucosidase addition was also decided based on the findings achieved in the laboratory. Initial results indicated that addition of 30 CBU of β-glucosidase gave maximum ethanol yield with CC as well IC preparation (data not shown). Therefore, 30 CBU of β-glucosidase g−1 substrate was added to the samples containing CC and IC, respectively. Total system volume was 20 mL (Citrate buffer, pH 4.8). The contents of the bottles were placed in a rotary shaker and pre-hydrolysis was carried at 50 °C at 16 rpm and fermentation was performed with Kluyveromyces marxianus J1 at 42 °C. In fed batch experiments, four different approaches were employed to start the enzymatic hydrolysis studies. The solid loading was kept constant whereas the enzyme loadings were varied in all the four approaches. In batch hydrolysis, the solid biomass was fed at the beginning of hydrolysis and the final solid loading was similar to that of fed batch method. Other conditions were kept constant.
The details of the four substrate approaches were as follows: In the beginning of experiment, the enzymatic hydrolysis studies were started with 5 % (1.0 g) solid loadings, gradually increased the solid loading to reach the final solid loadings of 10 % with the addition of 2.5 % (0.50 g) of fresh pre-treated solid biomass at regular intervals with a gap of 12–24 h. As in case of 15 % solid loading, the enzymatic hydrolysis studies were started with 5 % (1.0 g) solid loadings as it was done in 10 % solid loading, gradually increased the solid loading to reach the final solid loadings of 15 % with the addition of 3.3 % (0.66 g) of fresh pre-treated solid biomass at regular intervals with a gap of 12–36 h.
The details of the four enzyme approaches were as follows: In A-I, addition of cellulase enzyme mixture was done at the start of the experiment in both the solid loadings. In A-II, 25 % of the total cellulase enzyme mixture was added at the start up and the rest 75 % was added along with substrate with a gap of 12, 24 and 36 h in case of 10 and 15 % solid loadings, respectively. In A-III, 35 % of the total cellulase enzyme mixture was added at the start up and the rest 65 % was added along with substrates as mentioned above. In A-IV, cellulase enzyme loadings were added at a proportionate to the solid biomass loadings with a gap of 12, 24 and 36 h in case of 10 and 15 % solid loadings, respectively (Fig. 1).
Pre-hydrolysis was continued for another 12 h i.e. till 36 and 48 h for 10 and 15 % solid loadings, respectively for better conversion of cellulosic biomass. Later newly isolated yeast strain Kluyveromyces marxianus J1 was added under sterile conditions and fermentation was carried out at 42 °C till 108 h. Sampling was done at different time points for estimating total reducing sugars as described earlier [5].
Statistical Analysis
Repeated measures analysis for the data obtained in the present study was carried out by Dunnett’s Post Hoc Annova test using Statistical Package for the Social Sciences (SPSS) software, version 19.0.
Analysis
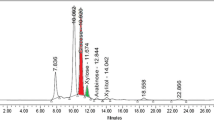
The activities of total celluase (FPA), endoglucanase, β-glucosidase and xylanase were measured as described earlier [11]. One unit of enzyme activity was defined as the amount of enzyme required to liberate 1 µmol of glucose, p-nitrophenol and xylose per min under the standard assay conditions [9]. Chemical composition of untreated and pre-treated rice straw for cellulose, hemicellulose and lignin content was done as described by Goering and Vansoest [12]. The samples were removed at regular intervals and analysed for cellobiose, glucose, xylose, acetic acid, glycerol and ethanol on Aminex-HPX-87H column (Biorad, Hercules, USA, CA) using High Performance Liquid Chromatography. The column temperature was maintained at 65 °C and the samples were eluted using 5 mM H2SO4 with the flow rate of 0.6 mL min−1. All the estimations were performed in duplicates. Error bars presented were standard deviation of duplicate experiments. Ethanol yields were calculated as described earlier [5].
where (EtOHt) = Concentration of ethanol at time t; (EtOHo) = Initial ethanol concentration; f = , Glucan fraction of dry biomass; Biomass = dry mass concentration; 1.11 = Conversion factor for glucan to glucose.
Results and Discussion
Large Scale Enzyme Production
Low value substrates such as WB and SB were used for the production of enzymes in large scale under solid state fermentation by Aspergillus terreus. The maximum production was achieved at 35:65 ratios of WB and SB on 5th day at 45 °C, pH 5.5, substrate to moisture ratio 1:4 and inoculum size 2 % (v/v). Under these optimum conditions the in-house fungal strain produced higher β-glucosidase (120.6 U g−1) and endoglucanase (243.1 U g−1), respectively (Table 1) as compared to the activities reported by us earlier and but at the same time the FP activity remained alike when the same fungal strain was cultivated on single substrate i.e. rice straw [11]. The cellulase activities obtained by researchers are comparable with the current study. The enzyme activities were higher by Aspergillus fumigatus during the growth on mixed substrate containing equal parts of WB and rice straw [13, 14]. Combination of growth substrates with two equal parts of WB and kinnow peel and one part of corn bran using Aspergillus niger under solid state fermentation yielded in higher cellulase activity of 10.81 U g−1 [14]. Increase in FP 1.29 U mL−1 and β-glucosidase 41 U mL−1 were also obtained with the combination of wheat bran and avicel in 7 L stirred tank bioreactor by Penicillium oxalicum [15]. Higher cellulase activities in these substrate mixtures may be because the texture of these substrate mixtures remains free/movable even in humid condition providing increased water retention capacity, sufficient nutrients and higher surface area [10].
One of the most important process parameter for solid state fermentation in cellulase production is the moisture content [16]. Moisture content plays a vital role on the production of cellulase as it was observed with an increase in the activity of enzymes directly related with the increase in moisture content during solid state fermentation. In the present study maximum activities achieved at substrate to moisture of 1:5 has been given in Table 2. The main disadvantages of higher moisture content than the optimum level results in low porous substrate, sticky surface with change in structure of the particles, increased aerial mycelia and reduced oxygen transfer [17]. High water stress in the substrate due to lower moisture content reduces the fungal growth and subsequently it affects the cellulase production [18].
Another important process parameter in solid state fermentation is inoculum preparation. Among the different methods available for inoculum preparation, the commonly used methods reported in literature were spore suspension, mycelia disc and mycelial suspension [19]. Researchers mostly preferred mycelia suspension as the best one for preparation of inoculum in cellulase enzyme production under solid state fermentation process as it has the advantage over spore suspension by eliminating the lag phase. Results from current work are similar to the work reported earlier [20, 21]. According to Pandey [22] the fungal growth during solid state fermentation can be influenced by inoculum size and thereby the enzyme production efficacy. In the present study maximum cellulase enzyme activities were found when the inoculum size was 3 % (v/v) (Table 3). The increase in inoculum size above the optimum level results in reduced cellulase activities which might be due to the non-availability of required sugar and oxygen transfer time and release of enzymes. It has also been reported that initially an increase in lag phase and moisture content in small and large inoculum size were observed, respectively to a large extent [23]. The presence of free additional liquid results in the reduction of fungal growth and correspondingly the production of enzymes due to the reduced diffusion forced by the solid biomass [11]. In order to yield enhanced enzyme productivity, an optimized inoculum size and moisture contents are essential.
Cellulases accounts for about 40 % of the total cost of bioethanol production from lignocellulosic biomass. As there is a demand for cost effective enzyme technology in-house produced crude enzyme is far economical than the commercial enzymes. At the same time large scale cellulase enzyme production also demands robust cellulase producing microorganisms with a good blend of cellulolytic/hemicellulolytic enzyme mixtures for better cellulosic biomass conversion. Therefore, presence of xylanase (1634 U g−1) in the in-house crude cellulase enzyme preparation is an important finding which might ensure the conversion of hemicelluloses fraction available in biomass into xylose sugars.
Compositional Analysis of Mild Alkali Pre-treated Rice Straw
The compositional analysis shows that before pre-treatment the rice straw had cellulose, hemicellulose, lignin, moisture and ash content of 41.02 ± 1.45 %; 28.47 ± 1.91 %; 8.40 ± 1.12 %; 7.04 ± 1.21 %; and 15.13 ± 1.15 %, respectively. Pre-treated rice straw had 58.50 ± 1.38 % cellulose; 22.91 ± 1.96 % hemicellulose and 3.40 ± 1.19 % lignin, respectively. The results revealed that in the present study, after pre-treatment the cellulose content was increased by 48.95 %, reduction in the lignin by 59.5 % and solid residue recovery was 65 to 67 %. Even though lignin resists degradation of lignocellulosic biomass it is not obligatory to eliminate all lignin to accomplish maximum enzymatic hydrolysis. Lignin removal around 20–65 % is assumed to be satisfactory to increase the availability of the cellulose to enzyme [24]. This is vital because efforts to remove additional lignin could end up losing more of carbohydrate and the release of inhibitory compounds eventually hamper the hydrolysis process [25].
Batch and Hybrid Fed Batch SSF at 10 % Solid Loading
In the present investigation, fed batch mode significantly improved the ethanol yield instead of batch mode. In all the four approaches of SSF fed batch mode, the enzyme feeding approach had an influence on the ethanol yield. Out of the four approaches, A- III i.e. addition of right amount of the enzyme mix together with the substrate feed, seems to be beneficial as compared to adding the enzyme at the beginning of experiment (A-I) or by differing the enzyme loading (A- II and IV). In A- III, after 84 h fermentation, glucose concentration decreased to less than 5.79 and 3.35 g L−1, and ethanol concentration increased to 30.55 and 28.66 g L−1 (Corresponding glucose to ethanol yield were 92.24 and 86.54 %) from CC+Cβ and IC+Cβ, respectively (Fig. 2a; Table 4). The possible reason for higher ethanol yields in fed batch mode (A-III) could be because of the addition of substrate and enzyme in a well ordered manner that might have eased the free flow of the reaction mixture inside the vessel with proper mixing and improved mass transfer rate, which induced the quick conversion of cellulose to glucose for ethanol production. The other reason could be that the viscosity reduction in the fermentation broth during pre-hydrolysis with the production of glucose which helps in the growth of yeast cells [26]. The current outcomes are in agreement with others findings that fed batch approach (A- III) has revealed maximum fermentation rate and attained higher ethanol concentration as compared to batch approach [1].
a Comparison of batch with fed batch SSF (A-III) at 10 % solid loading with CC and IC at different time intervals. Values of means of duplicate experiments. Errors presented here were standard deviation of duplicate experiments, b Comparison of batch with fed batch SSF (A-I) at 10 % solid loading with CC and IC at different time intervals. Values of means of duplicate experiments. Errors presented here were standard deviation of duplicate experiments
Whereas fed batch SSF A-I, II and IV produced maximum ethanol of 26.18, 24.99; 24.96, 18.91; 24.97, 21.02 g L−1 (Corresponding glucose to ethanol yield were 79.06, 75.47; 75.38, 57.11; 75.41, 63.48 %) from CC+Cβ and IC+Cβ, respectively (Figs. 2b, 3a, b).
a Comparison of batch with fed batch SSF (A-II) at 10 % solid loading with CC and IC at different time intervals. Values of means of duplicate experiments. Errors presented here were standard deviation of duplicate experiments, b Comparison of batch with fed batch SSF (A-IV) at 10 % solid loading with CC and IC at different time intervals. Values of means of duplicate experiments. Errors presented here were standard deviation of duplicate experiments
While the present results have also shown that the ethanol yields from SSF fed batch A- II and IV were comparable with batch mode when the CC+Cβ were used. Maximum ethanol concentration of 24.41, 24.96; 24.41, 24.97 g L−1 (Corresponding glucose to ethanol yield were 73.72, 75.38;73.72, 75.41 %) were achieved after 84 h of incubation period at 10 % solid loading in batch mode and fed batch SSF A- II and IV, respectively (Figs. 2b, 3b). The current results of SSF fed batch A-II and IV are well in agreement with the studies reported that conducting SSF in fed batch mode has shown no significant difference in ethanol yields from batch mode [1, 27]. Thus substrate/enzyme adding approach is very crucial to achieve higher ethanol yields in fed batch mode.
Batch and Fed Batch Hybrid SSF at 15 % Solid Loading
At 15 % solid loading higher ethanol concentrations were achieved with batch and fed batch compared to 10 % solid loading, but the conversion efficiencies were found to be low as compared to 10 % solid loading. After 84 h fermentation, fed batch SSF A-I, II, III and IV produced maximum ethanol of 28.90, 26.71; 27.95, 25.12; 34.75, 30.78; 28.19, 24.64 g L−1 (Corresponding glucose to ethanol yield were 58.18, 53.77; 56.27, 50.57; 69.96, 61.96; 56.75, 49.60 %) from CC+Cβ and IC+Cβ, respectively (Table 5). CC+Cβ produced slightly higher ethanol yields as compared IC+Cβ in batch as well fed batch SSF processes. The main advantage of fed-batch mode over batch mode at high solid loads are the free movement of reaction mixture i.e. substrate and enzyme by gradual addition to the reactor which maintains the desired level of viscosity therefore, reduces the clogging of fibrous substrate suspension and improves the mass and heat transfer rates [6].
When the measurements are made more than two times repeatedly over a period of time on the same dependent variable repeated measure ANOVA should be used. The use of standard ANOVA method to compare group means is inappropriate in this kind of study design, as it does not consider dependencies between observations within subjects in the analysis. Repeated measures analysis deals with response outcomes measured on the same experimental unit at different times or under different conditions [28]. To confirm the findings of the present study multiple comparisons were made using Dunnett’s Post Hoc Annova test. The data shows that fed batch A-III with CC+Cβ found to be significant over the other combinations tried. The second best was IC+Cβ (Tables 6 and 7). Profile mean plots of batch and fed batch SSF combinations using CC and IC were made in three separate time lines (Fig. 4).
Besides enzyme and solid loading approach, β-glucosidase as the vital enzyme component of cellulase also played an important role in hydrolysing cellobiose to glucose (Table 4). Cellobiose concentration was in the range of 3.95 g L−1 for fed batch process and 4.27 g L−1 for batch process. Additional supplementation of β-glucosidase led to improved cellobiose conversion to glucose, lessen the problems associated with product inhibition as the yeast strain metabolized the glucose monomers released during SSF simultaneously and resulted into higher ethanol yields. This result is in well agreement with our earlier report and as well with other researchers [5, 29]. With the addition of 1 % Tween 80, higher glucose concentrations were observed till 60 h and were reduced to less than 5 g L−1 in fed batch SSF A-III and 8.34 g L−1 in batch after 84 h fermentation. This indicates that the addition of additives improves the enzymatic digestibility [5]. Similar observation was also reported by the addition of Tween 40 enhanced the enzymatic hydrolysis and produced higher concentrations of glucose and xylose from aqueous ammonia pre-treated corn stover at the beginning of the simultaneous saccharification and co-fermentation process [26]. But at the same time the results also revealed that the xylose fraction remains unutilized. It is generally accepted that C6 fermenting yeast strains are unable to assimilate D-xylose in the presence of mixed sugars, unless they undergo some genetic modifications [30].
In the present investigation, by-products i.e. acetic acid and glycerol were also formed during the batch and fed batch SSF trials. Final acetic acid concentrations in batch and fed batch SSF A-III with CC+Cβ and IC+Cβ were less than 0.3 g L−1. Hydrolysis of pre-treated switchgrass with Accellerase 1500 also released less than 0.5 g L−1 acetic acid [31]. Since the pre-treated material contained 22 % hemicellulose, xylose was detected in the fermentation broth (with CC around 14.22 g L−1 and with IC 14.10 g L−1). The lower acetic acid concentrations during fermentation may be due to the metabolism of yeast strain (Table 4). The final glycerol concentrations were decreased as the fermentation time increased. The final glycerol concentrations after 84 h fermentation found to be below 1.10 g L−1 (16.78 mg g−1). Keikhosro et al. [32] reported 27 and 48 mg g−1 of glycerol in aerobic SSF and 117.3 mg g−1 of glycerol on anaerobic SSF of the pre-treated rice straw (Table 4).
The present study demonstrates that pre-hydrolysis along with enzyme loading approach played a major role in conversion of glucose monomers by attaining above 92 and 86 % theoretical ethanol yields with CC+Cβ and IC+Cβ, respectively (Fig. 5a, b). Slightly lower maximum theoretical ethanol yield of 81.3 % were obtained with aqueous ammonia pre-treated rice straw as compared to the present study by using Sacharomyces cerevisiae D5A at 15 FPU of cellulase and 30 CBU of β-glucosidase g−1 glucan, respectively after 72 h [24]. Comparison of ethanol yields of different batch and fed batch SSF processes with different pre-treatment and fermentation conditions shown in Table 8. Seeing the best ethanol yields in the current work, 1 tonne of rice straw can yield higher ethanol yields of 198 and 186 kg from CC+Cβ and IC+Cβ were used as an enzyme source (Fig. 6). The liquid fraction (wastewater) generated during mild alkali pretreatment was used as an influent to four anaerobic hybrid reactors to generate biogas as described in the earlier studies [35]. Thus, 731 million tonnes of rice straw produced annually in the world can be utilized for production of 170–180 billion L of ethanol and 406 billion kL of biogas per year. Besides, any improvement in utilizing xylose fraction of the mild alkali pre-treated rice straw will enhance the overall yield of ethanol. Experiments are in progress towards this direction.
a Theoretical ethanol yield of batch and fed batch SSF (A-III) at 10 % solid loading with CC and IC at different time intervals. Values of means of duplicate experiments. Errors presented here were standard deviation of duplicate experiments, b Theoretical ethanol yield of batch SSF and fed batch SSF (A-III) at 15 % solid loading with CC and IC at different time intervals. Values of means of duplicate experiments. Errors presented here were standard deviation of duplicate experiments
Conclusions
-
As there is a demand for cost effective enzyme technology which is necessary for producing viable biofuels, the present results demonstrated that use of low cost substrate mixtures are feasible alternative sources for on-site enzyme production by Aspergillus terreus.
-
A permutation and combination of regular loading of substrate and enzyme and pre-hydrolysis improved the overall performance of SSF fed batch process in comparison to regular batch SSF at 10 % solid loading by yielding higher ethanol concentrations.
-
The present study has proven that β-glucosidase addition was also found to favour faster reaction with the yeast strain.
-
The conceptual design of fed batch SSF A-III may be recommended because the best maximum theoretical ethanol yields of 92 and 86 % were obtained with CC+Cβ and IC+Cβ, respectively.
References
Singhania, R.R., Sukumaran, R.K., Patel, A.K., Larroche, C., Pandey, A.: Advancement and comparative profiles in the production technologies using solid state and submerged fermentation for microbial cellulases. Enz. Microb. Technol. 46, 541–549 (2010)
Chandel, A.K., Chandrasekhar, G., Silva, M.B., Silva, S.S.: The realm of cellulases in biorefinery development. Cri. Rev. Biotechnol. 32, 187–202 (2012)
Das, A., Paul, T., Halder, S.K., Jana, A., Maity, C.S.K., Das Mohapatra, P.K., Pati, B.R., Mondal, K.C.: Production of cellulolytic enzymes by Aspergillus fumigatus ABK9 in wheat bran-rice straw mixed substrate and use of cocktail enzymes for deinking of waste office paper pulp. Bioresour. Technol. 128, 290–296 (2013)
Zhao, X., Dong, L., Chen, L., Liu, D.: Batch and multi –step fed-batch enzymatic saccharification of formalin pre-treated sugarcane bagasse at high solid loadings for high sugar and ethanol titers. Bioresour. Technol. 135, 350–356 (2013)
Paulova, L., Patakova, P., Rychtera, M., Melzoch, K.: High solid fed batch SSF with delayed inoculation for improved production of bioethanol from wheat straw. Fuel 122, 294–300 (2014)
Mesa, L., Gonzales, E., Romero, I., Ruiz, E., Cara, C., Castro, E.: Comparison of process configurations for ethanol production from two-step pre-treated sugarcane bagasse. Chem. Eng. J. 175, 185–191 (2011)
Moreno, A.D., Ibarra, D., Ballesteros, I., Gonzalez, A., Ballesteros, M.: Competing cell viability and ethanol fermentation of the thermotolerant yeast Kuvelromyces marxianus and Saccharomyces cerevisiae on steam-exploded biomass treated with laccase. Bioresour. Technol. 135, 239–245 (2013)
Narra, M., James, J., Balasubramanian, V.: Simultaneous saccharification and fermentation of delignified lignocellulosic biomass at high solid loadings by a newly isolated thermotolerant Kluyveromyces sp. for ethanol production. Bioresour. Technol. 179, 331–338 (2015)
Ohgren, K., Bura, R., Lesnicki, G., Saddler, J., Zacchi, G.: A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam-pre-treated corn stover. Process Biochem. 42, 834–839 (2007)
Chen, M., Xia, L., Xue, P.: Enzymatic hydrolysis of corncob and ethanol production from cellulosic hydrolysate. Biodeter. Biodegr 59, 85–89 (2007)
Narra, M., Dixit, G., Madamwar, D., Shah, A.R.: Production of cellulases by solid state fermentation with Aspergillus terreus and enzymatic hydrolysis of mild alkali-treated rice straw. Bioresour. Technol. 121, 355–361 (2012)
Goering, H.K., Van Soest, P.J.: Forage Fiber Analyses (Apparatus, Reagents, Procedures and Some Applications). US Agricultural Research Service, Washington (1970)
Sherief, A.A., EI-Tanash, A.B., Atia, N.: Cellulase production by Aspergillus fumigatus grown on mixed substrate of rice straw and wheat bran. Res. J. Microbio. ISSN 1816-4935 (2010)
Kumar, S., Sharma, H., Sarkar, B.: Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged and solid state fermentation. Food Sci. Biotechnol. 20, 1289–1298 (2011)
Saini, R., Saini, J.K., Adsul, M., Patel, A.K., Mathur, A., Tuli, D., Singhania, R.R.: Enhanced cellulase production from Pencillium oxalicum for bioethanol application. Bioresour. Technol. 188, 240–246 (2015)
Ramesh, M.V., Lonsane, B.K.: Critical importance of moisture content of the medium in alpha-amylase production by Bacillus licheniformis M27 in a solid-state fermentation system. Appl. Microbiol. Biotechnol. 33, 501–505 (1990)
Narahara, H., Koyama, Y., Yoshida, T., Pichangkura, S., Ueda, R., Taguchi, H.: Growth and enzyme production in a solid-state culture of Aspergillus oryzae. J. Fermen. Technol. 60, 311–319 (1982)
Poorna, C.A., Prema, P.: Production of cellulase-free endoxylanase from novel alkalophilic thermotolerant Bacillus pumilus by solid-state fermentation and its application in waste paper recycling. Bioresour. Technol. 98, 485–490 (2007)
Li, W.Y., Teck, N.A., Gek, C.N., Adeline, S.M.C.: Fungal solid state fermentation and various methods of enhancement in cellulase production. Biomass Bioenergy 67, 319–338 (2014)
Shrestha, P., Rasmussen, M., Khanal, S.K., PomettoIII, A.L., van Leeuwen, J.: Solid-substrate fermentation of corn fibers by Phanerochate chrososporium and subsequent fermentation of hydrolysate into ethanol. J. Agr. Food Chem. 56, 3918–3924 (2008)
Deswal, D., Khasa, Y.P., Kuhad, R.C.: Optimization of cellulase production by a brown rot fungus Fomitopsis sp RCK2010 under solid state fermentation. Bioresour. Technol. 102, 6065–6072 (2011)
Pandey, A.: Solid state fermentation: an overview. In: Pandey, A. (ed.) Solid State Fermentation, pp. 3–10. Wiley Eastern Publishers, New Delh (1994)
Sharma, D.K., Tiwari, M., Behera, B.K.: Solid state fermentation of new substrates for production of cellulase and other biopolymer hydrolysing enzymes. Appl. Biochem. Biotechnol. 51, 495–500 (1995)
Kyong, K.J., Seop, J.B., Woo, M.J., Jin, L.H., Geol, C.I., Hyun, K.T.: Ethanol production from rice straw using optimized aqueous ammonia soaking pre-treatment and simultaneous saccharification and fermentation processes. Bioresour. Technol. 100, 4374–4380 (2009)
Jiele, X.U., Cheng, J.J., Ratna, R., Joseph, C.B.: Sodium hydroxide pretreatment of switch grass for ethanol production. Eng. Fuels 24, 2113–2119 (2010)
Zhu, J.Q., Lie, Q., Li, B.Z., Yuan, Y.J.: Simultaneous saccharification and co-fermentation of aqueous ammonia pre-treated corn stover with an engineered Saccahromyces cerevisiae SyBE005. Bioresour. Technol. 169, 9–18 (2014)
Hoyer, K., Galbe, M., Zacchi, G.: Effects of enzymatic feeding strategy on ethanol yield in fed-batch simultaneous saccharification and fermentation of spruce at high dry matter. Biotechnol. Biofuels 3, 14 (2010)
Vineeta, S., Rakesh Kumar, R., Richa, S.: Analysis of repeated measurement data in clinical trials. J Ayurveda Integr Med. 4, 77–81 (2013)
Hari Krishna, S., Janardhan, R., Chowdary, G.V.: Simultaneous saccharification and fermentation of lignocellulosic wastes to ethanol using a thermotolerant yeast. Bioresour. Technol. 77, 193–196 (2001)
Van Zyl, C., Prior, B.A., Kilian, S.G., Kock, J.K.: D-Xylose utilization by Saccharomyces cerevisiae. J. Gen. Microbiol. 135, 2791–2798 (1989)
Naveen, K.P., Hasan, K.A., Mark, R.W., Danielle, D.B., Ibrahim, M.B.: Simultaneous saccharification and fermentation of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: The effect of enzyme loading, temperature and higher solids. Bioresour. Technol. 102, 10618–10624 (2011)
Keikhosro, K., Giti, E., Mohammad, J.T.: Ethanol production from dilute acid pre-treated rice straw by simultaneous saccharification and fermentation with Mucor indicus, Rhizopus oryae and Saccharomyces cerevisiae. Enz. Microb. Technol. 40, 138–144 (2006)
Varga, E., Klinke, H.B., Reczey, K., Thomsen, A.B.: High solid simultaneous saccharification and fermentation of wet oxidized corn stover to ethanol. Biotechnol. Bioeng. 88, 567–574 (2004)
Zhang, M., Wang, F., Su, R., Qi, W., He, Z.: Ethanol production from high dry matter corncob using fed-batch simultaneous saccharification and fermentation after combined pre-treatment. Bioresour. Technol. 101, 4959–4964 (2010)
Narra, M., Balasubramanian, V., Mehta, H., Dixit, G., Madawar, D., Shah, A.R.: Performance evaluation of anaerobic hybrid reactors with different packing media for treating wastewater of mild alkali treated rice straw in ethanol fermentation process. Bioresour. Technol. 152, 59–65 (2014)
Acknowledgments
The authors wish to thank the Director, Sardar Patel Renewable Energy Research Institute (SPRERI), VallabhVidyanagar, Gujarat for providing facilities to perform research work at SPRERI. Funding from Indian Council of Agricultural Research (VVN/EAAI/DRET-BCT/2014/1; CRP/VVN/2015/2) and Department of Biotechnology (DBT) (D.O. No. BT/PR10103/PBD/26/131/2007), Government of India, New Delhi, 110 003 is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Narra, M., James, J.P., Divecha, J. et al. Simultaneous Saccharification and Fermentation of Low Strength Sodium Hydroxide Pre-treated Rice Straw for Higher Ethanol Yields: Batch and Fed Batch Hybrid Approach. Waste Biomass Valor 8, 1089–1103 (2017). https://doi.org/10.1007/s12649-016-9671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9671-5