Abstract
In the present study, scale-up systems for cellulase production and enzymatic hydrolysis of pre-treated rice straw at high-solid loadings were designed, fabricated and tested in the laboratory. Cellulase production was carried out using tray fermentation at 45 °C by Aspergillus terreus in a temperature-controlled humidity chamber. Enzymatic hydrolysis studies were performed in a horizontal rotary drum reactor at 50 °C with 25 % (w/v) solid loading and 9 FPU g−1 substrate enzyme load using in-house as well commercial cellulases. Highly concentrated fermentable sugars up to 20 % were obtained at 40 h with an increased saccharification efficiency of 76 % compared to laboratory findings (69.2 %). These findings demonstrate that we developed a simple and less energy intensive bench scale system for efficient high-solid saccharification. External supplementation of commercial β-glucosidase and hemicellulase ensured better hydrolysis and further increased the saccharification efficiency by 14.5 and 20 %, respectively. An attempt was also made to recover cellulolytic enzymes using ultrafiltration module and nearly 79–84 % of the cellulases and more than 90 % of the sugars were recovered from the saccharification mixture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulose is the most abundant plant material resource and available throughout the globe in large quantities. Rice straw is one of the abundant surplus crop residues in the world. Cellulose, the major fraction of lignocellulosic biomass, can be hydrolysed to glucose by cellulase enzyme. The hydrolysis can be affected by porosity of lignocellulosic biomass, cellulose fiber crystallinity, lignin and hemicellulose content. A pre-treatment process is, therefore, essential in order to remove lignin and hemicellulose, reduce cellulose crystallinity and to increase the porosity of the materials [1]. The most commonly used alkali sodium hydroxide (NaOH) has been extensively studied for many years, and it has been shown to disrupt the lignin structure of the lignocellulosic biomass, increasing accessibility of enzymes to cellulose and hemicelluloses. The cellulose fraction of lignocellulosic biomass can be converted into ethanol by either simultaneous saccharification and fermentation or separate enzymatic hydrolysis and fermentation processes.
Enzymatic hydrolysis at high-solid loadings (≥15 % solids, w/v), potentially offers many advantages over conversions performed at low- or moderate-solid loadings. Higher substrate loads have positive effect on the economics of the process in terms of reactor size, concentrated sugar syrups and eventually lower distillation costs [2]. At least two factors limit the feasibility of a saccharification system working at high-solid loads: the catalytic ability of the enzyme at high substrate loading and the mixing efficiency of the system to allow proper contact between the enzyme and the substrate. The need of the hour is to improve entire conversion process of lignocellulosic biomass to concentrated fermentable sugars which may be used for the production of biofuels by finding or developing enzymes which can take high-solid loads and inexpensive method of production.
The demand of cellulase enzyme is increasing especially in the emerging biofuel industry, which prompts the development of effective and economical methods to produce cellulase in large scale. Although cellulase is produced via submerged fermentation commercially, these enzymes can also be produced via solid-state fermentation. Solid-state fermentation (SSF) also offers advantages in terms of higher concentration of enzymes, higher fermentation productivity and lower demand on the sterility of the equipment’s [3]. The latter has the potential to be up-scaled for greater volume production [4]. Most of the reports are on production of cellulases by fungi in Erlenmeyer flasks, there are limited detailed studies on production of cellulases in a bioreactor system. Cellulases can be produced in tray bioreactor, packed bed bioreactor, rotary drum bioreactor and fluidized bed reactor [4].
It is also necessary to develop efficient methods to produce renewable fuels from lignocellulosic biomass. The high cost of enzymes remains a significant barrier to the economical production of ethanol from lignocellulosic biomass [5]. Therefore, it is necessary to reduce the amount of enzymes required for the enzymatic hydrolysis step. A variety of methods have been suggested to achieve increased hydrolysis yields, including surfactant addition [6] gradual substrate loading [7] or advance reactor configurations coupled with product removal to avoid inhibition [8]. One method that may reduce the amount of enzyme used and increase enzymatic productivity is to recover/recycle the enzymes [9]. Approaches have been demonstrated where free enzymes were recovered from the liquid fraction by membrane filtration [9]. Similarly, the enzymes associated with the solids have been recovered by washing with excess volumes of buffer, sometimes with surfactants to desorb the enzymes, which were then concentrated and then added to the fresh substrate [10]. These methods have shown different levels of success under controlled laboratory conditions.
In the context of the above discussion, efficacy of indigenously produced cellulases by Aspergillus terreus using tray fermentation were tested for enzymatic saccharification of alkali pre-treated rice straw at 25 % (w/v) solid loading in a bench scale horizontal rotary drum reactor for the production of fermentable sugars. Role of commercial β-glucosidase and commercial hemicellulases to the concentrated cellulase mixture were studied and reported in the present paper. The present study also demonstrates recovery of the cellulases from enzymatic hydrolasate obtained from bench scale reactor using tangential flow filtration (TFF) system (Table 1).
Methods
Substrate, chemicals and microorganism
Rice straw used for enzyme production and chemical pre-treatment studies was processed as reported earlier [11]. All chemicals and media components were of analytical grade and were purchased locally. A. terreus isolated from decomposed rice straw was used for large-scale cellulase production [11].
Chemical pre-treatment of rice straw
Based on the optimized data [11], pre-treatment was carried out at room temperature for 24 h in a batch mode. During each pre-treatment studies, one kilogram (1 kg) of dried rice straw with 5 mm mesh size was mixed with twenty liters (20 L) of 0.5 % (w/v) NaOH to obtain a final solid:liquid ratio of 1:20. The mixture from each batch was passed through screw press for solid and liquid separation and the solid residue was neutralized with 1 N HCl. The solid residue was dried at 60 °C till constant weight was achieved. After the pre-treatment, weight loss and the contents of lignin, hemicellulose and cellulose were determined. The total holocellulose (cellulose and hemicellulose) and recovery of solid biomass were also calculated. The dried pre-treated biomass was then used for the saccharification studies. The wastewater was collected and was used for biomethanation studies using anaerobic hybrid reactors [12].
Development of scale up systems for enzyme production and enzymatic hydrolysis studies
Walk-in humidity chamber
The walk-in humidity chamber was constructed with double wall, PUF insulated, leak proof modular panels with cam and lock arrangement for easy on-site assembly.
The chamber consists of following features:
Inner and outer chambers were pre-coated with GI sheet; linoleum carpet at the base; viewing window size 1 ft × 1 ft; florescent light for illumination; microprocessor based PID controller with digital display; long lasting U-shaped SS-tubular heaters with SS fins. The system also consists of reservoir tank and humidifier tank, which were fitted with two nos. of humidifier heaters—one works as primary and other as standby. The humidity was maintained by steam injection. Emerson (Kirloskar) make CFC free cooling system consisting of hermetically sealed compressor coupled with evaporation coil and condenser. Audio-visual alarms for temperature and humidity deviations. Safety devices were provided for temperatures overshoot/undershoot and humidity overshoot. The total capacity of the humidity chamber was 20,800 L with external dimensions of (2160 × 4160 × 2760 mm) and thickness of 160 mm. Temperature and humidity were in the range of 20–60 °C and 40–95 % RH, respectively. The chamber was provided with two racks which can accommodate 40 trays each of (250 × 150 × 210 mm) size (Fig. 1).
Bench scale horizontal rotary drum reactor
Based on the laboratory data on high-solid saccharification [13], a bench scale horizontal rotary drum reactor with structural support in SS was designed and fabricated with PLC controlled, LCD display and completely made up of SS 316. The total volume of the reactor was 7.5 L. The H:D ratio was 2.5:1. The design pressure and the working pressures were 4.0 and 3.0 kg cm−2 at full vacuum, respectively. Working temperature of the system was 15–60 °C. The reactor was supported by variable speed drive for speed variation and hydraulic power press (inside the drum) for separating liquid and solid after saccharification. The reactor was also provided with microprocessor controlled chiller/heater circulator with control temperature of 0–50 °C with an accuracy of 0.5 °C to maintain the temperature in jacket. Detailed drawings of the system and its components are shown in Fig. 2a.
Large-scale enzyme production, extraction and concentration
Two fifty gram (250 g) physically pretreated rice straw (5 mm mesh) was moistened with modified Mandels and Weber medium as described earlier [11] and sterilized at 121 °C for 15 min at 15 lbs pressure. Substrate to moisture ratio maintained was 1:6. The trays each of (250 × 150 × 210) mm were UV sterilized separately for 1 h. After sterilization, 3 % (v/v) of seed inoculum was added to the pre-sterilized mix. The trays were incubated at 45 °C for 6 days (relative humidity of 75 %) in a temperature controlled humidity chamber. Seed inoculum was prepared in potato dextrose broth by transferring the mycelial growth from a 2 days grown agar slants and then incubated in an orbital shaker with 150 rpm at 45 °C for 48 h. Enzyme extraction was carried out by removing the contents of each tray with 1500 mL of 0.05 M sodium acetate buffer (pH 4.8) and filtered through a double-layered muslin cloth by thorough squeezing. The crude cellulases were pre-clarified by centrifugation at 10,000×g for 10 min at 4 °C. The clear supernatant was concentrated by using TFF unit with a molecular weight cut off 10 kDa, Pall membrane, Mumbai, fitted with polyethylene sulfonate membrane and under vacuum using rota evaporator at 40 °C. The enzyme concentrate obtained by both the methods were stored at 4 °C until further use.
Influence of temperature, thermo stability and storage stability of concentrated enzymes
For determining the effect of temperature on concentrated enzymes, enzyme activities were estimated at different temperatures (30–80 °C). The thermostability was determined by pre-incubating the concentrated enzyme at 50–70 °C in 0.05 M sodium acetate buffer. Residual filter paper (FP), β-glucosidase and endoglucanase activities were assayed as mentioned earlier [11]. The storage stability of concentrated enzyme was studied at room temperature (30–35 °C) and under refrigeration (4 and −20 °C) conditions. Residual enzyme activities were measured intermittently for a period of 5 months using standard assay conditions.
Enzymatic saccharification of pre-treated rice straw at high-solid loads in bench scale horizontal rotary drum reactor
Based on the preliminary laboratory data obtained from 15 to 30 % (w/v) with different enzyme loads (9 and 12 FPU g−1 substrate) [13], bench scale studies were performed using TFF and vacuum concentrated enzymes from an in-house isolate A. terreus and commercial cellulases from Trichoderma reesei ATCC 26291 (Sigma) in a 7.5 L free-fall horizontal rotary drum reactor at 25 % (w/v) solid loadings. Bench scale high-solid saccharification system is shown in Fig. 2b. Five hundred grams (500 g) of pre-treated rice straw was mixed with an enzyme load of 9 FPU g−1 substrate with addition of 1.0 % Tween 80. Total volume of the system was 2000 mL (0.05 M citrate buffer, pH 4.8). The enzyme was over layered on the substrate and then the reactor was rotated at 16 rpm, at 50 °C for 40 h. Based on the data from TFF, vacuum concentrated and commercial cellulases, further experiments were carried out by supplementing commercial cellobiase from A. niger and commercial hemicellulase (Sigma) to the TFF concentrated in- house cellulases. The commercial cellulase preparation, which was from T. reesei had cellulase activity of 25 FP U mL−1, commercial cellobiase had β-glucosidase activity of 266 U mL−1 and commercial hemicellulase had an activity of 2487 U mL−1. Other conditions were kept constant as described earlier [11]. The contents of reactor after saccharification were squeezed using hydraulic press and centrifuged at 10,000×g for 15 min and the supernatant was analyzed for total reducing sugars.
Recovery of cellulases
Cellulases were recovered from enzymatic hydrolysate (1700 mL) obtained from mild alkali treated rice straw at 25 % solid load using TFF. The hydrolysate was separated from solid biomass by squeezed through a double-layered muslin cloth and centrifuged at 10,000×g for 15 min at 4 °C. The clear hydrolysate was passed through 5 L capacity TFF system. The supernatant (retentate-R1) was washed twice with sodium acetate buffer (0.05 M, pH 4.8) to remove residual sugar from the hydrolysate. The retentate-R1 and the filterate (permeate-P1) were analyzed for the recovered enzyme activities and sugar content. Further, the enzymes associated with the solids have also been recovered by adding Tween 80 1 % (v/v) and incubated the contents at room temperature for 1 h to desorb the enzymes. The solid residue was squeezed through a double-layered muslin cloth and the hydolysate was passed through TFF to recover maximum extent of sugar and cellulases. Both the retentate-R2 and permeate-P2 were again assayed for recovered enzyme activity and sugar content as described earlier [11].
Analytical methods
FP,endoglucanase, and β-glucosidase activities were measured as described earlier [11]. Reducing sugars were determined by dinitrosalicylic acid method [14]. The contents of cellulose, hemicelluloses and lignin in untreated and pretreated rice straw were determined according to Goering and Van Soest [15]. Saccharification efficiency was calculated as described previously [11].
Results and discussion
Chemical pretreatment of RS
Untreated rice straw contained 41.02 ± 1.45 % cellulose; 28.47 ± 1.91 % hemicellulose; 9.20 ± 1.12 % lignin; 7.04 ± 1.21 % moisture; and 15.13 ± 1.15 % ash, respectively. After pretreatment, the solid biomass of rice straw contained 61.10 ± 1.58 % cellulose; 21.01 ± 1.91 % hemicellulose and 3.40 ± 1.16 % lignin, respectively. The alkaline pre-treatment step led to reduction in lignin (59 %) content and increase in cellulose content (48.95 %) in the pre-treated biomass feedstock. The recovery of solid biomass was 65–67 % and the holocellulose content of lignocellulosic biomass was 82.11 %. These findings were consistent with earlier reported laboratory results [11].
Enzyme production and concentration
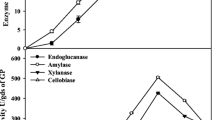
Morphological identification and molecular studies revealed that the fungal strain belongs to A. terreus (NCBI GenBank accession number: KF971363) and it was submitted for safe deposition at Microbial Culture Collection (MCC), National Center for Cell Science (NCCS), Pune, India with strain number A. terreus D34. The crude enzyme obtained by tray fermentation contained all the key enzymes almost in the same proportion viz. FP activity (0.94 ± 0.17 U mL−1), β-glucosidase (5.6 ± 0.37 U mL−1), endoglucanase (16.2 ± 0.40 U mL−1) and hemicellulase (179 ± 0.39 U mL−1) as earlier reported [11]. Many researchers have tried various bioreactors with appropriate designs to improve the cellulae production by overcoming the heat and mass transfer problem which becomes more prevalent in large scale as earlier reported [16–18]. Even though, cellulases activities are higher in some filamentous fungi particularly Trichoderma sp. but the cellulases from Trichoderma lacks β-glucosidase. Therefore, good blend of functionally different enzymes are required for better enzymatic hydrolysis of biomass (Table 2). Production of cellulases by different types of bioreactors are summarized in Table 2 [19–21]. Among the bioreactors examined for cellulase production, tray bioreactor is the most widely employed in lab, pilot and even industrial scale. The current results also in accordance with other researchers who found the application of tray bioreactor in the industrial koji process by adapting SSF for stability and feasibility for large-scale production as earlier reported [22]. Inoculum preparation is the important aspect in SSF. There are several ways of preparing fungal inoculum for SSF. The commonly applied inoculum preparation methods for SSF include spore suspension, mycelial disc and mycelial suspension as earlier reported [4]. During laboratory-scale studies, spore suspension was used which took 7 days for the fungus to form spores as earlier reported [11]. Whereas in scale up studies mycelial suspension was used as an inoculum which reduced the time from 7 to 2 days. Mycelial suspension was also used as an inoculum by many researchers and it has become the most popular choice of inoculum preparation method in cellulase production via SSF and which will also eliminate the lag phase experienced by cultivating the fungi from its spore suspension. The present results are well in agreement with other researchers as earlier reported [23, 24]. It was observed that the number of days required for enzyme production was also reduced from 7 to 6 days as compared to laboratory findings.
To perform saccharification studies at high substrate loads, different methods for enzyme concentration viz. TFF and vacuum evaporation were tried. It was observed that using TFF, higher recovery of the cellulase enzymes was obtained compared to vacuum evaporation (Table 2). The yields of FP, β-glucosidase and endoglucanase from TFF was found to be 95.85, 93.23 and 94.87 %, respectively, whereas for vacuum evaporation it was found to be 91.91, 86.78 and 85.55 %, respectively.
Influence of temperature, thermo stability and storage stability of concentrated enzymes
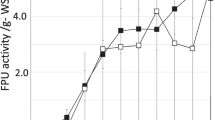
All the three enzymes from TFF and vacuum evaporation showed almost the same trend and exhibited maximum activity at 50 °C and at pH 4.8. FP, β-glucosidase and endoglucanase retained up to 90 % of its maximum activity after 90 min of incubation and more than 80–85 % up to 120–150 min of incubation at 50 °C. At 60 °C, FP, β-glucosidase and endoglucanase retained 52–69 % up to 30 min and 36–41 % activity for 1 h, thereafter there was activity loss. The three enzymes were rapidly inactivated at 70 °C and retained only 10–20 % activity after 15-min incubation (Fig. 3). The effect of storage of TFF and vacuum concentrated enzymes were determined as it is an important parameter for commercial utilization of the enzyme. FP retaining 50 % initial activity after storage for 4 months β-glucosidase and endoglucanase retained 70 % initial activity after storage for 5 months at room temperature. More than 90 % initial activity of all the three enzymes after storage for 4 months at 4 and −20 °C, respectively.
Enzymatic hydrolysis at high-solid loads (25 % w/v) in bench scale reactor
The laboratory data indicated that the system could with stand up to 25 % (w/v) solid loading with a saccharification efficiency of 69.2 and 60.71 % when TFF and vacuum concentrated cellulases were used as an enzyme source, respectively, after 40 h of incubation period. When higher substrate loads were tested [>25 % (w/v)], the efficiency of the system with TFF and vacuum concentrated cellulases fell to 41.49 and 36.21 %, respectively. The same trend was observed with UF concentrated cellulases were tested at 30 % solid loading [13]. This is because of increase in substrate concentration limits the saccharification yield because of poor mixing and heat transfer problem due to the rheological properties of a dense fibrous suspension, which ultimately cause insufficient adsorption of the cellulase to the cellulose as earlier reported [25].
Since bioreactor conditions promote higher conversions with the same amount of enzymes used in flask, hence further experiments were carried out in order to evaluate if it is possible to produce more concentrated sugars which will eventually reduce the ethanol production costs. Bench scale reactor was operated at 25 % w/v solid loading for 48 h. During operation, it was observed that the liquefaction of the contents inside the reactor occurred within 12 h of start of the hydrolysis. Once liquefaction has started, free-fall mixing of the contents also begun. Higher saccharification efficiency of 76.00 % and 66.34 %, respectively, was achieved in 40 h with TFF and vacuum concentrated enzymes in the scaled up system compared to laboratory findings (69.2 and 60.71 %, respectively). As reported in literature, gravitational or free-fall mixing and horizontal oriented rotating drum provides many advantages over typical vertical stirred tank reactors. These types of reactors are also easily scalable from bench scale to pilot scale and larger. The other challenge specific to high-solid enzymatic hydrolysis is typically thought to be the bottleneck of the entire bioconversion process in terms of both time and money, since the reaction time needed for most enzymes to convert lignocellulose into sufficient glucose concentrations for fermentation is on the order of days (usually ≥3 days) as earlier reported [26].
In the present study, the optimum conditions for higher yield was temperature 50 °C, rpm 16, enzyme load 9 FPU g−1 substrate, time 40 h, Tween 80 1.0 % (v/v) and the solid load 25 % (w/v). Under these conditions the efficiency was increased by 9.8 % and produced up to 20 % concentrated sugar syrup in less than 2 days using in-house TFF concentrated cellulases alone without addition of external β-glucosidase. The horizontal orientation might have provided better free-fall and thorough mixing of the contents inside the vessel. Thus, minimizing the particle settling and local accumulation of reaction products within the reactor, as well as ensuring better enzyme distribution leads to higher efficiency in scale-up system compared to laboratory systems [27]. It has also been reported that the enzyme produced using the same biomass if used for bioconversion proved to be more efficient than the one produced on other cellulosic substrate. The present results are in accordance with Lynd et al. [27].
The results also revealed that for 25 % (w/v) substrate load with 9 FPU g−1 substrate, the in-house enzyme (no addition of commercial cellulases, i.e., celluclast 1.5 L) was able to release almost the same amount of sugars when commercial cellulases was completely replaced (Fig. 4). The maximum reducing sugars released from TFF concentrated cellulases and commercial cellulases were 693 and 713 mg g−1 substrate, respectively. The reported literature indicates that the hydrolytic efficiency of a multi-enzyme complex for lignocellulose saccharification depends both on properties of individual enzymes and their ratio in the multi-enzyme cocktail [28, 29]. The present results revealed that the in-house enzyme cocktail might also be having balanced ratios of all the three enzymes along with other accessory enzymes which showed better saccharification efficiency. The proportions and ratios of FP:β-glucosidase:endoglucanase for in-house cellulases were show in Table 3. The ratios of FP:β-glucosidase activity (1:5.79 and 1:5.63) was higher compared to the reported values of 1:1.5 [30], 1:2 [31] and 1:4 [32]. These ratios are not always maintained during enzymatic hydrolysis and depend on nature of substrate [33].
The results further revealed that addition of 60 % each of commercial β-glucosidase and commercial hemicellulases separately to TFF concentrated in-house cellulases increased the reducing sugar yield by 14.5 and 20.0 %, respectively (Fig. 5). There were reports on different solid loadings higher than 25 % (w/v) using different lignocellulosic materials, but to the best of our knowledge no reports on hydrolysis of rice straw at 25 % w/v substrate load without addition of any external enzymes to produce concentrated fermentable sugars up to 20 % in a scale up system [25, 34, 35].
Recovery of cellulases after saccharification
In the present investigation, an attempt was made to recover cellulolytic enzymes used for the hydrolysis of mild alkali treated rice straw using TFF unit (Table 4). The results showed that a significant amount of enzymes remained in the hydrolysate as well as being adsorbed onto the unhydrolysed residual substrate, which was recovered by using simple methods such as repeated washing with buffer/or addition of surfactant to the residual substrate. Repeated washings with 0.05 M sodium acetate buffer (retentate-1) around 41 % FP, 71 % β-glucosidase and 42 % endoglucanase activities were recovered from the hydrolysate. Addition of Tween 80 to the unhydrolysed residual straw (retentate-2) showed 38 % FP, 14 % β-glucosidase and 37 % endoglucanase activities (Table 4). The amount of enzyme activity which was successfully recovered was less than the total amount of enzyme which was initially added to the hydrolysis. This loss in overall enzyme activity may be due either to thermal deactivation and precipitation of the enzyme or loss of soluble enzyme in the liquid fraction [10].
Overall, the results indicating that nearly 79–84 % of cellulases and more than 90 % sugars were recovered from saccharification mixture. Studies have been carried out where free enzymes were recovered from the liquid fraction by membrane filtration [9]. Similarly, the enzymes associated with the solids have been recovered by washing with excess volumes of buffer, sometimes with surfactants to desorb the enzymes, which were then concentrated and added to the fresh substrate [10]. Ana et al. [36] and Xue et al. [37] used the ultrafiltration device (Amicon, 10 kDa polysulfonate membrane) for the recovery of enzymes from the enzymatic hydrolysis of steam pre-treated wheat straw and reported addition of surfactant as a part of the solids washing.
With the results obtained by pretreatment, separate hydrolysis and fermentation, anaerobic digestion to methane, 1 kg rice straw at the rate of 65 % recovery during pre-treatment (0.659 kg of pre-treated rice straw) could be converted into 0.5775 kg of fermentable sugars including glucose (0.3282 kg) and xylose (0.2492 kg). This amount of sugars eventually produced ethanol of 143 g or 181 mL from glucose and 36.5 g or 46.2 mL from xylose, respectively (data not shown). Besides ethanol, 1 kg of dry rice straw during pretreatment produces 82 L of biogas. At the same time, 1 kg rice straw as a solid substrate gives 8 L of crude enzyme and 0.6 kg of solid residue, which can produce 78 L of biogas. Therefore, by using zero waste approach 227 mL of ethanol and 160 L of biogas can be produced from rice straw [38]. Overview of process flow chart which includes pre-treatment, saccharification, ethanol fermentation and biogas production from waste streams of ethanol fermentation process of rice straw is shown in Fig. 6.
Conclusion
The present study demonstrates that the bench scale system developed for high-solid enzymatic hydrolysis was simple, easy to handle, less energy intensive and also has shown increase in saccharification efficiency by 9.8 % compared to laboratory findings and produced up to 20 % concentrated sugar syrup in less than 2-day time without addition of external β-glucosidase. Thus, the use of high-solid operations would make biofuels more economical and more price-competitive with petroleum. The present study also demonstrated that around 80 % cellulase could be recovered after saccharification which would improve the process economics of ethanol fermentation process significantly.
References
Sun Y, Cheng JJ (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Modenbach AA, Nokes SE (2013) Enzymatic hydrolysis of biomass at high solids loadings—a review. Biomass Bioenergy 56:526–544
Holker U, Hofer M, Lenz J (2004) Biotechnological advantages of laboratory scale solid state fermentation with fungi. Biochem Eng J 22:211–219
Li WY, Teck NA, Gek CN, Adeline SMC (2014) Fungal solid state fermentation and various methods of enhancement in cellulase production. Biomass Bioenergy 67:319–338
Weiss N, Borjesson J, Pedersen LS, Meyer AS (2013) Enzymatic lignocellulose hydrolysis: improved cellulase productivity by insoluble solids recycling. Biotechnol Biofuels 6:5
Borjesson J, Peterson R, Tjerneld F (2007) Enhanced enzymatic conversion of softwood lignocellulose by polyethylene glycol addition. Enzyme Microbiol Technol 40:54–762
Rosgaard L, Pedersen S, Meyer AS (2007) Comparison of different pretreatment strategies or enzymatic hydrolysis of wheat and barley straw. Appl Biochem Biotechnol 143:284–296
Yang J, Zhang X, Yong Q, Yu S (2010) Three-stage hydrolysis to enhance enzymatic saccharification of steam-exploded corn stover. Bioresour Technol 101:4930–4935
Tjerneld F (1994) Enzyme-catalyzed hydrolysis and recycling in cellulose bioconversion, vol 288. In: Harry Walter GJ (ed) Methods in enzymology. Academic Press, Waltham, pp 549–558
Tu M, Zhang X, Paice M, MacFarlane P, Saddler JN (2009) The potential of enzyme recycling during the hydrolysis of a mixed softwood feedstock. Bioresour Technol 100:6407–6415
Narra M, Dixit G, Divecha J, Madamwar D, Shah AR (2012) Production of cellulases by solid state fermentation with Aspergillus terreus and enzymatic hydrolysis of mild alkali-treated rice straw. Bioresour Technol 121:355–361
Narra M, Velmurugan BS, Mehta H, Dixit G, Madamwar D, Shah AR (2014) Performance evaluation of anaerobic hybrid reactors with different packing media for treating wastewater of mild alkali treated rice straw in ethanol fermentation process. Bioresour Technol 152:59–65
Dixit G, Shah AR, Madamwar D, Narra M (2015) High solid saccharification using mild alkali-pretreated rice straw by hyper-cellulolytic fungal strain. Bioresour Bioprocess 2(46):1–8
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Goering K, Van Soest PJ (1970) Forage fiber analysis. Agriculture Research series handbook, p 379
Kalogeris E, Fountoukides G, Topakas E, Christakopoulos P, Kekos D, Macris BJ (2003) Performance of an intermittent agitation rotating drum type reactor for soli-state fermentation of wheat straw. Bioresour Technol 86:207–213
Alam MZ, Mamn AA, Qudsieh IY, Muyibi SA, Salleh HM, Omar NM (2009) Solid state bioconversion of oil palm empty fruit bunches for cellulase production using a rotary drum bioreactor. Biochem Eng J 46:61–64
Kim S, Kim CH (2012) Production of cellulase enzymes during the solid-state fermentation of empty palm fruit bunch fiber. Bioprocess Biosyt Eng 35:61–67
Xia L, Cen P (1999) Cellulase production by solid state fermentation on lignocellulosic waste from the xylose industry. Process Biochem 34:909–912
Kalogeris E, Fountoukides G, Kekos D, Macris BJ (1999) Design of solid-state bioreactor for thermilic microorganisms. Bioresour Technol 67:313–315
Fujian X, Hongzhang C, Zuohu L (2002) Effect of periodically dynamic changes of air on cellulose production in solid-state fermentation. Enzyme Microb Technol 30:45–48
Durand A (2003) Bioreactor designs for solid state fermentation. Biochem Eng J 13:113–125
Shrestha P, Rasmussen M, Khanal SK, PomettoIII AL, van Leeuwen J (2008) Solid-substrate fermentation of corn fibers by Phanerochate chrososporium and subsequent fermentation of hydrolysate into ethanol. J Agr Food Chem 56:3918–3924
Deswal D, Khasa YP, Kuhad RC (2011) Optimization of cellulase production by a brown rot fungus Fomitopsis sp RCK2010 under solid state fermentation. Bioresour Technol 102:6065–6072
Chen HZ, Xu J, Li ZH (2007) Temperature cycling to improve the ethanol production with solid state simultaneous saccharification and fermentation. Appl Biochem Microbiol 43:57–60
Dasari RK, Dunaway K, Berson RE (2009) A scraped surface bioreactor for enzymatic saccharification of pre-treated corn stover slurries. Energy Fuels 23:492–497
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Rev 66:506
Gusakov AV, Salanovich TN, Antonov AI, Ustinov BB, Okunev ON, Burlingame Emalfarb M, Baez M, Sinitsyn AP (2007) Design of highly efficient cellulose mixtures for enzymatic hydrolysis of cellulose. Biotechnol Bioeng 97:1028–1038
Agarwal R, Gaur R, Mathur A, Kumar R, Gupta RP, Tuli DK, Satlewal A (2015) Improved saccharificatio nof pilot scale acid pretreated wheat straw by exploiting the synergistic behaviour of lignocelluloses degrading enzymes. RSC Adv. doi:10.1039/c5ra13360b
Mandles M, Medeiros JE, Anderson RE, Bisset FH (1981) Enzymatic hydrolysis of cellulose, evaluation of culture filtrates under use conditions. Biotechnol Bioeng 23:2009–2026
Samayan IP, Schall CA (2010) Saccharification of ionic liquid pretreated biomass with commercial enzyme mixtures. Bioresour Technol 101:3561–3566
Cochet N (1991) Cellulases of Trichoderma reesei: influence of culture conditions upon the enzymatic profile. Enzyme Microbiol Technol 13:104–109
Gao J, Weng H, Zhu D, Yuan M, Guan F, Xu Y (2008) Production and characterization of cellulolytic enzymes from the thermo acidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresour Technol 99:7623–7629
Zhang Q, Cai W (2008) Enzymatic hydrolysis of alkali-pretreated rice straw by Trichoderma reesei ZM4-F3. Biomass Bioenergy 32:1130–1135
Larsen J, Petersen MO, Thirup L, Li HW, Iversen FK (2008) The IBUS process -lignocellulosic bioethanol close to a commercial reality. Chem Eng Technol 31:765–772
Ana CR, Alexandre FL, Susana M, Claus F, Miguel G (2012) Recycling of cellulases in lignocellulosic hydrolysates using alkaline elution. Bioresour Technol 110:526–533
Xue Y, Jameel H, Park S (2012) Strategies to recycle enzymes and their impact on enzymatic hydrolysis for bioethanol production. Bioresour Technol 7:602–615
Narra M, Velmurugan BS (2015) Utilization of solid and liquid waste generated during ethanol fermentation process for production of gaseous fuel through anaerobic digestion—zero waste approach. Bioresour Technol 180:376–380
Acknowledgments
The authors are thankful to the Director, Sardar Patel Renewable Energy Research Institute (SPRERI), Vallabh Vidyanagar, Gujarat for providing facilities and resources to carry out this research at SPRERI. The financial support from Department of Biotechnology (DBT), Government of India is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Narra, M., Balasubramanian, V. & James, J.P. Enhanced enzymatic hydrolysis of mild alkali pre-treated rice straw at high-solid loadings using in-house cellulases in a bench scale system. Bioprocess Biosyst Eng 39, 993–1003 (2016). https://doi.org/10.1007/s00449-016-1578-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1578-9