Abstract
In the present work V2O5 xerogel thin films are deposited on quartz substrate by using a cost effective sol–gel based dip coating method. Films of thickness ~122, 224 and 284 nm are grown as the number of dipping varies from one to three. Phase analyses of the thin films are investigated by X-ray diffraction technique. The microstructural characterization is carried out by electron microscopy techniques. Crystalline, phase pure V2O5 thin films with uniform nanoporous microstructure are grown in the present work. Solar transmittance and reflectance of films are measured by UV–VIS–NIR spectrophotometer. The optical band gap, refractive index and extinction coefficient of the films are also evaluated. Further, the average solar absorptance and IR emittance are evaluated by solar spectrum reflectometer and emissometer, respectively. The solar transmittance and optical band gap decrease with the increase in film thickness from 122 to 284 nm while reverse trend is observed for solar absorptance. Refractive index (~1.95) and IR emittance (~0.75) of the nanoporous V2O5 xerogel thin films on quartz substrate are almost independent of film thickness. The sheet resistance value slightly decreases from ~1.6 × 106 to ~2.6 × 105 ohm/cm2 as the thickness of the V2O5 xerogel thin film increases from 112 to 284 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vanadium pentoxide (V2O5) thin films are very well known today for its immense technological applications. Among many others, the most emerging applications include gas and chemical sensing [1, 2], catalysis [3, 4], cathodes in lithium ion batteries [5, 6], smart windows [7], organic solar cell [8], thermochromic [9] and electrochromic [10] device applications. The synthesis methods of these thin films are as wide as reactive magnetron sputtering [11], pulsed spray pyrolysis [12], thermal evaporation [13], ultrasonic spraying [14], atomic layer deposition [15], chemical vapour deposition [16] and sol–gel [17–22]. Most of these methods [11–16] except the sol–gel technique [17–22] demand very high vacuum condition and other complex instrumental attachments. Even then only small scale production is achievable by these methods. In contrast, the V2O5 xerogel thin films synthesized by the inexpensive sol–gel route are very well known for many advanced applications e.g., supercapacitors [23], electrochromic devices [24] and components in energy storage devices [25].
Thus, in the present work a cost-effective dip coating method is employed to deposit V2O5 xerogel thin films. Further, systematic and in-depth phase analysis, microstructure, thermo-optical properties and sheet resistance evaluation are conducted for the deposited films.
2 Experimental details
V2O5 powder was synthesized from precursor ammonium metavanadate, NH4VO3 (Thermo Fisher Scientific India Pvt. Ltd.). In a typical synthesis, approximately 5 g of NH4VO3 was dissolved in 200 ml distilled water in a glass beaker under the condition of constant stirring at 400 rpm with gentle heating at 50 °C on a hot plate magnetic stirrer. Subsequently constant stirring (400 rpm) at 90 °C, a 0.2 M HCl solution was added drop by drop to it to make the solution acidic (pH ~ 2). As a result, orange colour powder precipitated and then the same was filtered and washed repeatedly with distilled water followed by drying in air. This air dried precipitate was post heat treated at 450 °C for 1 h in air [26]. Next, 1 gm of the synthesized heat treated powder was dissolved in 60 ml of 15 % H2O2 (Merck Specialities Pvt. Ltd., India) solution with vigorous agitation at about 500 rpm at room temperature (e.g., ~30 °C) to prepare the sol. Next, the sol was heated for about 20 min at ~70 °C in a water bath with constant stirring at 500 rpm to form the gel. The pH of the gel was ~1.5. Quartz substrates of size 40 mm × 40 mm were used for the deposition of the V2O5 xerogel thin films. Finally, the dried substrates were dipped into the gel at room temperature (e.g., ~30 °C). Films of three thicknesses were grown by varying the number of dipping i.e., one, two and three. To obtain uniform xerogel thin film deposition, the rate of immersion into and withdrawal from the gel was instrumentally controlled at a rate of about 1.67 mm s−1 for all the substrates. All the dip coated xerogel films were subsequently cured at 150 °C for 20 min in an air oven.
The thickness of the deposited thin films was measured by the profilometry technique (3400E, Bruker, USA). The phase analysis of the synthesized xerogel thin films was performed by the X-ray diffraction (XRD: monochromatic Cu Kα1 radiation, 35 mA, 40 kV, PANalytical X’pert Pro MPD Diffractometer, The Netherlands) technique. Microstructural characterizations were carried out by field emission scanning electron microscopy (FESEM: Supra VP35 Carl Zeiss, Germany) and transmission electron microscopy (TEM: Model Tecnai G2 30, S-Twin, 300 kV, FEI, The Netherlands). Transmittance and reflectance of the thin films were measured as a function of wavelength by a UV–VIS–NIR spectrophotometer (Cary 5000, Agilent Technologies, USA) in solar region (e.g., 200 nm to 2.3 μm) of the spectral window. The absorption coefficients (α) of the xerogel thin films were calculated [27–31] utilizing the transmittance spectra. The optical band gap was also estimated employing conventional ‘Tauc extrapolation plot’. The refractive indices of the films were evaluated following the method described in elsewhere [31] from the corresponding reflectance spectra. For this purpose the reference refractive index of the quartz substrate was taken as 1.45 [31]. The extinction coefficients of the films were calculated according to the relation reported in [27–31].
The average solar absorptance (α s ) was evaluated by the solar spectrum reflectometer (SSR) in transmittance mode (SSR-E, Devices and Services Co., USA) as per ASTM C1549-09. Further, the average IR emittance (ε ir ) of the deposited films on quartz were measured by an emissometer (AE, Devices and Services Co., USA) using the standard ASTM C1371–04a.
A portable two point resistance probe meter (e.g., Model 152-1, Trek Inc., USA) was utilized to measure sheet resistance. It took care of the contact resistance and evaluated the sheet resistance of only the material. Probe electrode test voltage was kept at a constant magnitude of 10 V. The measurement was done in a standard clean room environment. The two probes i.e., the electrodes length of 25.4 mm were kept perpendicular to the specimen surface and parallel to each other with a constant distance of 25.4 mm to form a square area. At least 5 measurements were taken in four corners and at the middle of a given specimen. Thus, the average sheet resistance data of at least 5 measurements are reported here.
3 Results and discussion
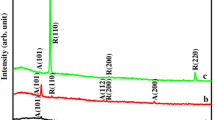
The V2O5 xerogel thin films grown by single dipping are 122 nm thick. The films grown by dipping twice are 224 nm thick. However, the films grown by three successive dippings are 284 nm thick. The typical XRD patterns of the deposited xerogel thin film are shown in Fig. 1. The films show crystalline nature. The XRD data agree well with the literature [32]. The films are primarily grown along (001) orientation with the crystallite size of 20, 21.1 and 20.7 nm corresponding to the one, two and three dip films, respectively. A uniform nanoporous surface morphology is observed for a single dipped film [Fig. 2(a)]. The inset of Fig. 2(a) shows a typical low magnification FESEM image of the microstructure. Similar features are also observed for two and three dipping and for the sake of brevity the other microstructures are not included. Typical TEM bright field photomicrograph is shown in Fig. 2(b). Further, Fig. 2(b) depicts the distribution of fine particles. The average equivalent diameter of the particles size is measured as 15.7 ± 6.2 nm. The present TEM based data matched reasonably well with the crystallite size measured by the XRD based technique.
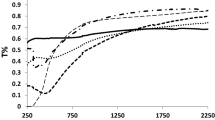
Transmittance spectra of the different V2O5 xerogel films are shown in Fig. 3(a) as a function of wavelength. Particularly, at near IR region a significant decrease in transmittance (i.e., from ~60 to ~45 %) is observed while the thickness of the film is increased from 122 to 284 nm, as expected [33]. A similar behavior is reported by other researchers [33]. In general, it is reported that amorphous V2O5 shows transmittance higher in comparison to that of the crystalline one [34, 35]. For instance, amorphous V2O5 thin films deposited by sol–gel based spin coating method [32, 33] shows a transmittance value as high as e.g., 70–80 % [36, 37] while nanocrystalline V2O5·nH2O thin films developed by sol–gel based dip coating method shows a typical transmittance that is as low as e.g., about 35 % [38]. Thus, the present dip coated V2O5 xerogel thin films have transmittance intermediate to those reported in literature e.g., [32–34]. Most interestingly, a shift in fundamental absorption edge towards the higher wavelength is noticed as the film thickness is increased as shown in Fig. 3(a). According to the data presented in Fig. 3(a), the fundamental absorption edge begins at 430 nm for the film thickness of 122 nm. Further, it is shifted to a higher wavelength of 445 nm for the film thickness of 224 nm. Finally it reaches up to a wavelength as high as 470 nm for the 284 nm thick film. The wavelength at which the transmission first starts in the transmittance spectra is known as the fundamental absorption edge. The fundamental absorption edge defines the absorption occurring at a particular wavelength which always differs depending on the thickness of material. As thickness increases, the energy required to excite an electron reduces because the photon energy required to excite an electron at a particular wavelength is always inversely proportional to the particular wavelength [39]. This phenomenon leads to the decrease in corresponding optical band gap energy values which are shown in Table 1 and the corresponding Fig. 4. Accordingly, the optical band gap value is gradually decreased from 2.71 to 2.42 eV as the film thickness increases from 122 to 284 nm. A similar behavior is also reported for sputtered vanadium oxide thin films [33]. The range of the optical band gap energy values (i.e., 2.71–2.42 eV) of the present V2O5 xerogel thin films matches with literature data [14, 15, 36–42]. The increase in V2O5 film thickness (from ~35 to ~80 nm) reduces the optical band gap energy value from ~2.7 to 2.35 eV [15]. In the case of spin coated V2O5 films, the increase in film thickness from ~150 to 670 nm reduces the optical band gap value from ~2.5 to 2.25 eV [40]. In general, the optical band gap energy value of V2O5 is a strongly sensitive function of thickness enhancement [15, 40], substrate temperature [15, 34, 39], molar ratio of the dopant [38] and oxidation state of the coating [41]. The corresponding reflectance spectra of these films are shown in Fig. 3(b). Several maxima and minima are observed in the spectra.
The variations of the optical absorption coefficient (α) of the V2O5 xerogel thin films are shown as a function of photon energy in Fig. 4(a)–4(c) for the films of 122, 224 and 284 nm thicknesses, respectively. The corresponding refractive index values along with the earlier mentioned optical band gap energy values are also summarized in Table 1.
The refractive index (e.g., ~1.95) of the present V2O5 xerogel thin films is not affected by the increase in thickness, as expected [40]. The spin coated V2O5 thin films have refractive index as 2.31 [32], 2.11 [33] and 2–2.66 [40]. So both novelty and superiority of the present work are established here. On the other hand, the dip coated V2O5 thin films have refractive index in the range of 1.9–2.38 [38]. The sputtered V2O5 thin films have refractive index of about 1.8–1.9 [34] and 2.18 [41]. In general, the refractive index alters due to molar ratio of the dopant [38], change in oxidation state of vanadium oxide [40] and the substrate temperature [34]. In fact increase in dopant (e.g., potassium) molar ratio can tune the refractive index down to even as low as e.g., 1.9 [38]. Lower packing density also reduces the refractive index to e.g., about 1.8–1.9 [34]. In the present work, the refractive index of the V2O5 xerogel film is evaluated to be a little on the lower side e.g., about 1.95. This may also happen due to the nanoporous microstructure as shown in Fig. 2. The smaller magnitude of refractive index signifies lesser density of the V2O5. However, in the present case we have not used any dopant and yet we have achieved the refractive index of as low as about 1.95 which may be beneficiary for future application such as e.g., a potential candidate for fast switching in electrochromic devices [36]. So, here are the novelty and superiority of the present work which should neither be scientifically denied nor technically ignored.
The variation of the extinction coefficient as a function of wavelength is shown in Fig. 5. Higher extinction coefficient values are observed at lower wavelength region, however it decreases significantly at higher wavelength region. The average solar absorptance and IR emittance values of the V2O5 xerogel films deposited on quartz substrate are summarized in Table 2. Very low (~0.02) solar absorptance is found for the bare quartz substrate. However, after coating of V2O5, significant increase in solar absorptance is observed. In fact, it further increases from ~0.5 to ~0.8 as the V2O5 xerogel film thickness increases from 122 nm to 284 nm.
The bare quartz substrate shows average IR emittance of 0.79. The average IR emittance of the V2O5 coated quartz is ~0.76. So, with respect to the IR emittance of the bare quartz substrate itself (e.g., ~0.76) there is not much of a significant change in that (e.g., ~0.79) of the present V2O5 thin films on quartz substrates. This information implies that the present V2O5 xerogel thin films are IR transparent [33, 43]. This fact suggests further that such films can be a potential candidate also for applications that require IR transparency. As far as process efficacy is concerned it is indeed the most interesting point to note that the average IR emittance value (e.g., ~0.76) of the present V2O5 xerogel thin films dip coated on quartz substrates matches with that (e.g., ~0.78) reported for the r.f. sputtered 243 nm V2O5 thin films on quartz substrates [33]. For xerogel V2O5 films in particular, prior to the present work the in-depth and systematic investigation of thermo-optical properties such as IR emittance, solar transmittance, reflectance and absorptance and the evaluation of optical constants were not explored. In the present work, we have attempted the same. Exhaustive review of existing literature [1–36] also supports this viewpoint. So, here again both the novelty of the present work are established beyond any doubt.
The sheet resistance values of different V2O5 xerogel thin films are shown in Table 1. As the V2O5 film thickness increases from 122 nm to 284 nm, the sheet resistance value is slightly decreased from ~1.6 × 106 to ~2.6 × 105 ohm/cm2. The present data on sheet resistance agree well with the data reported in literature [33]. The small standard deviations of the experimental data as shown in Table 1 strongly suggest that the coated surfaces are reasonably uniform as far as the sheet resistance is concerned.
4 Conclusions
By using a cost effective dip coating technique, the present work achieves the deposition of phase pure, crystalline V2O5 xerogel thin films on quartz substrates. Thin films of 122–284 nm thickness are deposited by varying the number of dipping. The deposited films possess a nanoporous microstructure. A noteworthy decrease in transmittance (i.e., ~60 to ~45 %) is observed as the thickness of the film increases. Due to shift in fundamental absorption edge towards the higher wavelength the optical band gap decreases from 2.71 to 2.42 eV with increase in film thickness. Comparatively lower refractive index (~1.95) value is found for the xerogel V2O5 thin films. The reduction in refractive index may be linked with the nanoporous microstructure of the films. Most importantly for the present films of 112–284 nm thickness the average solar absorptance is ~0.8 which is much higher than that of the bare quartz (e.g., about 0.02) substrates. The other significant achievement is that the average IR emittance (e.g., about 0.76) is almost as good as that (e.g., ~0.79) that of the bare quartz substrate. In other words these xerogel V2O5 thin films can be useful for applications that require high IR transparency. The IR emittance is also almost insensitive to variations in film thickness. Finally, the sheet resistance value slightly decreases from ~1.6 × 106 to ~2.6 × 105 ohm/cm2 as the thickness of the V2O5 xerogel thin film increases from 112 to 284 nm.
References
J Huotari, J Lappalainen, J Puustinen and A L Spetz Sens. Actuators B Chem. 187 386 (2013)
Y Vijayakumar, G K Mani, M V R Reddy and J B B Rayappan Ceram. Int. 41 2221 (2015)
E A Khudaish and A T Al-Hinai J. Electroanal. Chem. 587 108 (2006)
S Moon, T Kim, S Park, J Jung and S Hong Stud. Surf. Sci. Catal. 110 1231 (1997)
D Chen, H Quan, S Luo, X Luo, F Deng and H Jiang Phys. E 56 231 (2014)
C Liang, D Fang, Y Cao, G Li, Z Luo, Q Zhou et al. J. Colloid Interface Sci. 439 69 (2015)
M Kamalisarvestani, R Saidur, S Mekhilef and F S Javadi Renew. Sustain. Energy Rev. 26 353 (2013)
G T Escobar, J Pampel, J M Caicedo and M L Cantu Energy Environ. Sci. 6 3088 (2013)
N Wang, S Magdassi, D Mandler and Y Long Thin Solid Films 534 594 (2013)
S Zanarini, N Garino, J R Nair, C Francia, P J Wojcik, L Pereira et al. Int. J. Electrochem. Sci. 9 1650 (2014)
R M Öksüzoğlu, P Bilgiç, M Yıldırım and O Deniz Opt. Laser Technol. 48 102 (2013)
C E Patil, N L Tarwal, P S Shinde, H P Deshmukh and P S Patil J. Phys. D Appl. Phys. 42 25404 (2009)
R Santos et al. Appl. Surf. Sci. 282 590 (2013)
Y Wei, M Li, J Zheng and C Xu Thin Solid Films 534 446 (2013)
E Østreng, O Nilsen and H Fjellvåg J. Phys. Chem. C 116 19444 (2012)
N K Nandakumar and E G Seebauer Thin Solid Films 519 3663 (2011)
D V Raj, N Ponpandian, D Mangalaraj and C Viswanathan Mater. Sci. Semicond. Process. 16 256 (2013)
A A Bahgat, F A Ibrahim and M M El-Desoky Thin Solid Films 489 68 (2005)
D Grosso J. Mater. Chem. 21 17033 (2011)
D R Ceratti, B Louis, X Paquez, M Faustini and D Grosso Adv. Mater. doi:10.1002/adma.201502518 (2015)
C Glynn et al. Sci. Rep. 5 11574 (2015)
C Glynn, D Creedon, H Geaney, J O’Connell, J D Holmes and C O’Dwyer ACS Appl. Mater. Interfaces 6 2031 (2014)
K Jeyalakshmi, S Vijayakumar, S Nagamuthu and G Muralidharan Mater. Res. Bull. 48 760 (2013)
D McNulty, D N Buckley and C O’Dwyer J. Power Sources 267 831 (2014)
E A Olivetti, J H Kim, D R Sadoway, A Asatekin and A M Mayes Chem. Mater. 18 2828 (2006)
H K Koduru, H M Obili and G Cecilia Int. Nano Lett. 24 1 (2013)
B R Kumar and T S Rao J. Ovonic Res. 7 115 (2011)
P Kumar, M K Wiedmann, C H Winter and I Avrutsky Appl. Opt. 48 5407 (2009)
Y Wang, I Ramos and J J Santiago-Aviles J. Appl. Phys. 102 93517 (2007)
K Eunah, J Zhong-Tao and N Kwangsoo Jpn. J. Appl. Phys. 39 4820 (2000)
I N Reddy et al. Ceram. Int. 40 9571 (2014)
V Petkov, P N Trikalitis, E S Bozin, S J L Billinge, T Vogt and M G Kanatzidis J. Am. Chem. Soc. 124 10157 (2002)
D Porwal et al. RSC Adv. 5 35737 (2015)
L J Meng, R A Silva, H N Cui, V Teixeira, M P dos Santos and Z Xu Thin Solid Films 515 195 (2006)
C V Ramana, R J Smith and O M Hussain Phys. Status Solidi (a) 199 R4 (2003)
N Ozer Thin Solid Films 305 80 (1997)
M Benmoussa, A Outzourhit, R Jourdani, A Bennouna and E L Ameziane Act. Passive Electron. Compon. 26 245 (2003)
H A Mady, S E Negm, A S Abdel Moghny, A S Abd-Rabo and A A Bahgat J. Sol-Gel Sci. Technol. 62 18 (2012)
Z S El Mandouh and M S Selim Thin Solid Films 371 259 (2000)
F N Dultsev, L L Vasilieva, S M Maroshina and L D Pokrovsky Thin Solid Films 510 255 (2006)
M Benmoussa, E Ibnouelghazi, A Bennouna and E L Ameziane Thin Solid Films 265 22 (1995)
V Bondarenka, S Kačiulis, Z Martūnas, A Rėza, G J Babonas and A Pašiškevičius Lith. J. Phys. 48 341 (2008)
L Jacques Coord. Chem. Rev. 391 190 (1999)
Acknowledgments
B.B. and A.K.M. gratefully acknowledge the kind support and encouragements of Director, CSIR-CGCRI during the course of the present work. Financial support of CSIR is also gratefully acknowledged by B.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bera, B., Esther, A.C.M., Dey, A. et al. Structural, optical and electrical properties of V2O5 xerogel thin films. Indian J Phys 90, 687–692 (2016). https://doi.org/10.1007/s12648-015-0807-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-015-0807-4