Abstract
In the present study, two different processes, separate hydrolysis and fermentation (SHF), and simultaneous saccharification and fermentation (SSF) were compared. Three different lignocellulosic biomass viz. rice straw (RS), wheat straw (WS), and sugarcane bagasse (SB) were pretreated with dilute acid at two different concentrations (2 and 4 % H2SO4 w/v) and at two different time intervals, i.e., 30 and 60 min. RS, WS, and SB with 4 % H2SO4 at 121 °C for 30 min yielded maximum reducing sugars (110, 90, and 95 g l−1). Delignification of the solid residues were carried out with 0.5 % NaOH, at 121 °C for 30 min. In-house cellulase produced by Aspergillus terreus was used for separate hydrolysis studies at 10 % solid loading and 9 FPU g−1 substrate enzyme loading for 0–48 h at 42 °C. Maximum yield of reducing sugars from RS, WS, and SB were 266, 242, and 254 mg g−1 substrate, respectively. Acid and enzymatic hydrolysates from RS, WS, and SB produced 5.1, 4.9, 5.2 g l−l, and 14.0, 13.9, 12.9 g l−1 of ethanol with Pichia stipitis and Saccharomyces cerevisiae in 24 and 36 h, respectively. Whereas SSF at 10 % solid loading and 9 FPU g−1 substrate enzyme loading for different time intervals 0–72 h at 42 °C was carried out using in-house thermotolerant yeast strain Kluyveromyces sp. RS, WS, and SB yielded maximum ethanol of 23.23, 18.29, and 17.91 g l−1, respectively. Ethanol yield was enhanced by addition of Tween 80 1 % (v/v) by 8.39, 9.26, and 8.14 % in RS, WS and SB, respectively.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Bioethanol can be produced from any lignocellulosic biomass such as RS, WS, and SB as they are readily available renewable resources of carbohydrates for biological conversion to fuels and chemicals (Borbala et al. 2013). One of the most abundant lignocellulosic biomass in the world is RS. About 731 million tones of RS is produced annually which are distributed in Africa (20.9 million tones), Asia (667.6 million tones), Europe (3.9 million tones), America (37.2 million tones), and Oceania (1.7 million tones). Around 205 billion liters bioethanol per year can be potentially produced from this quantity of RS, which is the largest amount from a single biomass (Faveri et al. 2004). Sugarcane industries also generate huge amount of bagasse annually and some of this residue is currently used for energy cogeneration in sugar mills while the surplus being stockpiled.
Lignocellulosic biomass primarily consists of cellulose, hemicellulose, and lignin and its composition varies with different feed materials used. They are very complex materials; hence a single pretreatment method cannot be applied to all lignocellulosic biomass. Various pretreatment methods have been developed including chemical, physical, physico-chemical, and biological for lignocellulosic biomass and commonly used methods are steam, dilute acid, alkaline, and oxidative pretreatment methods. At current scenario, an up-to-date-technology like dilute acid pretreatment is used for pretreating any lignocellulosic biomass. In acid hydrolysis, removal of hemicellulosic content with small fraction of lignin takes place and the remaining part of lignin remains fixed to the cellulosic content (Kaya et al. 2000). Delignification of lignocellulosic solid biomass is essential to achieve maximum cellulosics hydrolysis due to the greater affinity of cellulase components, β-glucosidase, and endoglucanase towards lignin than to the carbohydrates, resulting in lower saccharification efficiency during enzymatic hydrolysis. Agricultural residues such as RS and herbaceous crops are very effectively pretreated using alkali agents. (Chen et al. 2007). Advantages of alkali pretreatment over other pretreatment technologies include lower temperature, pressure, and time requirement. Sodium hydroxide (alkali) has been widely studied for many years to increase the ease of access of cellulases towards cellulose and hemicelluloses by disrupting the lignin structure of the lignocellulosic biomass.
During enzymatic hydrolysis, addition of cellulases to pretreated material containing holocellulosic (cellulose and hemicelluloses) material converts into monomeric sugars and subsequent addition of yeast ferment these sugars to ethanol. SHF is a two step process where enzymatic hydrolysis is followed by fermentation. However, in SSF using single reactor both the process steps can be carried out simultaneously. SSF is more advantageous compared to SHF as it reduces the cost of reactors by performing saccharification and fermentation in single vessel and better ethanol yields by reducing the product inhibition exerted by saccharification products (Scordia et al. 2013).
The need of the hour is to develop a suitable technology for bioethanol production as a partial replacement of gasoline from lignocellulosic biomass as most of these materials are found in surplus and burnt in the open fields, thereby creating environmental pollution. In order to have an economically viable ethanol plant, the primary focus should be on low cost pretreatments, novel enzymes with higher activities, innovations on fermentation technologies for complete sugar utilization, bioreactors design as well as strains for SSF to provide higher ethanol productivity. Hence, it is essential to develop an indigenous technology with low capital cost and operational expenditure.
In this study, two different processes, SHF and SSF were compared with respect to production of ethanol from dilute acid pretreated lignocellulosic biomass. Both SHF and SSF were carried out at 10 % solid loading and 9 FPU g−1 substrate enzyme loading at 42 °C by the in-house cellulase produced by Aspergillus terreus for different time intervals 0–48 h and using thermotolerant in-house yeast strain Kluyveromyces sp. for different time intervals 0–72 h, respectively. Comparative performance of both the processes was reported in this paper.
2 Materials and Methods
2.1 Lignocellulosic Biomass, Media and Chemicals
Raw materials such as RS and WS were collected from local farmers, Anand, Gujarat, India. SB was procured from a local sugar factory. After collection, the raw materials were exposed to physical pretreatment, i.e., passing through 5 mm mesh in hammer mill prior to chemical pretreatment (Finex, India). The physically pretreated lignocellulosic biomass were washed thoroughly with tap water, air dried, and stored at room temperature in air tight containers. All the chemicals, reagents, and media of analytical grade were purchased from local vendors.
2.2 Acid Pretreatment
One fifty grams of pre-sized RS, WS, and SB were mixed with 750, 1500, and 3000 ml of 2 and 4 % H2SO4. Substrate to acid ratio (w/v) maintained were 1:5, 1:10, and 1:20, respectively. Pretreatment of RS, WS, and SB were conducted at temperature 121 °C for time period 30 and 60 min as described earlier (Narra et al. 2015). Double layered muslin cloth was used to filter the acid hydrolysate. The acid hydrolysate was detoxified with calcium hydroxide as described by Kuhad (2010) and analyzed for sugars, phenolics, and furans. The leftover solid residues after acid pretreatment were thoroughly washed with continuous flow of water till neutral pH and dried under sunlight. The dried acid pretreated solid residues were further subjected to delignification using 0.5 % NaOH at 121 °C for 30 min of 1:20 substrate to alkali ratio. The cellulosic material separated from lignin portion were filtered using double layered muslin cloth and washed thoroughly with tap water till neutral pH and sun dried. These dried solid residues were further used for SHF and SSF studies or stored at 4 °C in air tight bags.
2.3 Microorganisms and Culture Conditions
Standard cultures for SHF were viz. Saccharomyces cerevisiae 3570 and Pichia stipitis NCIM 3499 were procured from National Chemical Laboratory (NCL), Pune, India whereas, newly isolated thermotolerant yeast strain isolated from fruit waste was used for SSF. Partial sequencing of purified strain was carried out at National Collection for Industrial Microorganisms (NCIM), Pune, India. The hexose fermenting yeast strains were maintained on agar slants containing (g−1 l): glucose, 30.0; yeast extract, 3.0; peptone, 5.0; agar, 20.0 at pH 6.0 ± 0.2, and Pichia stipitis was maintained on agar slants containing (g−1 l) xylose, 20.0; yeast extract, 4.0; peptone, 5.0; KH2PO4, 1.5; MgSO4, 7H2O, 0.5; agar, 20.0 at pH 5.0 ± 0.2 and temperature 30 °C, respectively. The cultures were stored at 4 °C.
2.4 Yeast Inoculum Preparation
The Saccharomyces cerevisiae inoculum was grown for 12 h at 30 ± 2 °C in a culture medium containing (g−1 l); glucose, 30.0; yeast extract, 3.0; peptone, 5.0; (NH4)2HPO4, 0.25 at pH 6.0 ± 0.2 (Chen et al. 2007). Kluyveromyces inoculum was prepared at 42 ± 2 °C for 12 h. After incubation, the flask contents were aseptically collected, centrifuged, and used for SHF and SSF studies. An optical density of 0.6–0.8 at 620 nm was used for cell culture. Inoculum of Pichia stipitis was prepared as described by Nigam (2001) using (g−1 l); xylose, 50.0; yeast extract, 3.0; malt extract, 3.0; peptone, 5.0 at pH ± 0.2, and temperature 30 °C. A 12 h seed culture of Pichia stipitis with 1 % v/v were inoculated in separate flasks.
2.5 SHF of Cellulosic Residue
2.5.1 Cellulase Preparation
Crude cellulase used for separate hydrolysis and SSF was indigenously produced by Aspergillus terreus under solid state fermentation as described earlier (Narra et al. 2012). RS was used as a substrate for cellulase production and the crude enzyme contained FP activity, β-glucosidase, and endoglucanase, of 0.98 ± 0.13, 5.2 ± 0.30, and 14.2 ± 0.40 U ml−1, respectively.
2.5.2 Enzymatic Hydrolysis of Delignified Cellulosic Residue
Enzymatic hydrolysis was carried out with cellulosic solid residues of RS, WS, and SB at 42 °C in 50 ml capacity oak ridge wide mouth bottles at 16 rpm for 4–40 h with an enzyme load of 9 FPU g−1 substrate. Total volume of the system was 20 ml (0.05 M citrate buffer, pH 4.8). Other conditions were kept constant as described earlier (Narra et al. 2012). At regular intervals, the supernatant samples were analyzed for total reducing sugars by DNSA method (Miller 1959) after centrifugation at 10,000 g for 15 min. Saccharification efficiency was calculated as mentioned previously (Narra et al. 2012).
2.5.3 Ethanol Fermentation
The enzymatic and acid hydrolysate were fermented with Saccharomyces cerevisiae and Pichia stipitis, respectively, in a 100 ml stoppered flask at 30 ± 2 °C for 40 h. Nutrients containing NH4Cl, 0.5, KH2PO4, 0.15, yeast extract, 3.0 were added to the acid hydrolyse (20 ml) containing (20.0 g l−1) sugars and the pH was adjusted to 5.5 ± 0.2. While the cellulosic hydrolysate having 40.0 g l−1 sugar was supplemented with yeast extract 3.0 g l−1 and (NH4)2HPO4, 0.25 g l−1. The flasks were inoculated with 10 % (v/v) inoculum and incubated at 30 °C for 60 h at 150 rpm.
2.6 SSF
SSF was performed with cellulosic solid residues of RS, WS, and SB in 50 ml capacity oak ridge wide mouth bottles at 42 °C. The total volume of the system maintained was 20 ml (0.05 M citrate buffer, pH 4.8). Crude cellulases were used to hydrolyze the cellulosic substrates at 10 % solid loading and 9 FPU g−1 substrate enzyme loads. After 6 h of hydrolysis at 42 °C, an in-house yeast strain Kluyveromyces sp. was added under sterile conditions for better conversion of cellulosic material. The experimental flasks placed in a rotating assembly and were rotated at 16 rpm for 60 h as described earlier (Narra et al. 2015).
2.7 Analytical Methods
Endoglucanase activity was assayed using 2 % carboxymethyl cellulose (CMC, Sigma Chemical Co.) in 0.05 M sodium acetate buffer, pH 4.8 as substrate. The release of reducing sugars in 20 min at 50 °C was determined by DNSA (Miller, 1959). β-glucosidase assay was carried out using p-nitrophenyl—β-D-glucopyranoside (PNPG, Sigma Chemical Co.) as substrate at 50 °C for 30 min. The reaction was terminated by addition of 4 ml NaOH-glycine buffer (0.2 M, pH 10.6). FP activity was measured as describer earlier (Narra et al. 2012). Lignin, cellulose, and hemicellulose contents of the untreated and pretreated RS, WS, and SB were analyzed according to Goering and Vansoest (1975).
The samples from SHF and SSF were withdrawn at regular intervals, centrifuged at 10,000 × g for 15 min, and the supernatant was analyzed for residual sugars by DNSA method as described earlier. Ethanol was estimated using high performance liquid chromatography (Shimadzu, Japan) equipped with a refractive index detector (RID) and packed with an Aminex-HPX-87 column (Biorad, Hercules, USA, CA) with dimension of 300 mm × 7.8 mm. Samples were eluted using 5 mM H2SO4 with the flow rate of 0.6 ml min−1. Column temperature was maintained at 65 °C. The saccharification efficiency and the theoretical yield of ethanol were calculated as described by Narra et al. (2012, 2015).
3 Results and Discussion
3.1 Biomass Composition Analysis
RS, WS, and SB contained cellulose content of 41.02 ± 1.45 %, 38.50 ± 1.07 %, 39.00 ± 1.83 %; hemicellulose content of 28.47 ± 1.91 %, 27.00 ± 1.36 %, 25.00 ± 1.44 %; lignin content of 9.20 ± 1.12 %, 12.82 ± 1.27 %, 14.21 ± 1.62 % and moisture content of 7.04 ± 1.21 %, 6.93 ± 1.17 %, 8.14 ± 1.15 %, respectively. After pretreatment, the cellulose, hemicellulose, and lignin contents of RS, WS, and SB were (80.00 ± 1.58 %; 67.00 ± 1.60 %; 70.00 ± 1.32 %); (3.00 ± 1.91 %; 6.54 ± 1.42 %; 4.36 ± 1.44 %); (2.01 ± 1.16 %; 4.98 ± 1.97 %; 5.13 ± 1.48 %), respectively (Narra et al. 2015).
3.2 Dilute Acid Hydrolysis of Lignocellulosic Biomass
RS yielded maximum amount of reducing sugars (110 g l−1) followed by WS and SB (90 and 95 g l−1) when treated at optimum conditions, i.e., 4 % H2SO4 at 121 °C for 30 min. The substrate to acid ratio maintained was 1:5 (w/v). As the treatment time increased from 30 min to 60 min, there was no substantial difference in the reducing sugar yield (102, 85, and 91 g l−1). The acid hydrolysate obtained at optimum conditions of hydrolysis contained major amount of xylose (90.23 ± 2.34 g l−1), glucose (5.01 ± 1.29 g l−1), arabinose (7.48 ± 2.69 g l−1), phenolics, furfural, and HMF. The major degradation products of pentose, hexose sugars, and lignin are furfural, HMF, and phenolics, respectively (Kuhad et al. 2010). These toxins have the ability to decrease the activities of several yeast enzymes, e.g., alcohol dehydrogenase, aldehyde dehydrogenase, and pyruvate dehydrogenase during fermentation (Modig et al. 2002). In order to reduce the inhibitor concentrations in acid hydrolysates, sequential addition of overliming and activated charcoal was used. Overliming followed by activated charcoal treatment resulted in reduction of furfural (96.31, 95.15, and 96.20 %), HMF (93.36, 92.18, and 92.19 %), and phenolic (93.36, 92.18, and 92.19 %) in RS, WS, and SB, respectively. As the acid hydrolysate was rich in xylose it was used to produce ethanol using pentose utilizing yeast strain Pichia stipitis NCIM 3499.
3.3 Delignification of Lignocellulosic Biomass
Delignification of acid pretreated solid biomass was carried out with 0.5 % NaOH at 121 °C for 30 min to maximize reducing sugar yield. The cellulose content in the pretreated biomass increased substantially with simultaneous reduction in lignin content after alkaline pretreatment (Narra et al. 2015). The cellulose content was found to be increased by 95.02, 74.02, and 79.48 %, while the lignin removals were 78.16, 61.15, and 63.90 % in RS, WS, and SB, respectively. It was also observed that during the treatment most of the pentosan was solubilized. The increase in cellulose content during pretreatment might be due to removal of lignin which might have increased the enzyme effectiveness by eliminating nonproductive adsorption sites and increasing access to cellulose and hemicelluloses (Lu et al. 2002). Kumar et al. (2009) reported that solubilization of other components in the aqueous alkali solution is also responsible for increase in the cellulose content. These findings were consistent with earlier published data on effects of delignification of different lignocellulosic biomass RS, WS, and rapeseed straw by alkaline pretreatment (Narra et al. 2012; Nopparat et al. 2013).
3.4 Enzymatic Saccharification of Delignified Cellulosic Material
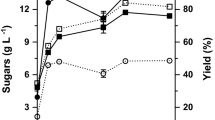
When delignified cellulosic substrates were subjected to enzymatic saccharification, an increase in reducing sugar concentration was observed till 40 h time period and thereafter it remained almost constant. Maximum yield of saccharification from RS, WS, and SB was 266, 242, and 254 mg g−1 substrate, respectively (Fig. 1). Tween 80 1 % (v/v) addition also found to increase the saccharification yield by 12.1, 11.4, and 10.6 %, respectively (Fig. 2). According to Kaar and Holtzapple (1998), all through the enzymatic saccharification process, thermal deactivation of enzymes was prevented by the addition of Tween. This outcome may be due to the surface activity of Tween which resulted in shorter enzyme contact with air-liquid interface. The decrease in surface tension of the solution not only permits the saccharifying exoglucanase more active sites to cellulose, but it also prevents the nonproductive part of the exoglucanase to the lignin surface which yielded in increased sugar level (Hematinejad et al. 2002).
3.5 Fermentation of Hemicellulosic and Enzymatic Hydrolysate
Maximum amount of ethanol production from RS, WS, and SB was obtained up to 5.1, 4.9, 5.2 g l−l, respectively. Table 1 shows the profile for ethanol fermentation by Pichia stipitis 3499 NCIM. It was observed that there was increase in ethanol and increase in the growth of fermenting yeast but the consumption of reducing sugars was relatively poor. The incomplete utilization of reducing sugars by fermenting yeast may be due to some kind of inhibitors present in the acid hydrolysate which might have not been removed during the detoxification process. Similar observation was also made by Kuhad et al. (2010) that the yeast could not tolerate the higher amount of sugar concentration. The ethanol yield obtained from cellulosic hydrolysate (RS, WS, and SB) using Saccharomyces cerevisiae was 0.35, 0.35, and 0.32 g g−1, respectively (Table 2).
3.6 SSF
Maximum ethanol yield achieved at 60 h with 10 % solid load from RS, WS, and SB (23.23, 18.29, and 17.91 g l−1) which was equivalent to 51.29, 48.22, and 45.19 % of maximum theoretical yield (Fig. 3). The earlier reports have shown that increase in substrate load beyond certain extent could have caused decrease in hydrolysis rate due to the product inhibition. The extent of inhibition usually depends on the ratios of substrate to enzyme load (Wang et al. 2011; Xin et al. 2010). The present results also revealed that higher ethanol yields at lower solid loads may be due to lower glucose accumulation compared to higher solid loads. The current results were in accordance with Naveen et al. (2011) that at 8 % solid loading, higher ethanol yields were observed compared to 12 % solid loading when pretreated switchgrass was used for SSF studies by Kluyveromyces marxianus IMB3.
Enhanced ethanol yields were achieved by the addition of 1 % Tween 80 to the reaction mixture in comparison to the control (Fig. 4). The yields were increased by 8.39, 9.26, and 8.14 % with 10 % solid loading at 60 h from RS, WS, and SB, respectively. Maximum ethanol yield from RS, SB, and WS were found to be 25.18, 19.57, and 18.19 g l−1, respectively. Kaar and Holtzapple (1998), Zhu et al. (2014) similarly have found that addition of surfactant enhanced the rate and extent of hydrolysis as the surfactants could have changed the nature of the substrate either by increasing the available cellulose surface area or by eliminating inhibitory lignin.
The present results have shown that 1 kg untreated RS, WS, and SB contained 410, 370, 380 g, and 252, 205, 207 g cellulose and hemicelluloses, respectively. This amount can be theoretically enough for production of 232, 217, 215 g and 129, 105, 106 g ethanol from cellulose and hemicellulose, respectively. Based on the best yields attained in the current work, the ethanol production from RS, WS, and SB through SSF was found to be 201, 162, 156 g kg−1 raw biomass, respectively. Whereas, the ethanol production from RS, WS, and SB through SHF was found to be 95, 75, 82 g kg−1 raw biomass, respectively (Table 3). The present results revealed that SSF could prove enhanced ethanol production from delignified biomass compared to SHF. The reason could be that the optimum condition for separate enzymatic hydrolysis might be higher than 42 °C, and secondly the product inhibition exerted by saccharification might have occurred during the process. Whereas in case of SSF, pre-hydrolysis was carried out at 42 °C for 6 h followed by yeast addition to ease the problems caused by production inhibition.
4 Conclusion
Cellulosic ethanol is considered to be a globally accepted alternative fuel. The current results demonstrated that SSF could ascertain improved ethanol production from delignified lignocellulosic biomass compared to SHF in terms of total ethanol production time and ethanol yield. RS yielded higher amount of ethanol followed by WS and SB using in-house cellulases produced by Aspergillus terreus and in-house thermotolerant yeast strain Kluyveromyces sp.
References
Borbala E, Mats G, Guido Z (2013) Simultaneous saccharification and co-fermentation of whole wheat in integrated ethanol production. Biomass Bioenergy 56:506–514
Chen HZ, Xu J, Li ZH (2007) Temperature cycling to improve the ethanol production with solid state SSF. Appl Biochem Microbiol 43:57–60
Faveri DD, Torre P, Perego P, Convarti A (2004) Statistical investigation on the effects of starting xylose concentration and oxygen mass flow rate on xylose production from RS hydrolysate by response surface methodology. J Food Eng 65:383–389
Goering K, Van Soest PJ (1975) Forage fiber analysis-agriculture research series. Handbook 379
Hemantinejad N, Vahabzadeh F, Koredestani SS (2002) Effect of surfactant on enzymatic hydrolysis of cellulosic fabric. Iranian Poly J 11:333–338
Kaar WE, Holtzapple MT (1998) Benefits from tween during enzymatic hydrolysis of corn stover. Biotechnol Bioeng 419–427
Kaya F, Heitmann JA, Thomas WJ (2000) Influence of lignin and its degradation products on enzymatic hydrolysis of xylan. J Biotechnol 80:241–247
Kuhad RC, Gupta R, Khasa YP, Singh A (2010) Bioethanol production from Lanata Camara (red sage): pretreatment, saccharification and fermentation. Bioresour Technol 641–654
Kumar R, Mago G, Balan V, Wyman CE (2009) Physical and chemical characterization of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour Technol 100:3948–3962
Lu Y, Yang B, Gregg D, Saddler J, Mansfield S (2002) Cellulase adsoption and an evaluation of enzyme recycling during hydrolysis of steam-exploded soft wood residues. Appl Biochem Biotechnol 98–100
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Modig T, Liden G, Taherzadehm MJ (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J 363:769–776
Narra M, Dixit G, Madamwar D, Shah AR (2012) Production of cellulases by solid state fermentation with Aspergillus terreus and enzymatic hydrolysis of mild alkali-treated RS. Bioresour Technol 121:355–361
Narra M, James J, Balasubramanian V (2015) SSF of delignified lignocellulosic biomass at high solid loadings by a newly isolated thermotolerant Kluyveromyces sp. for ethanol production. Bioresour Technol 179:331–338
Naveen KP, Hasan KA, Mark RW, Danielle DB, Ibrahim MB (2011) SSF of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: The effect of enzyme loading, temperature and higher solids. Bioresour Technol 102:10618–10624
Nigam JN (2001) Ethanol production from WS hemicellulose hydrolysate by Pichia stipitis. J Biotechnol 87:17–27
Nopparat S, Khatiya W, Navadol L, Verawat C (2013) Optimize simultaneous saccharification and co-fermentation of RS for ethanol production by Saccharomyces cerevisiae and Scheffersomyces stipitis co-culture using design of experiments. Bioresour Technol 142:171–178
Scordia D, Cosentino SL, Jeffries TW (2013) Enzymatic hydrolysis, simultaneous saccharification and ethanol production of oxalic acid pretreated giant reed (Arundodonax L.). Ind Crops Prod 49:392–399
Wang W, Kang L, Wei H, Arora R, Lee Y (2011) Study on the decreased sugar yield in enzymatic hydrolysis of cellulosic substrate at high solid loading. Appl Biochem Biotechnol 164:1139–1149
Xin F, Geng A, Chen ML, Gum MJM (2010) Enzymatic hydrolysis of sodium dodecyl sulphate (SDS) pre-treated newspaper for cellulosic ethanol production by Saccharomyces cerevisiae and Pichia stipitis. Appl Biochem Biotechnol 162:1052–1064
Zhu JQ, Lie Q, Li BZ, Yuan YJ (2014) Simultaneous saccharification and co-fermentation of aqueous ammonia pretreated corn stover with an engineered Saccharomyces cerevisiae SyBE005. Bioresour Technol 169:9–18
Acknowledgements
The authors are thankful to the Director, Sardar Patel Renewable Energy Research Institute (SPRERI), Vallabh Vidyanagar, Gujarat for allowing us to carry out research at SPRERI. The financial support from Department of Biotechnology (DBT), Government of India is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer India
About this paper
Cite this paper
Madhuri Narra, James, J.P., Velmurugan Balasubramanian (2016). Comparison Between Separate Hydrolysis and Fermentation and Simultaneous Saccharification and Fermentation Using Dilute Acid Pretreated Lignocellulosic Biomass. In: Kumar, S., Khanal, S., Yadav, Y. (eds) Proceedings of the First International Conference on Recent Advances in Bioenergy Research. Springer Proceedings in Energy. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2773-1_1
Download citation
DOI: https://doi.org/10.1007/978-81-322-2773-1_1
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2771-7
Online ISBN: 978-81-322-2773-1
eBook Packages: EnergyEnergy (R0)