Abstract
In this work, study the influence of the thermochromic capability of tungsten-doped vanadium oxide thin film at high-treating temperature. The films were prepared by following sol–gel technique starting from the incorporation of vanadium (V) oxytripropoxide with anhydrous isopropanol. Tungsten chloride, at a concentration of 4 at %, used in the recipe as a doping material. The films deposited, on (10 × 5 × 3 mm) quartz substrate utilizing spin coating method at spinning speed of 4000 rpm. All samples were heat-treated at a rate of 1 °C/min reaching 500 °C, 1000 °C, 1500 °C and 2000 °C then kept at these temperatures for 30 min. Structural and spectral characterization, UV–VIS-NIR, SEM, and XRD examinations were used to evaluate the resultant films. The thermochromic transition temperature of the film dropped to as low as 70 °C, while the visible transmittance maintains at 75%. This intrinsically high-treating temperature process, practically, makes the tungsten-doped vanadium oxide films more potentially in thermochromic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermochromic windows present the employing of intelligent, smart glass to provide design solutions with increased energy consumption. Above the critical temperature, the crystal structure shifts toward a rutile structure and the material shows reflective properties for mid- and far-IR radiation [1]. Frequently, through temperature controlling, the glass shifts from allowing light to pass through to blocking certain part (or all) light wavelengths. Photochromic, thermochromic, and electrochromic [2], as well as micro-blind and polymer dispersed liquid crystal devices [3], are some of the most recent smart glass technologies. In terms of practicality, smart windows are those that partially exclude undesirable solar radiation. It can be improved in cold climates by increasing heat gain and decreasing it in hot climates by dynamically taking on glasses irradiative and thermal characteristics [3]. By managing the falling solar heat flow, an appropriate absorbing layer on the surface of the glass can affect the optical properties of the glass [4]. Low-emissive coatings are spectrally selective films that allow visible light to pass through while blocking infrared and ultraviolet wavelengths [5].
Monoclinic vanadium dioxide in nanostructure is a first material for understanding correlation effects in solids with completely reversible phase transitions, as well as for further applications in smart devices [6]. The most crucial vanadium oxide phases are VO, V2O3, VO2, and V2O5, which reveal different oxidation states V2+, V3+, V4+, and V5+ respectively. The first discovery of the VO2 transition was in 1959 by F. J. Morin. It exhibits a monoclinic crystal structure at low temperatures [7]. When this oxide is heated over the transition temperature (about 60–70 °C), the lattice changes from monoclinic to tetragonal, allowing the material to become IR reflective. This high temperature is unsuitable for many uses. To make the VO2 more commercially feasible, commonly, dopants added to reduce its transition temperature, [8]. Several dopants incorporate into vanadium (IV) oxide many experimental investigations on the W × V1 × O2 systems have been carried out, with the goal of presenting some mechanistic and structural explanations [9,10,11]. The charge-transfer mechanism takes place, given the fact that tungsten (VI) ion replaces vanadium (IV) ion.
This mechanism is assumed to be both through the placing extra electrons into the vanadium d band, plus the larger ionic radius of tungsten over vanadium [12]. Several studies, Booth et al. [11], Whittaker et al. [9] and Patridge et al. [13] and Netsianda et al. [14], according his research suggested that dopants cause a local structural deformation changing the structure from asymmetric monoclinic phase toward high symmetry of VO2 rutile phase.
The preparation of vanadium oxide films is quite complicated since it is influenced by some growth parameters, practically, the annealing time and temperature in the final step [15]. Up to 800 °C, some of surface vanadium oxide species showing are stable state, while surface vanadia species to crystalline V2O5 conversions occur between 800 and 900 °C [16].
The paper aims to examine the effect of heat-treated temperature on the phase transformation of vanadium oxide films and thermochromic capability, starting from vanadium alkoxides and heat treated under an inert atmosphere.
Experimental
Material
The significant chemicals used, in this work, were vanadium oxytripropoxide (VOTP) 98%, and tungsten hexachloride ≥ 99.99% all supplied from Sigma-Aldrich. Anhydrous isopropyl alcohol ≥ 98 supplied from Chemistry lab. The deionized water used as a solvent.
Procedure
Vanadium oxide films prepared via sol–gel technique through mixing vanadium oxytripropoxide in anhydrous isopropyl alcohol under stable stirring for 45 min at room temperature. To prepare the impurity-doped films, at 4% of WCl6, was dissolved in ethanol then introduce into the vanadium solution. The colorless sol herein turned to bright yellow upon blending WCl6, after stirring for 45 min, the solution color then shifts from yellow to blue. The shifted occurs because W6+ (yellow) is reduced to W5+ (blue) by means of an organic solvent.
The films were coated on the quartz substrates at a spin velocity of 5000 rpm for 60 s, then dried in air for 45 min at 40 °C to drive off the solvent. Finally, the films were then heat treated at several temperatures degrees 500 °C, 1000 °C, 1500 °C, and 2000 °C in the air at a rate of 1 °C/min, kept at these temperatures for 30 min, Specimens are cooled to room temperature inside the furnace.
Characterization
Using a double beam (Ultrospec. 4300 pro) UV–Visible spectrophotometer in range of (300–1000) nm transmittance spectra been measured, and in the IR region until 2500 nm using a Perkin Elmer UV Win Lab, Lambda 900. XRD spectra were recorded using an instrument from (XRD: Philips, model: X'Pert MPD), which was operated at (40 kV) and (30 A Field-Emission Scanning Electron Microscope (model/S41-60 from “Hitachi”,) was used to examine the morphology and microstructure of the films.
Results
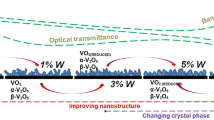
Different crystal sites and sizes are formed at different heat-treating temperatures, resulting in a variety of crystal sites and sizes, this process, usually, cause variation in the film’s transmittance. This variation may be due to the scattering phenomenon, particularly in the visible region, Fig. 1. As a consequence of smart window requirements, it is essential to keep the entrance of visible light throws the windows as much as possible. Apparently, at 2000 °C, the film shows almost unvaried transmittance, ranges about 60% in the entire visible as well as in the near IR region. As a result, it may be the most popular option in this case.
SEM investigation
Figure 2 shows SEM images of a non-doped vanadium oxide film heat-treated at 2000 °C. The film has a well-distributed multilayer crystalline structure with well-joined layers. Layer by layer, the structure appears to be repeating itself, accumulating tiny pyramid forms. The vanadium oxide layer was condensing into rather big particles (0.5–2.0 m) at this high temperature.
However, increasing the heat-treating temperature till 2000 °C can well arrange these large particles regardless of the grain’s alignment in the films. Furthermore, the film’s optical transmittance may be reduced due to the irregular alignment of these grains in the microstructure. Nevertheless, rather than creating a mass of adjacent crystals, this recipe resulted in significant, isolated crystals of uniform striped cones shape. Several separated nanoparticles arranged as a star like shaped are covered the large particles affected by the crystal site but lays on the top of the crystal surface. The initiated of these nanoparticles may attribute to the existing of several vanadium oxidation states.
Figure 3 presents the images of tungsten chloride-doped film heat-treated at several temperatures, the images appeared to show distinct crystal sites at first glance, which was to be expected given that tungsten chloride was used.
At low heat-treated temperature 500 °C, the lack of sufficient energy may not give a chance to the initiated vanadium oxide to interact with each other resulting in new oxidation state nor linked with tungsten. At 1000 °C the image reflects how vanadium oxide bits and pieces start from pointing together. At 1500 °C two distinct phases were shown, however, the sticks like V2O5 phase dominate. At 2000 °C the vanadium oxide nanoparticles dispersed on the prior mentioned striped cones due to the high treating temperature, whereas, it may utilize tungsten ions to boost their development, resulting in well-defined tree leaves that act as crystal sites. On the film surface, these well-structured crystal sites predominated, and they may have obliterated the prior striped cones sites. None of these films have a consistent particle size distribution, and we shouldn’t expect them to. Several crystal locations made of various vanadium oxides connect with tungsten oxide with varying degrees of likelihood.
We prepared a thick film of 4 at % tungsten chloride-doped vanadium oxides through five spin-coated layers. This film crashed, and the resulting powder was heat treated at 2000 °C then its XRD spectrum is recorded, Fig. 4a.
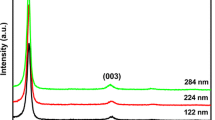
Naturally, the extracted powder reflects well definite peaks. As dictated by chart inserted, JCDPS card No. 82-0661, Fig. 4a, the pattern belongs to monoclinic VO2 (M), almost peaks identically present.
The annealing temperature influence on the vanadium oxide crystal structure is present in Fig. 4b. Several crystal sites can be present in the films XRD spectra as the heat-treated temperature rises from 500 to 800 °C corroborated by their SEM images. As an example, the peaks 2θ (21.50, 47.55) may relate to V2O5 according to (9–0387). Whereas, the broad peak is located at 2θ (40.91) which related may be to V2O3 according to (26–278). Finally, the broad peak is also located at 2θ (35.7) and a sharp peak at 2θ (37.8) may relate to VO2 according to (31–1439) and 82–0661 respectively. XRD spectra reveal that as the heat-treating temperature raising the number of the crystal sites were decreased, accomplishing approximately just two sites at 2000 °C. These crystal sites were related to the oxidization state of VO2 and V2O5 as confirmed by SEM images Fig. 3.
XRD investigation
We prepared a thick film of 4 at % tungsten chloride-doped vanadium oxides through five spin-coated layers. This film crashed, and the resulting powder was heat treated at 2000 °C then its XRD spectrum is recorded, Fig. 4a.
Naturally, the extracted powder reflects well definite peaks. As dictated by chart inserted, JCDPS card No. 82-0661, Fig. 4a, the pattern belongs to monoclinic VO2 (M), almost peaks identically present.
The annealing temperature influence on the vanadium oxide crystal structure is present in Fig. 4b. Several crystal sites can be present in the films XRD spectra as the heat-treated temperature rises from 500 to 2000 °C corroborated by their SEM images. As an example, the peaks 2θ (21.50, 47.55) may relate to V2O5 according to (9–0387). Whereas, the broad peak located at 2θ (40.91) may be related to V2O3 according to (26–278). Finally, the broad peak is located at 2θ (35.7) and a sharp peak at 2θ (37.8) may relate to VO2 according to (31–1439) and 82–0661 respectively. XRD spectra reveal that as the heat-treating temperature raising the number of the crystal sites were decreased, accomplishing approximately just two sites at 2000 °C. These crystal sites were related to the oxidization state of VO2 and V2O5 as confirmed by SEM images Fig. 3
Thermochromic investigation
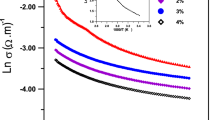
The thermochromic capability investigated through two categories. Firstly; by recording the UV–VIS transmission spectra for the vanadium films under two different temperatures; 30 °C and 80 °C. These two temperatures demonstrate the room temperature and above the transition temperature, 68 °C. Secondly; by recording the IR transmission at specific IR wavelength (1300 nm). This test was done at different temperatures starting from room temperature (30 °C) rising each 10 °C up to (80 °C). The thermochromic capability of these films will be investigation via drawing their hysteresis loop by way the metal-to-semiconductor transition.
The transmittance spectra of films heat-treated at four different temperatures 500 °C, 1000 °C, 1500 °C, and 2000 °C, are illustrated in Fig. 5 a, b, c, and d respectively. It appears that 2000 °C sample expose high visible transmittance, particularly below 500 nm, about 50% with an approximately adequate thermochromic response.
As Adle mentioned [16], a first-order transition occurs when the free energy of the metallic vanadium oxide state is less than the local minimum of its semiconducting state. On the other hand, if this local minimum exists indefinitely up to the temperature of the second-order transition, a metastable condition may result. When the material heated during their semiconducting condition, this hysteresis would emerge as a local minimum of free energy. If the transition is second-order, hysteresis does not occur; nevertheless, VO2 undergoes first-order transitions [16]. When tungsten is used to dope vanadium oxides, a charge-transfer process occurs because the tungsten (VI) ion replaces the vanadium (IV) ion. The mechanism is considered to include the introduction of extra electrons into the vanadium d-band, in addition to the larger ionic radius of tungsten over vanadium [12].
This width should be narrow enough to optimize the power advantage of using thermochromic films of this sort. This discrepancy might be attributed to a higher number of defects in the VO2 lattice, which would result in a larger divergence in the temperatures at which various areas of the film would transition, making the material even more appropriate for an innovative material coating [17].
As can be seen from Table 1, the results we achieved by varying the types and percentages of materials used are summarized in Figs. 6 and 7 Vanadium dioxide thin films were effectively deposited on quartz substrates using the VOTP low-temperature sol–gel process.
Conclusion
Using the VOTP low-temperature sol–gel method, vanadium dioxide thin films were successfully produced on quartz substrates under optimal conditions. Perhaps the most important outcome of this research is the lowering of the tungsten-doped vanadium oxide transition temperature from 95 to 70 °C degrees Celsius, which is often observed outside buildings in several countries, especially during the summer season. SEM results reveal that there is always existing of the secondary phase of vanadium oxides at all heat-treating temperature. The XRD results showed that by increasing the film heat treating temperature, about 2000 °C, the grain size increased significantly, that is why peak width decrease accordingly. The optical properties demonstrate roughly dependence on the film’s nanostructure.
References
C.-G. Granqvist, Oxide electrochromics: why, how, and whither. Sol. Energy Mater. Sol. Cells 92(2), 203–208 (2008)
S. Huang, Thin solid films, determination of optical constants of functional layer of online low-E glass based on the Drude theory. Thin Solid Films 516, 79–83 (2008)
Z. Ren, Y. Huang, M. Shen, C. Song, W. Weng, G. Han, N. Ma, P. Du, Electrical and corrosion properties of the Ti5Si3 thin films coated on glass substrate by APCVD method. J. Non-Cryst. Solids 357(15), 2802–2809 (2011)
A.M. Alattar, B.T. Chiad, W.A. Twej, R.A. Mohammed, Effects of TEOs aerogel particles size of TEOS aerogel on its mesoporous structure and thermal behavior via supercritical drying and high temperature. Iraqi J. Sci. pp. 119–128. (2019)
R. Chen, L. Miao, C. Liu, J. Zhou, H. Cheng, T. Asaka, Y. Iwamoto, S. Tanemura, Shape-controlled synthesis and influence of W doping and oxygen nonstoichiometry on the phase. Sci. Rep. 5(1), 1–12 (2015)
F.J. Morin, Oxides which show a metal-to-insulator transition at the neel temperature. Phys. Rev. Lett. 3(1), 34 (1959)
W. Pierce, B. Goodenough, Structure of orthorhombic V0.95 Cr0.05 O2. Phys. Rev. B 5(10), 4104 (1972)
L. Whittaker, T. Wu, C. Patridge, G. Sambandamurthy, S. Banerjee, Distinctive finite size effects on the phase diagram and metal–insulator transitions of tungsten-doped vanadium(iv) oxide. J. Mater. Chem. 21, 5580 (2011)
C. Tang, P. Georgopoulos, F.M.E. Fine, J.B. Cohen, M. Nygren, G.S. Knapp, A. Aldred, Local atomic and electronic arrangements in WxV1–xO2. Phys. Rev. B 31, 1000–1011 (1985)
J.M. Booth, S.P. Casey, Anisotropic structure deformation in the VO2 metal-insulator transition. Phys. Rev. Lett. 103, 086402 (2009)
P. Jin, S. Nakao, S. Tanemura, Tungsten doping into vanadium dioxide thermochromic films by high-energy ion implantation and thermal annealing. Thin Solid Films 324, 151–158 (1998)
C.J. Patridge, L. Whittaker, B. Ravel, S. Banerjee, Elucidating the influence of local structure perturbations on the metal-insulator transitions of V1–xMoxO2 nanowires: mechanistic insights from an X-ray absorption spectroscopy study. J. Phys. Chem. C 116(5), 3728–3736 (2012)
M. Netsianda, E.P. Ngoepe, C.C. Richard, A. Catlow, M.S. Woodley, The displacive phase transition of vanadium dioxide and the effect of doping with tungsten. Chem. Mater. 20, 1764–1772 (2008)
S.A. Corr, M. Grossman, J.D. Furman, B.C. Melot, A.K. Cheetham, K.R. Heier, R. Seshadri, Controlled reduction of vanadium oxide nanoscrolls: crystal structure, morphology, and electrical properties. Chem. Mater. 20, 6396–6404 (2008)
J.M. Jehng, I.E. Wachs, F.T. Clark, M.C. Springman, Raman characterization of alumina supported MO-V-Fe catalysts: influence of calcination temperature. J. Mol. Catal. 81, 63–75 (1993)
D. Adler, Mechanisms for metal-nonmental transitions in transition-metal oxides and sulfides. Rev. Mod. Phys. 40, 714–736 (1968)
A.M. Alattar, Spectral and structural investigation of silica aerogels properties synthesized through several techniques. J. Non-Cryst. Solids 571, 121048 (2021)
Acknowledgements
I would like to express my gratitude to the Iraqi Ministry of Higher Education and Scientific Research for their help. I'd also want to thank AL-Karkh University of Science for their invaluable support
Funding
The current study received no financing from any public, commercial, or non-profit sources. As a result, its personal effort and statement of interests are entirely mine, and its support has been subjective during the three years of research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alattar, A.M. Optical and nanostructure properties of the tungsten chloride doped of vanadium oxide xerogel thin film under high temperature. J Opt 52, 424–430 (2023). https://doi.org/10.1007/s12596-022-00905-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12596-022-00905-0